Abstract
Background: Cabotegravir (GSK1265744) is an integrase strand transfer inhibitor in development as a long-acting (LA) intramuscular injectable suspension for HIV-1 pre-exposure prophylaxis (PrEP).
Objective: We report participant outcomes from the phase IIa ECLAIR study related to tolerability, acceptability, and satisfaction of cabotegravir LA.
Methods: The ECLAIR study (ClinicalTrials.gov identifier, NCT02076178) was a randomized, placebo-controlled study in healthy men not at high risk of acquiring HIV-1. Participants were randomized (5:1) to once-daily oral cabotegravir 30 mg or placebo tablets for 4 weeks, followed by gluteal intramuscular injections of cabotegravir LA 800 mg or saline placebo every 12 weeks. The primary objective was to evaluate the safety of cabotegravir LA over three injection cycles (to Week 41). Secondary objectives assessed the tolerability, satisfaction, and acceptability of cabotegravir LA.
Results: Among 115 participants who received injections in the cabotegravir (n = 94) and placebo (n = 21) groups, 93% (n = 87) and 95% (n = 20) completed the injection phase, respectively. Injection intolerability led to withdrawal in 4 participants (4%) receiving cabotegravir LA. The most frequently reported Grade ≥2 adverse event was injection-site pain. Most participants (74% [n = 67]) receiving consecutive injections favored cabotegravir LA vs oral cabotegravir. Most participants were satisfied with cabotegravir LA (75% [n = 64]), were willing to continue (79% [n = 68]), and would recommend (87% [n = 75]) the therapy.
Conclusions: While Grade ≥2 injection-site pain was common, most participants reported overall satisfaction with and preference for cabotegravir LA, with few discontinuations due to injection intolerance. These findings support investigation of cabotegravir LA as an alternative to daily oral PrEP regimens.
Introduction
Limited pre-exposure prophylaxis (PrEP) strategies for prevention of HIV-1 infection exist for men and women at high risk of HIV-1 exposure. Vulnerable populations at risk for HIV-1 exposure include men who have sex with men (MSM), incarcerated individuals, people who inject illicit drugs, sex workers, and transgender people. The World Health Organization recommends preventive strategies to reduce the risk of HIV-1 infection that include male and female condom use with condom-compatible lubricants, instituting needle, and syringe programs for those who inject illicit drugs, and use of oral PrEP.Citation 1 No single prevention strategy has been shown to be successful for all individuals; hence, alternative intervention strategies should be considered and made available for PrEP. Oral tenofovir disoproxil fumarate + emtricitabine (TDF/FTC) is approved by the US Food and Drug AdministrationCitation 2 for PrEP, and reductions in HIV incidence ranging from 44% to 86% have been demonstrated in PrEP clinical trials among specific populations, including MSM (iPrEX, PROUD, and ANRS IPERGAY trials), heterosexual men and women (TDF2 trial), serodiscordant heterosexual couples (Partners PrEP), and users of injection drugs (Bangkok Tenofovir study).Citation 3–8 However, challenges with daily, oral TDF/FTC have provided opportunities for improvement with the development of alternative PrEP agents. A recent meta-analysis demonstrated that TDF is associated with small, subclinical decreases in bone mineral density and occasional renal and liver function impairment when used as PrEP.Citation 9 Side effects from the TDF/FTC combination for PrEP can affect patient compliance, which is critical for reducing the risk of HIV-1 transmission, as demonstrated by the strong relationship between clinical efficacy and adherence to PrEP.Citation 4–6 , Citation 10 Alternate forms of administration, such as an injectable medication, may provide an opportunity for improving compliance in individuals who may have difficulty adhering to a daily oral PrEP regimen.
Cabotegravir is a long-acting (LA) injectable HIV-1 integrase strand transfer inhibitor in phase III clinical development for the treatment and prevention of HIV-1 infection. Phase I studies demonstrated that cabotegravir LA has a half-life of 25–54 days, making it possible to dose individuals monthly to once every 2 months using an aqueous suspension for injectable administration.Citation 11 For HIV treatment, cabotegravir is being evaluated as part of a novel two-drug combination with the non-nucleoside reverse transcriptase inhibitor rilpivirine as LA intramuscular maintenance therapy in patients infected with HIV.Citation 12 For HIV prevention (PrEP), cabotegravir is being evaluated as monotherapy and may be an ideal candidate for PrEP because its long half-life enables use of longer dosing intervals and may be an attractive option for individuals with challenges to adhering to other preventive options.
Because an LA injectable may address some of the compliance issues previously associated with daily oral medication regimens,Citation 13 , Citation 14 it is also important to understand patient acceptance and satisfaction associated with injectable administration. Herein we present patient-reported outcomes (PROs) from the ECLAIR study that assessed participant acceptance of and satisfaction with cabotegravir LA.
Methods
Study design
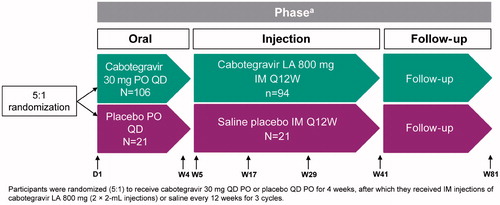
ECLAIR was a phase IIa, randomized, multisite, two-arm, double-blind study conducted in the United States in healthy men, including MSM who were not at high risk of acquiring HIV at the time of screening (ClinicalTrials.gov identifier, NCT02076178).Citation 15 The study design of ECLAIR has been previously described.Citation 15 Briefly, eligible patients were randomized at a ratio of 5:1 to receive cabotegravir LA or saline placebo at 10 study centers, including in New York and San Francisco (). Participants who entered the injection phase received cabotegravir 800 mg or saline intramuscular (IM) injections every 12 weeks for three cycles (36 weeks). At each injection visit, site staff administered two 2-mL intramuscular injections of cabotegravir LA (400 mg) or saline placebo with a 1.5- to 2-inch, 25-gauge needle in the ventrogluteal site (gluteus medius).
Figure 1 ECLAIR study design.
D, day; IM, intramuscular; LA, long-acting; PO, oral administration; Q12W, every 12 weeks; QD, once daily; W, week.
aNot all scheduled study visits are shown in the flowchart.
IM injections were given at Weeks 5, 17, and 29.
The primary objective of ECLAIR was to evaluate the safety and tolerability of cabotegravir LA in men not infected with HIV. Secondary objectives included assessments of the satisfaction, tolerability, acceptability, and pharmacokinetics of cabotegravir injections during Weeks 5–41.
Ethics committee approval was obtained at all participating centers according to the principles of the 2008 Declaration of Helsinki.
Study participants
Before enrollment in this study, written informed consent was obtained from each eligible patient. Entry and exclusion criteria for ECLAIR have been previously described.Citation 15
Volunteers could withdraw from the study at any time. Reasons for participants to withdraw included confirmed HIV infection after enrollment, adverse event (AE), protocol deviation, lost to follow-up, investigator discretion, study volunteer or investigator noncompliance, or intolerability of injections.
Assessment of ISRs
The safety population included participants who received ≥1 dose of study medication and was included in the analysis of PROs. Investigator evaluation of injection-site reactions (ISRs) included assessments of pain, tenderness, pruritus, warmth to touch, infections, rash, erythema, swelling, induration, and nodules (granulomas or cysts). Information about symptoms associated with ISRs (e.g. pain, itching, warmth to touch, redness, swelling, documentation of whether the participant had to seek medical advice) were collected by the participant in diaries. A 7-day postinjection diary was dispensed at the injection visit at Weeks 5, 17, and 29 and returned to the respective clinics 1 week later. An ISR postinjection diary covering Weeks 2–12 was distributed at Weeks 6, 18, and 30, and participants were asked to bring it to each clinic visit so that it could be reviewed by clinical staff.
Data were analyzed using SAS software (version 9.1 or higher; SAS, Cary, NC, USA).
PRO assessments
The Study Medication Satisfaction Questionnaire (SMSQ) is a self-reported measure that evaluates the satisfaction to study medication (e.g. cabotegravir LA) for prevention of HIV in uninfected patients.Citation 16 The SMSQ was adapted from the HIV Treatment Satisfaction Questionnaire and included 11 questions relevant to injectable treatments administered in ECLAIR.Citation 17 The most relevant changes to the questionnaire were to exclude questions specifically related to HIV treatment and to avoid mention of “HIV,” as the study participants were HIV negative with a low to medium risk of acquiring HIV infection.Citation 16 Two versions of the SMSQ, the status version (SMSQs; Supplemental Table 1) and change version (SMSQc) were used (Supplemental Table 2). These questionnaires assessed the change in treatment satisfaction over time within the cabotegravir and placebo groups (SMSQs) and compared the current level of satisfaction of cabotegravir LA at Week 18 with oral cabotegravir taken during the 4-week induction (SMSQc). The SMSQs had seven response options scored on a Likert scale that ranged from 0 (very dissatisfied) to 6 (very satisfied). The SMSQc also had seven response options that ranged from –3 (greatest decrease in satisfaction) to 3 (greatest increase in satisfaction). The overall SMSQs score could range from 0 to 66, and the questionnaire was administered at Weeks 6, 18, and 30 and at withdrawal. The overall SMSQc score could range from –33 to 33 and the questionnaire was administered at Week 18 only. The seven response options were recoded to simplify reporting of the SMSQs results, with a response of 0–2 classified as “dissatisfied,” a response of 3 classified as “neutral,” and a response of 4–6 classified as “satisfied”; for the SMSQc, a response of –3 to –1 was classified as “dissatisfied,” a response of 0 was classified as “neutral,” and a response of 1–3 was classified as “satisfied.”
Table 1 ISR-related symptoms during injection phase.Table Footnote a
The Study Medication Questionnaire (SMQ) consists of one 4-part question and evaluates participants’ adherence with their medication regimen (Supplemental Table 3). The SMQ was administered at Weeks 6, 18, and 30 and at withdrawal. The SMQ had seven response options ranging from 0 (none of the time or none at all) to 6 (all of the time or a great deal), with the exception of question 1a, which provided six response options that corresponded to frequency of dosing, ranging from daily to every 12 weeks or did not know. For participants answering ≥50% of the questions (≥6 questions) for all questionnaires, missing responses were determined by averaging the available values and multiplying by 11.
In a separate substudy of participants in ECLAIR, qualitative interviews were conducted with 26 volunteers at two sites (New York, n = 15; San Francisco, n = 11) to determine participants’ experience with cabotegravir LA.Citation 18 Oral consent was obtained from the Johns Hopkins Bloomberg School of Public Health Institutional Review Board onsite.
Results
Study disposition
Healthy males (n = 205) were screened for inclusion in the study, and 127 were randomly assigned (5:1) to receive injections with either cabotegravir (n = 106) or placebo (n = 21). The median age of the participants was 31 years. The majority were white (56%); African Americans comprised 31% of participants. Compared with the placebo group (76%), more participants in the cabotegravir treatment group (85%) had an HIV risk factor of homosexual contact. The cabotegravir group included fewer participants with an HIV risk factor of heterosexual contact (21%) compared with the placebo group (29%). Nineteen participants withdrew from the cabotegravir group during the study. One participant withdrew before treatment, and 11 participants withdrew during or after the oral dosing phase but prior to injections.Citation 15 Of the 94 participants in the cabotegravir group included in the injection phase, seven participants withdrew during this phase of the study, four participants (4%) of whom withdrew because of intolerability to injections (not considered an AE).Citation 15 One participant in the placebo group withdrew from the study; the withdrawal occurred during the injection phase due to HIV infection.
Safety and tolerability
Overall AEs reported at any time during the oral and injection phases occurred with similar frequency in both treatment groups (cabotegravir, 96%; placebo, 90%). In the cabotegravir group, 80% (n = 75) of participants reported a Grade ≥2 AE compared with 48% (n = 10) in the placebo group (P < 0.01).
During the oral phase, 56 AEs occurring in ≥5% of participants were reported in the cabotegravir group (53%) compared with eight in the placebo group (38%), with the most frequently reported AEs being diarrhea (cabotegravir, 8%; placebo, 10%) and headache (cabotegravir, 9%; placebo, 5%). However, the overall frequency of Grade 2–4 AEs reported was similar for both groups (cabotegravir, 23%; placebo, 19%). Treatment-related AEs were reported by 36% of participants in the cabotegravir group (n = 38) compared with 14% in the placebo group (n = 3), with the majority of events being of Grade 1/2 in severity. No serious AEs were reported in the oral phase.
During the injection phase, participants in the cabotegravir (n = 94) and placebo (n = 21) groups underwent a total of 272 and 62 injections, respectively. In the cabotegravir group, 87 participants (93%) experienced ISR events compared with 12 (57%) in the placebo group (). In the cabotegravir treatment group, 80% (n = 75) and 56% (n = 53) of participants experienced ISR-related AEs that were Grade 1 or 2 in severity, respectively; 19% (n = 18) experienced ISR-related AEs that were Grade 3; no events were reported as Grade 4. All ISRs reported by participants in the placebo group were Grade 1 (n = 11; 52%) or 2 (n = 1; 5%). In the cabotegravir group, 92% of ISRs were attributed to injection-site pain compared with 27% of ISRs in the placebo group. The mean duration of injection-site pain was 5.4 days for the cabotegravir group versus 2.0 days for placebo. In addition, the number of events of injection-site pain (Grades 1–3) reported by the cabotegravir group decreased over the three injections (injection 1, 95 events; injection 2, 82 events; injection 3, 73 events), with the caveat that the number of participants receiving injections also decreased. In both treatment groups, all ISR-related AEs resolved, no ISR resulted in withdrawal, and study medication was continued for all participants. Although no ISR-related AEs were categorized as directly leading to withdrawal, patient-reported injection intolerability led to withdrawal in four participants (4%) who received cabotegravir during the injection phase of the study.
PROs
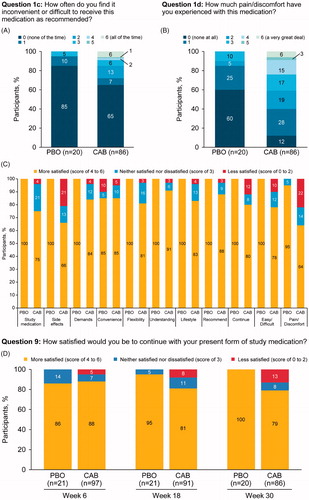
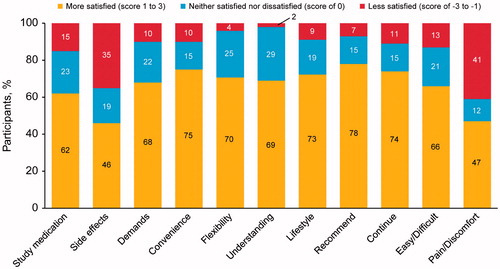
At Week 30, the majority of participants in both groups (cabotegravir, n = 56 [65%]; placebo, n = 17 [85%]) found it neither inconvenient nor difficult (SMQ score of 0) to receive the study medication as recommended (). A total of 12% of participants in the cabotegravir group reported no pain or discomfort on the SMQ (scores of 0–2) compared with 60% in the placebo group. In the cabotegravir treatment group, 6% of participants reported pain/discomfort “a very great deal” compared with 0% in the placebo group. At Week 30, participants were generally satisfied with the study medication based on the SMSQs (placebo, 100%; cabotegravir, 74%). Overall, participants reported a willingness to recommend the treatment (cabotegravir, 87%; placebo, 100%) and continue the study medication (cabotegravir, 79%; placebo, 100%). Tolerability assessments of cabotegravir injections showed that 66% (n = 57) of participants were satisfied with the side effects and 64% (n = 55) were satisfied with the amount of pain/discomfort. Throughout the course of the study, ≥79% of participants in the cabotegravir group reported a level of satisfaction sufficient to continue with the study medication (Week 6, 88%; Week 18, 81%; Week 30, 79%). When comparing views of treatment at Week 18 using the SMSQc, participants were more satisfied with cabotegravir LA than with oral cabotegravir, particularly on items related to convenience, flexibility, and lifestyle (). Approximately 25% of participants in the cabotegravir treatment group reported dissatisfaction with the amount of discomfort or pain associated with the study medication during the injection phase. However, 74% (n = 67/91) of participants were satisfied to continue treatment with cabotegravir LA, and few (4%; n = 4/94) withdrew from the study because they reported injection intolerability.
Discussion
The only pharmacologic option approved for PrEP is daily oral TDF/FTC, which has demonstrated variable rates of efficacy and protection, correlating with patient noncompliance.Citation 13 , Citation 14 , Citation 19 Alternative options for PrEP regimens, such as the cabotegravir LA injectable evaluated in ECLAIR, may be useful for improving patient adherence. Results from ECLAIR showed that participants receiving cabotegravir 800 mg every 12 weeks for three cycles were largely satisfied with the treatments and were willing to continue in the study despite the associated transient injection-site pain.
With respect to tolerability, significantly more Grade ≥2 AEs were observed in the cabotegravir treatment group (80%) compared with placebo (48%; P < 0.01), primarily attributed to the different number of Grade 2–3 injection-site pain events reported. The majority of ISRs reported in the cabotegravir treatment group were Grade 1 or 2 pain, with lower numbers of participants reporting Grade 1 pruritus, nodules/bumps, warmth to touch, bruising, and induration. The mild-to-moderate severity of ISRs in participants treated with cabotegravir was consistent with previous reports.Citation 11 , Citation 20 All of the ISR-related AEs resolved <1 week after the injection. Whereas no ISR-related AEs were categorized as directly leading to withdrawal, patient-reported injection intolerability led to withdrawal in four (4%) of 94 participants who received cabotegravir during the injection phase. Similar proportions of participants in the cabotegravir and placebo groups completed all three injection visits, demonstrating that differences in injection-site pain did not influence withdrawal rates between treatment groups during the injection phase. These results are consistent with results from the SMQs, in which ∼75% of participants reported no dissatisfaction with the amount of pain or discomfort they experienced with the study medication, and 74% reported a desire to continue treatment with cabotegravir LA. Additionally, 85% of participants almost never found it difficult to comply with the 12-week dosing schedule. Assessment of the tolerability of cabotegravir injections from the SMSQs showed that a majority of participants was satisfied with the side effects they experienced and tolerated the pain/discomfort.
The SMSQs indicated that overall satisfaction with the study medication remained consistent during the injection phase, with similar overall scores at Weeks 6, 18, and 30. A majority of participants reported that they were satisfied with the medication, were willing to continue treatment, and would recommend cabotegravir LA treatment. The SMSQc individual item scores assessed at Week 18 (1 week after the second injection) indicated that, with few exceptions, most participants in both treatment groups were more satisfied with the injectable medication compared with tablets administered during the oral phase of the study. Similarly, results from a recent qualitative study with gay and bisexual men in the United States suggest that 46% of participants preferred an LA injectable compared with oral PrEP (14%).Citation 21 Clinical data showed that the efficacy of oral PrEP significantly correlated with detectable drug levels in participants samples (Pearson correlation =0.86, P = 0.003), demonstrating the importance of treatment adherence.Citation 10 Therefore, options in addition to oral PrEP may provide opportunities for alternative forms of HIV prevention that accommodate each individual’s lifestyle.
In-depth interviews conducted in New York and San Francisco with a subgroup of 26 participants from ECLAIR provided additional information from participants. The qualitative interviews demonstrated the importance of the convenience of an LA injectable as providing “peace of mind,” an overall sense that the participants were interested in continuing treatment even with minor injection soreness, noting a perceived advantage of not “having to remember to adhere to a daily oral regimen.”Citation 18 All participants reported being satisfied with cabotegravir LA injection for PrEP, would recommend it as an alternative treatment, and would be interested in continuing when the option becomes commercially available. Disadvantages identified included the needles being relatively long and the location of the injection site was uncomfortable. However, participants mostly agreed that the AEs were worth the benefit of preventing HIV infection. In addition, several would have preferred the injections to occur at a less-frequent rate (e.g. every 6 months, once yearly) instead of every 3 months. People who may benefit from an LA injectable include those who would like to have confidence in LA protection from HIV infection or those with adherence challenges related to their work or lifestyle, such as those with an atypical routine or those at high risk (e.g. sex workers, serodiscordant couples, users of illicit intravenous drugs) for HIV-1 infection.
Although ECLAIR showed general tolerability and satisfaction for treatment with cabotegravir, certain limitations exist based on the characteristics of the study population and design. Statistical comparisons were challenging because of the sample size differences between the groups due to the 5:1 randomization to cabotegravir or placebo. Furthermore, enrolling only men (predominantly white) from US sites is not fully reflective of the global, US, or European HIV-positive population,Citation 22 so it is unclear whether these study outcomes can be generalized to other countries or to women. At present, it is unclear whether people at high risk of HIV infection would show different preferences than the lower-risk participants of ECLAIR. Lastly, participants agreeing to participate in ECLAIR were willing to receive an injectable treatment, so they may have been more likely to report satisfaction and acceptability of the injectable compared with the overall at-risk population.
The PROs from ECLAIR demonstrate the acceptability and tolerability of cabotegravir 800 mg every 12 weeks and provide a patient-centered perspective. Whereas Grade 1 and 2 injection-site pain was commonly associated with cabotegravir injections, participants experienced a high level of overall satisfaction and preference for an LA injectable on dimensions that included convenience, flexibility, and ease of use. These results support further development of cabotegravir LA injection as an alternative to oral PrEP in people at high risk of HIV infection, particularly the first phase III clinical study (HPTN 083; ClinicalTrials.gov identifier NCT02720094) that was initiated in December 2016 to compare cabotegravir LA injection every 2 months with daily oral TDF/FTC in HIV-uninfected cisgender men and transgender women who have sex with men.
Disclosure statements
MIM, KJH, PP, ARR, WRS, and DAM are currently employed by ViiV Healthcare and are shareholders in GlaxoSmithKline. MM reports grants from GlaxoSmithKline and personal fees from Gilead Sciences, Merck, and Bristol Myers Squibb. IF reports grants from ViiV Healthcare, personal fees from Gilead Sciences and ViiV Healthcare, and acknowledges support from award P30 AI 045008 from the Penn Center for AIDS Research. RMG reports receiving grants from ViiV Healthcare. KHM reports receiving grants from Gilead Sciences and ViiV Healthcare. BSS and SLF were employed by GlaxoSmithKline for a portion of the time that the study was being conducted.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplemental Material
Download MS Word (58.5 KB)Acknowledgments
All listed authors meet the criteria for authorship set forth by the International Committee of Medical Journal Editors. This study was funded by ViiV Healthcare. Editorial assistance was provided under the direction of the authors by Jeffrey Stumpf, Julie B. Stimmel, and Sherri Damlo, MedThink SciCom, and was funded by ViiV Healthcare.
Additional information
Funding
Notes on contributors
Miranda I. Murray
Miranda I. Murray, PhD, is the Head of the Health Outcomes group within the medical organization at ViiV Healthcare. Prior to joining ViiV Healthcare, Dr Murray worked at the English Health Protection Agency on Surveillance and Epidemiology of Healthcare Associated Infections. Her current research interests include behavioral research; patient/physician communication; and patient-reported outcomes measures in clinical trials. Dr Murray holds a PhD from Johns Hopkins University, Bloomberg School of Public Health.
Martin Markowitz
Martin Markowitz, MD, is the Clinical Director and Staff Investigator at the Aaron Diamond AIDS Research Center and an Aaron Diamond Professor at the Rockefeller University. His research interests include the pathogenesis and treatment of acute HIV-1 infection, the fitness and transmission of drug resistant HIV-1, and investigations of numerous novel antiretroviral agents, as well as pathogenesis-based interventional trials. Dr Markowitz earned his MD from Stanford University School of Medicine and completed clinical and research fellowships in hematology/oncology at Cornell Medical College and a clinical fellowship in the Cornell Medical College Division of Infectious Diseases.
Ian Frank
Ian Frank, MD, is an Attending Physician; Director, Antiretroviral Clinical Research; and Associate Professor, Department of Medicine in the Infectious Diseases Division of the Hospital of the University of Pennsylvania. His research interests include HIV vaccinology, prevention, treatment, and pathogenesis. Dr Frank earned his MD from Dartmouth Medical School and is a member of many professional and scientific societies, including the American Association for the Advancement of Science, the Infectious Diseases Society of America, and the International AIDS Society.
Robert M. Grant
Robert M. Grant, MD, directs the Gladstone-University of California, San Francisco (UCSF) Laboratory of Clinical Virology, serves as Associate Director of the UCSF Center for AIDS Research, directs a research laboratory, and has a clinical practice in the Division of Pulmonary and Critical Care Medicine at San Francisco General Hospital. His active research focuses on the clinical and immunologic consequences of virologic failure of antiretroviral therapy and antiretroviral resistance. Dr Grant received his MD and completed a research fellowship in Molecular Medicine at UCSF. He completed specialty training in internal medicine, pulmonary subspecialty, and research at UCSF and the Gladstone Institute of Virology and Immunology.
Kenneth H. Mayer
Kenneth H. Mayer, MD, is a Professor at Harvard Medical School and the Harvard School of Public Health and Attending Physician and Director of HIV Prevention Research at Beth Israel Deaconess Medical Center in Boston. He is the founder, Co-Chair, and Medical Research Director of The Fenway Institute, the research, training and health policy division of Fenway Health, the largest ambulatory facility caring for HIV-infected patients in New England. His research interests include international HIV/AIDS, gay and bisexual men’s health, HIV/AIDS prevention, microbicides, PrEP, PEP, vaccines, secondary prevention, HIV/AIDS treatment, and antibiotic use and molecular epidemiology of antibiotic resistance. He is a member of the Governing Council of the International AIDS Society and Co-Chair of the Scientific Advisory Board of the Center for Global Health Policy of the Infectious Disease Society of America.
Krischan J. Hudson
Krischan J. Hudson, PhD, MPH, joined the Infectious Diseases Unit at GlaxoSmithKline in 2014 and is currently a Clinical Development Director at ViiV Healthcare serving as the Clinical Investigative Lead for phase II and III HIV trials. He was previously with Pfizer, supporting diabetes, obesity, cardiovascular, and oncology programs. He gained early clinical trial experience through Study Manager positions held at the University of Miami Department of Family Medicine and the University of Virginia Cancer Center. Dr Krischan earned an MPH in epidemiology from Florida International University and a PhD in microbiology focused on host-pathogen interactions from the University of Virginia.
Britt S. Stancil
Britt S. Stancil, BS, is the Statistics Leader at GlaxoSmithKline (GSK). She has also worked at PAREXEL International, Inc., as Associate Director of Biostatistics. Britt has worked for over 20 years designing and analyzing clinical trials. Her focus is in the HIV therapeutic area where she works on both treatment and prevention indications. Britt Stancil earned her BS from North Carolina State University.
Susan L. Ford
Susan L. Ford, PharmD, is a Clinical Pharmacokineticist at GSK. Her focus is on antiviral clinical pharmacology modeling and simulation. Dr Ford earned her PharmD from the Medical College of Virginia and completed a PK/PD fellowship at the University of North Carolina at Chapel Hill in conjunction with GSK.
Parul Patel
Parul Patel, PharmD, is the Clinical Pharmacology Lead for the long-acting integrase inhibitor, cabotegravir at ViiV Healthcare. Her earlier work for GSK focused on early- and late-stage clinical development of HIV antiretroviral and antibacterial agents. Dr Patel received her PharmD at the University of Southern California and completed postgraduate training in pediatric pharmacology at the University of California, San Diego.
Alex R. Rinehart
Alex R. Rinehart, PhD, is the Director of Medical Communications for cabotegravir at ViiV Healthcare. Since joining the Global Medical Strategy group at ViiV Healthcare, he is responsible for developing and executing ViiV Healthcare’s global HIV prevention strategy. While formerly at Tibotec Therapeutics, he served as an Associate Medical Director and was involved in the successful launches of both darunavir and etravirine. Dr Rinehart received his PhD in Biochemistry and Molecular Biology from SUNY Stony Brook.
William R. Spreen
William R. Spreen, PharmD, is the Director of Research and Development at ViiV Healthcare and the Medicine Development Leader for the HIV integrase inhibitor cabotegravir, a long-acting injectable agent under investigation for both HIV prevention and treatment. In more than 20 years with GSK, he has led global development programs for a number of small-molecule antiretroviral agents, culminating in approval of dolutegravir, abacavir, and the combination products Trizivir® and Epzicom/Kivexa®. Dr Spreen and colleagues also led GSK’s efforts to validate the clinical utility of a genetic-based screening test for abacavir hypersensitivity reaction. He received his PharmD from The Ohio State University
David A. Margolis
David A. Margolis, MD, is the Director of Clinical Development and R&D at ViiV Healthcare and serves as the Project Physician Lead for the long-acting integrase inhibitor cabotegravir. At GSK and ViiV Healthcare, his research focus has been on HIV drug development and HIV clinical trials. Dr Margolis earned his MPH at the University of North Carolina at Chapel Hill and earned his MD at Duke Medical University School of Medicine. He was a resident in internal medicine at the University of Colorado and completed a fellowship in infectious diseases at the University of California, San Diego.
References
- WHO. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. 2016 update. http://www.who.int/hiv/pub/guidelines/keypopulations-2016/en. Accessed March 12, 2018
- Truvada [package insert]. Foster City, CA: Gilead Sciences; 2017.
- Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410.
- Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–2090.
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–434.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599.
- McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60.
- Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373(23):2237–2246.
- Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30(12):1973–1983.
- Abdool Karim S, Baxter C. Translating pre-exposure prophylaxis evidence into practice and public health impact. In: Eaton LA, Kalichman SC, eds. Biomedical Advances in HIV Prevention: Social and Behavior Perspectives. New York: Springer; 2014:29–40.
- Spreen W, Ford SL, Chen S, et al. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr. 2014;67(5):481–486.
- Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390(10101):1499–1510.
- Grant RM, Liegler T, Defechereux P, et al. Drug resistance and plasma viral RNA level after ineffective use of oral pre-exposure prophylaxis in women. AIDS. 2015;29(3):331–337.
- Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–422.
- Markowitz M, Frank I, Grant RM, et al. Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): a multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. Lancet HIV. 2017;4(8):e331–e340.
- Romaine J, Bradley C. The Study Medication Satisfaction Questionnaire (SMSQ) Status and Change Version User Guidelines. Egham, Surrey: Health Psycohology Research; 2015.
- Woodcock A, Bradley C. Validation of the revised 10-item HIV Treatment Satisfaction Questionnaire status version and new change version. Value Health. 2006;9(5):320–333.
- Kerrigan D, Mantsios A, Grant R, et al. Expanding the menu of HIV prevention options: a qualitative study of experiences with long-acting injectable cabotegravir as PrEP in the context of a phase II trial in the United States. AIDS Behav. 2017. [Epub ahead of print].
- Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–518.
- Spreen W, Williams P, Margolis D, et al. Pharmacokinetics, safety, and tolerability with repeat doses of GSK1265744 and rilpivirine (TMC278) long-acting nanosuspensions in healthy adults. J Acquir Immune Defic Syndr. 2014;67(5):487–492.
- Parsons JT, Rendina HJ, Whitfield TH, Grov C. Familiarity with and preferences for oral and long-acting injectable HIV pre-exposure prophylaxis (PrEP) in a national sample of gay and bisexual men in the U.S. AIDS Behav. 2016;20(7):1390–1399.
- UNAIDS. Latest statistics on the status of the AIDS epidemic. http://www.unaids.org/en/resources/fact-sheet. Accessed May 20, 2018.