Abstract
The antiretroviral drug efavirenz (EFV) has been linked to disordered sleep and cognitive abnormalities. We examined sleep and cognitive function and subsequent changes following switch to an alternative integrase inhibitor-based regimen. Thirty-two HIV-infected individuals on EFV, emtricitabine, and tenofovir (EFV/FTC/TDF) without traditional risk factors for obstructive sleep apnea (OSA) were randomized 2:1 to switch to elvitegravir/cobicistat/emtricitabine/tenofovir (EVG/COBI/FTC/TDF) or to continue EFV/FTC/TDF therapy for 12 weeks. Overnight polysomnography and standardized sleep and neuropsychological assessments were performed at baseline and at 12 weeks. No significant differences in change over 12 weeks were noted between the two arms in any sleep or neuropsychological test parameter. At entry, however, the rate of sleep disordered breathing (SDB) was substantially higher in study subjects compared to published age-matched norms and resulted in a high assessed OSA rate of 59.4%. Respiratory Disturbance Index (RDI), a measure of SDB, correlated with age- and education-adjusted global neuropsychological Z-score (NPZ) (r = −0.35, p = 0.05). Sleep Maintenance Efficiency, Wake after Sleep Onset, REM Sleep and RDI correlated with domain-specific NPZ for learning and memory (all p-values ≤ 0.05). Among HIV-infected individuals on EFV-based therapy and without traditional risk factors for OSA, sleep and neuropsychological abnormalities do not readily reverse after discontinuation of EFV. High baseline rates of SDB and abnormalities in sleep architecture exist in this population correlating with neuropsychological impairment. The role of HIV immuno-virologic or lifestyle factors as contributing etiologies should be explored. OSA may be an under-recognized etiology for cognitive dysfunction during chronic HIV.
Introduction
Efavirenz (EFV) is a potent non-nucleoside reverse transcriptase inhibitor used in the treatment of HIV. The drug is marketed in the US as a fixed dose combination tablet of EFV/emtricitabine/tenofovir (EFV/FTC/TDF, Atripla®) taken once-daily at bedtime. Integrase inhibitors have largely replaced EFV as a first line treatment option in developed regions, but the drug remains heavily utilized in low and middle-income countries.
Neuropsychiatric disturbances have been reported in 25–70% of patients receiving EFV.Citation 1 Many of these disturbances, such as vivid dreams, insomnia and/or frequent awakenings, suggest that sleep architecture is altered by this drug. While the rates and intensity of the symptoms are believed to improve within a few weeks post initiation of EFV, the symptoms persist in up to half of individuals for 6 months or longer.Citation 2 , Citation 3 Furthermore, concerns have been raised regarding potential neurotoxic properties of EFVCitation 4 and at least two cross-sectional studies have reported a linkage between EFV and cognitive impairment as assessed by neuropsychological testing.Citation 5 , Citation 6
While a few studies have examined changes in immunologic and various side effect profiles following a structured switch from an EFV-based to a non-EFV-based regimen, none have examined both sleep and neuropsychological performance in a controlled prospective design. The present study addressed this gap using overnight facility-based polysomnography (PSG) and standardized sleep and neuropsychological assessments at baseline and again after 12 weeks following randomization to either continue EFV/FTC/TDF or switch to open-label elvitegravir/cobicistat/emtricitabine/tenofovir (EVG/COBI/FTC/TDF, Stribild®).
Materials and methods
Subjects and study design of the switch study
This study, H027 ‘Change in Sleep Architecture and Neuropsychological Performance Following Switch from Atripla to Stribild’, was approved by the Committee on Human Subjects of the University of Hawaii (UH). Written informed consents were obtained from all participants. All study visits were completed at the UH Clinics at Kakaako, John A. Burns School of Medicine, UH at Manoa.
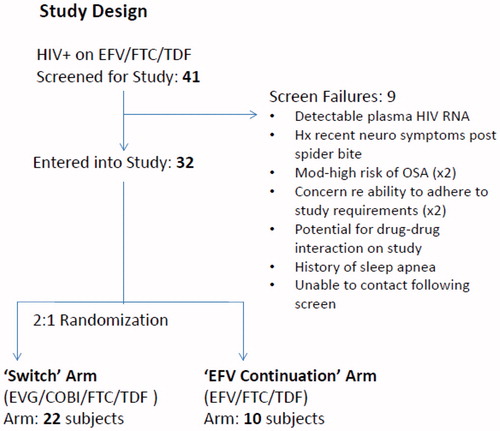
Entry inclusion criteria included age 18–70, and on EFV/FTC/TDF antiretroviral therapy (ART) for >12 months with documented plasma HIV RNA <50 copies/ml within 3 months of entry. Self-reported symptoms of sleep or cognitive difficulties were not required for enrollment. Exclusion criteria included any documented plasma HIV RNA >100 copies/ml within the past 6 months, untreated hepatitis C, any uncontrolled chronic illness or acute illness within 2 weeks of entry, creatinine clearance (Cockcroft and Gault) < 70 ml/min, hemoglobin <9.0 g/dL, absolute neutrophil count <500/μL, platelet count <40,000/μL, AST (SGOT) and ALT (SGPT) > 5× upper limit of normal (ULN), substance use disorder that would interfere with protocol compliance or data fidelity, pregnancy or breast-feeding, documented or likely viral resistance or contra-indication to the use of EVG/COBI/FTC/TDF, major depression based on the Beck Depression Inventory—II (BDI-2), or use of any immunomodulator, HIV vaccine or investigational therapy within 30 days of study entry. In addition, individuals with a documented history of obstructive sleep apnea (OSA) or moderate to high risk for OSA were excluded. Moderate to high risk of OSA was defined as body mass index (BMI) > 30 plus two of the following: history of habitual snoring, gasping/choking or observed apnea while sleeping, and neck circumference >17 inches.
Individuals meeting entry criteria were randomized 2:1 to either continue on EFV/FTC/TDF (EFV continuation arm) or switch to EVG/COBI/FTC/TDF (switch arm) for the 12-week course of the study. There was no placebo medication utilized in this study, and once randomized, both investigators and subjects were aware of the arm to which the subject had been randomized. All participants completed a facility-based PSG and neuropsychological testing at baseline and at the 12-week follow-up. The Epworth Sleepiness Score,Citation 7 Pittsburgh Sleep Quality Index (PSQI),Citation 8 and the RAND 36-Item Health Survey (version 1.0) were obtained at entry and at the end of the 12 week study. The questionnaires were administered and scored following standardized procedures. Adverse events on study were graded using the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events Version 1.0, December 2004; Clarification August 2009.Citation 9
Polysomnography and diagnosis of OSA
Overnight PSG was conducted at the American Academy of Sleep Medicine (AASM)-accredited Sleep Center at Queen’s Medical Center, Honolulu, Hawaii. Data acquisition was performed by a registered polysomnographic technologist. Studies were scored by a blinded registered polysomnographic technologist and read by a blinded board-certified sleep specialist (B. Soll) using the rules, terminology, and technical specifications described in the AASM Manual for the Scoring of Sleep and Associated Events, Version 2.1. The following parameters were recorded: EEG (F3-M2, F4-M1, C3-M2, C4-M1, O1-M2, O2-M1), E1-M2 and E2-M2, 3 submental EMGs, right and left anterior tibialis EMG, EKG, snore channel, nasal pressure, oral/nasal airflow, thoracic and abdominal inductive plethysmography, and oxygen saturation. Sleep disordered breathing (SDB) calculated as the apnea hypopnea index (AHI) and respiratory disturbance index (RDI) was scored according to AASM standards. Specifically, apnea was defined as a 90% reduction in airflow lasting 10 seconds or more. Apneic events were defined as obstructive in nature when accompanied by continued or increased inspiratory effort and defined as central in nature when accompanied by absent inspiratory effort. A mixed classification was defined by initial absence of inspiratory effort followed by resumed effort. Hypopnea was defined by a 30–90% reduction of nasal pressure lasting 10 seconds or longer with at least 4% desaturation. Respiratory effort related arousal (RERA) was defined as a sequence of respiratory events lasting 10 seconds or more accompanied by an increased effort to breath and a reduction in flow that did not meet criteria for apnea or hypopnea, followed by an arousal from sleep. OSA was defined using the AASM definition as an RDI >15 or an RDI 5–15 accompanied by symptoms or comorbidities. Sleep parameters assessed are as listed in .
Table 1 Baseline characteristics of switch study by arms.
Neuropsychological testing
Neuropsychological testing was conducted by trained psychometrists at the UH Clinics at Kakaako with quality assurance of testing procedures provided by neuropsychologist (R Paul). Subjects were tested in a quiet room to minimize distractions and provided breaks as needed. The neuropsychological battery involved a series of tests that were selected to assess the function of specific domains known to be sensitive to HIV-related cognitive dysfunction. Raw scores from individual assessments were converted to standardized Z-scores using published norms adjusted for age, sex, and education where applicable.Citation 10 , Citation 11 The Z-scores of individual tests were averaged according to domains to obtain domain-specific Z-scores. Z-scores from all assessments in the neuropsychological battery were aggregated and averaged to create a global measure of cognitive performance (NPZ-Global). The domains evaluated and the tests utilized for each domain and for the global score were as follows: Learning and Memory (CVLT Total, CVLT Long-Delay Free Recall, BVMT Total, BVMT Delayed Recall); Executive Function (FAS, Stroop, Trail B, Action); Working Memory (WAIS IV DIGIT Span Backward, CVLT B, WAIS IV Letter Number); Psychomotor Speed (WAIS IV Digit Symbol, Trail A, Grooved Pegboard Dominant, Grooved Pegboard Non-Dominant); Global (All components used in domains previously listed plus CalCap Choice, CalCap Sequential, and Timed Gait). Data on impairment in work or activities of daily living was not collected in this study, and clinical determination of cognitive impairment was therefore not possible to ascertain. However, the percentage of individuals who would have at least met criteria for asymptomatic neurocognitive impairment (ANI) based on the Frascati criteria was calculated using the criteria of average scores < −1.0 on 2 of the 4 domains.Citation 12
Separate HIV-infected comparison group on non-EFV containing regimens
Following awareness during the study of the high rates of sleep abnormalities noted at baseline in our study participants, we recruited a separate small cohort of HIV-infected individuals on non-EFV-containing regimens to preliminarily assess whether the extensive sleep abnormalities noted were specific to EFV-based therapy. These subjects underwent overnight PSG and standardized sleep and neuropsychological assessments similar to subjects who entered the switch study but on a one-time basis.
Statistical analyses
Demographic, clinical, and immunologic information was summarized by median (interquartile range) values for continuous variables and frequency (percentage) for categorical variables. Baseline characteristics of participants between the two arms and between subjects enrolled into the switch study and into the non-EFV comparison group were compared by Wilcoxon rank sum test. Fisher’s exact test was used to compare categorical variables between groups. Baseline characteristics of the study participants were compared to age-corrected normative data by Mitterling et al.Citation 13 using one-sample Wilcoxon Signed Rank Test. Normative values from this study were selected because this study was conducted utilizing the more recent AASM 2012 version 2.0 criteria and, similar to our study, excluded individuals at high risk of OSA.
Associations between variables were examined using Pearson correlations. Continuous variables with skewed distributions were log-10 transformed or square root transformed prior to analyses. For variables that were log-10 transformed, 0 values were first converted to 0.01 before transforming. Pearson correlation analyses for stage 3 sleeps were performed utilizing only the data from the subjects who had detectable stage 3 sleep (62.5%). All statistical analyses were conducted in SPSS (IBM, Version 24). A two-sided p-value < 0.05 was utilized to define statistical significance.
Effect sizes for Wilcoxon Rank Sum and Wilcoxon Signed Rank tests were calculated by dividing the Z-statistic by the square root of the sample size to obtain a Pearson r coefficient, and are denoted r W. Cramer’s V statistic is reported for the effect size of Fisher’s Exact Test. The effect sizes r W and V, as well as Pearson’s correlation coefficient r, are interpreted as small (effect size ≤ ±0.2), medium (±0.2 > effect size ≤ ±0.5), and large (effect size ≥ ±0.5).
Results
Baseline demographic and clinical patient characteristics and safety on study
A study flow diagram is shown in . Thirty-two participants entered and completed the study. These subjects were on EFV-containing regimens for a median (IQR) of 6.7 (5.3, 8.9) years. Baseline demographic and clinical characteristics (shown in ) were similar between the treatment arms. Subjects were all males, predominately Caucasian with a median age of 50 years and were all virologically suppressed.
Self-reported history of hypertension, diabetes, and depression were obtained in 34.4%, 6.3%, and 28.1% of subjects, respectively. No subject gave a history of diagnosed dementia or atherosclerotic disease. Eighteen (56.3%) individuals reported snoring but only 3 (15.8%) reported nightly or habitual snoring. Five (15.6%) reported regular use of pharmaceutical sleep aides; and 23 (71.8%) reported one or more awakenings each night, primarily to use the bathroom. No significant differences were noted in these parameters between the two arms of the study.
The study was well tolerated clinically. All subjects on entry had plasma HIV RNA <50, and at the end of the 12-week study, virologic suppression was maintained in all subjects except for one subject on the continuation arm with a value of 114 copies/ml who admitted to occasional non-compliance with his antiretroviral therapy. No serious adverse events (SAEs) occurred on study. Two grade II adverse events (AEs) [both cholesterol elevations] occurred in the switch arm and 3 grade II AE [one an elevation of AST, one LDL cholesterol elevation and one triglyceride elevation] occurred in the continuation arm.
Baseline sleep parameters
The median (IQR) values for sleep parameters at baseline among study subjects by arm are also shown in . All baseline sleep parameters were similar between the two randomized arms of the study.
A high rate of SDB was found at baseline in our study subjects. The overall median RDI (IQR) of the 32 subjects recruited at baseline was 8.3 (2.7, 19.8). Using AASM criteria, 11 (34%) subjects were classified as normal (RDI <5), 9 (28.1%) as mild (RDI 5– <15), 9 (28.1%) as moderate (RDI 15– <30), and 3 (9.4%) as severe (RDI ≥30). Based on these RDI values plus symptoms or co-morbidities, 59.4% of the participants met AASM criteria for OSA.
As possible, baseline sleep parameters were compared to age- and gender-adjusted normative data as reported by Mitterling et al.Citation 13 The median overall sleep efficiency (sleep time/study time) and sleep latency (lights out to first sleep period) were similar as was the Wake After Sleep Onset percent (WASO%). SDB was reported by AHI and not RDI in this publication. The overall median AHI at baseline in our study subjects was 4.0 (1.4, 10.8). This value was significantly higher than the median value of 2.6 reported for healthy individuals between the ages of 51–60 by Mitterling et al. (r W = 0.41, p = 0.018). In addition, our study subjects spent significantly less time in stage 3 sleep compared to reference norms (3.7% vs. 15.5%, r W = −0.77, p < 0.001) with more than one third (37.5%) recording no time in stage 3 sleep. REM sleep percentage was higher in study participants compared to norms (18.1% vs. 12.2%, r W = 0.53, p = 0.002) and REM latency was prolonged (119.3 vs. 97.5 minutes, r W = 0.40, p = 0.025).
Epworth scores of 11 or greater were observed in 3 (9.4%) individuals at baseline. Scores of 5 or greater indicative of poor sleep quality by PSQI were observed in 59.4% of the cohort. Neither the Epworth score nor the PSQI total score correlated with AHI, RDI or any sleep architecture parameters.
CD4 percent correlated positively with Sleep Maintenance Efficacy (r = 0.514, p = 0.004) and Sleep Efficacy (r = 0.463, p = 0.011) and negatively with WASO% (r = −0.532, p = 0.003) and REM latency (r = −0.410, p = 0.027).
Baseline neuropsychological performance and correlation to sleep parameters
Baseline global and domain-specific Z-scores (shown in the first 2 columns in ) were similar between the two randomized arms. Zero of 10 participants (0%) on the continuation arm and 4 of 22 participants (18%) on the switch arm met the criteria of ANI. Correlations between neuropsychological test performance and baseline sleep parameters are shown in . Poorer NPZ-Global correlated with higher RDI scores. Working Memory correlated positively with Sleep Maintenance Efficiency and negatively with WASO (%). Learning and Memory correlated positively with Sleep Efficiency, Sleep Maintenance Efficiency and REM sleep, and negatively with WASO (%) and RDI.
Table 2 Correlations between sleep and neuropsychological performance parameters in switch study subjects at baseline. baseline values from both arms were combined for analyses.
Change in sleep and neuropsychological performance over 12 weeks
Baseline to week 12 changes in sleep and neuropsychological performance by arm are shown in . No significant changes between the two arms were found for any sleep or neuropsychological parameter.
Table 3. Change in sleep and neuropsychological performance parameters over 12 weeks in switch study subjects by arm.
Sleep evaluation in HIV-infected individuals on non-EFV-containing regimens
Nine virologically suppressed HIV-infected individuals on non-EFV-containing ART were recruited to preliminarily assess whether the high rates of sleep abnormalities were unique to individuals on EFV-based therapy. Their demographic, clinical and immunologic data were similar to the combined baseline values of subjects on EFV-containing ART enrolled into the switch study. Sixty-seven percent were on Integrase Inhibitor-based regimens. Three individuals had a previous history of EFV-based therapy; however, all had discontinued EFV 12 months or more before enrollment.
Sleep Efficiency, Sleep Maintenance Efficiency, WASO (%) and Respiratory Effort Related Arousals (RERA) were worse, rather than better, compared to the baseline sleep parameters of the switch study participants on EFV (r W > ±0.27, p < 0.1) while percentage of REM, stage 3 sleep, and RDI values were similar (r W < ±0.17, p > 0.2). Comparing the baseline values between the all EFV subjects and these nine non-EFV subjects, there were no differences in NPZglobal, or in the domains of working memory, executive function or psychomotor speed. There was a significant difference in learning memory (median 0.04 in EFV subjects vs. 0.59 in non-EFV subjects, p = 0.035).
Discussion
Our study did not reveal any changes in any sleep or neuropsychological outcome following switch to a non-EFV containing treatment regimen. The one previously published interventional study that examined the impact of EFV on sleep using overnight PSG assessed these parameters in ART-naïve individuals with EFV used as part of first time therapy.Citation 14 This study did not measure SDB but noted an increase, rather than a decrease, in stage 4 sleep (now considered part of stage 3 sleep), and a modest increase in REM sleep. Considering that HIV immuno-virologic changes, such as lower CD4 count, are known to correlate with sleep disturbancesCitation 15 as well as with cognitive dysfunction,Citation 16 we reasoned that the drug effect of EFV could have been confounded by a ‘return to health’ phenomenon. Therefore, a better method for assessing possible outcomes of EFV treatment would be to determine whether these parameters improved when EFV-based therapy was discontinued in favor of a non-EFV-containing therapy. In respect to neuropsychological parameters, one small pilot study of 16 subjects reported that EFV discontinuation was unlikely to modify neurocognitive function.Citation 17 Our study similarly found a lack of ready reversal with EFV discontinuation. This may suggest a limited etiologic role for EFV in inducing sleep and cognitive dysfunction. It is possible that our inability to demonstrate differences may have been secondary to a small sample size. It is further possible that we may have recruited subjects more able to tolerate EFV considering their median duration of EFV use of close to 7 years. Finally, it is also possible that a component of irreversibility may exist in the disease process when continued for a long duration, as has been suggested for OSA-induced neuronal injury.Citation 18
Perhaps the most significant findings from this study were the high rates of SDB and OSA as well as disturbances in sleep architecture found at entry in this cohort of virally suppressed patients on EFV/FTC/TDF who were specifically selected because they did not have high traditional risk factors for OSA. The median RDI observed in our study was high (8.3), and well above the cutoff of <5 considered normal by AASM criteria. Based on RDI plus symptoms or co-morbidities, OSA was diagnosed in 59.4% of the subjects. OSA is a serious medical condition linked not only to excessive day-time sleepiness but to increased risk for cardio-metabolic disease including hypertension, myocardial infarction, stroke and diabetesCitation 19–23 as well as to risk of neurocognitive impairment.Citation 18 , Citation 24 It is therefore not surprising that in our study sleep abnormalities correlated with neuropsychological performance particularly in tests of learning and memory. Risks associated with OSA may be partially modifiable with the use of continuous positive airway pressure (CPAP),Citation 19 , Citation 21 , Citation 25 and consideration for possible CPAP therapy may be warranted as a next step in our subjects.
The high rate of OSA at baseline was unexpected as the study entry criteria excluded individuals with moderate to high risk for OSA. This may have important implications for clinical care. The use of traditional risk factors to screen for OSA may not be an effective screening strategy in individuals with chronic HIV. Many participants did not have a sleeping partner, limiting the ability to report snoring, gasping and chocking or observed apnea to their medical provider. Furthermore, the lack of correlations between PSG-defined AHI and RDI with the Epworth Sleepiness Scale and the PSQI suggests that these questionnaires may not be effective screening tools for OSA in a HIV-infected population. This is consistent with some studies in the general population that have concluded that these two questionnaires, while important for understanding subjective sleep quality, are not ideal indicators of OSA.Citation 26 , Citation 27 Collectively, our results indicate the importance of careful clinical attention in the diagnostic process and clinical care of OSA in individuals with chronic HIV.
Patient self-report and/or electronic medical records may be insufficient to determine OSA in HIV. A Veteran’s Administration study relying on electronic medical records and coded data found lower prevalence of OSA diagnoses in HIV-infected patients than HIV-uninfected patientsCitation 28 while the MACS and the Women’s Interagency HIV Study (WIHS), using pulmonary questionnaires, found higher reported diagnoses when adjusted for confounders such as age and BMI.Citation 29 Our findings are most consistent with a separate MACS study which utilized over-night PSG studies. The MACS found a rate of RDI >5 in 70.7% of their HIV + subjects on ART.Citation 30 Their population, unlike our study, however, did not exclude individuals with traditional risk factors for OSA.
Alterations in sleep architecture consisting of a decrease in stage 3 also known as slow wave sleep (SWS) and an increase in REM sleep were seen in our study subjects. These parameters may be related to OSA as it has been reported that OSA severity is associated with less time in deep sleep.Citation 31 The percent of stage 3 sleep in our cohort (median of 3.7%) was extremely low, with a surprising 37.5% of subjects having zero stage 3 sleep. Decreased SWS has been similarly described in a cross-sectional study of HIV-infected patients on EFV,Citation 32 and in HIV-uninfected individuals with histories of alcohol and illicit drug use.Citation 33 It has also been described in conjunction with increased REM sleep duration that is characteristic of depression.Citation 34 The significance of these sleep architecture alterations is unclear. Both REM and SWS have been implicated in memory consolidation. It has been proposed that SWS facilitates consolidation of declarative and spatial memories, whereas REM sleep facilitates consolidation of non-declarative memories.Citation 35 It has been recently reported that SWS disruption increases CSF amyloid-β levels,Citation 36 further suggesting a potential connection between sleep and cognitive problems.
Because of the high rates of sleep disturbances on entry in our subjects on EFV/FTC/TDF, we undertook sleep evaluations in a small cohort of individuals not on EFV-containing regimens and found similar rates of sleep disturbances in these individuals as well. This, together with the lack of traditional risk factors for OSA at entry in our study subjects and the lack of response to discontinuation of EFV, may indicate that factors other than EFV such as HIV immuno-virologic changes or other yet to be identified risks in individuals with chronic HIV may at least partially account for some of the sleep abnormalities found in our study. In our study, lower CD4 percent was associated with poorer sleep efficiency and sleep maintenance efficacy as well as increased sleep fragmentation as reflected by higher WASO%. SDB in untreated HIV has been reported to be associated with higher HIV viral loads as well as high CRP, TNFα, TNFRII, and IL-6 levels.Citation 37 , Citation 38 Even with the use of potent combination ART, HIV-associated immune activation may result in OSA via blunted neuromuscular control of the upper airway.Citation 39 It is also possible that lifestyle factors such as tobacco, alcohol and substance use may be partially responsible.Citation 40–42 Supportive of this hypothesis, the MACS sleep study found sleep abnormalities including RDI to be highly prevalent and increased not only in their HIV-infected subjects but also in their sero-negative subjects, concluding that their sample was not representative of the general population and may be at higher risk for sleep disordered breathing.Citation 30
Our study results may have been influenced by the small sample size, and the groups may have included individuals who were more capable of tolerating EFV as we did not recruit based on symptoms. Subjects in this study had a relatively long duration of use of EFV-based therapy prior to enrollment, and it is possible that a 12-week period was insufficient to detect change. Furthermore, we cannot address the potential interaction with sex, as the cohort included only males. The strengths of this study include the use of formal facility-based over-night PSG and concomitant evaluation of neuropsychological performance. Our study raises important questions about sleep and cognition that will require further investigation.
In summary, our study found that discontinuation of EFV in virally suppressed HIV-infected subjects on EFV/FTC/TDF therapy for 12 weeks did not readily reverse sleep or neuropsychological abnormalities. We found however surprisingly high rates of OSA, decreased stage 3 sleep, and increased REM sleep at entry in this group specifically selected to be without high traditional risk factors for OSA. Further studies are warranted to define the prevalence, risks, and diagnostic as well as treatment strategies for OSA among individuals with chronic HIV.
Acknowledgments
The authors thank the many patients who made this study possible and the staff of the Hawaii Center for AIDS, University of Hawaii and the Sleep Study at the Queen Medical Center, Honolulu, Hawaii. The study was made possible by the generous funding and drug support from Gilead Sciences, and clinical research support from RMATRIX (NIH U54MD007584). Clinical Trial: Registered on ClinicalTrials.gov, NCT02283563 as H027 ‘Sleep and Cognition after Atripla to Stribild Switch’.
Disclosure statement
None of the authors have any relevant conflicts of interest to disclose.
Additional information
Funding
References
- Maggiolo F. Efavirenz: a decade of clinical experience in the treatment of HIV. J Antimicrob Chemother. 2009;64:910–928.
- Hawkins T, Geist C, Young B, et al. Comparison of neuropsychiatric side effects in an observational cohort of efavirenz- and protease inhibitor-treated patients. HIV Clin Trials. 2005;6:187–196.
- Fumaz CR, Munoz-Moreno JA, Molto J, et al. Long-term neuropsychiatric disorders on efavirenz-based approaches: quality of life, psychologic issues, and adherence. J Acquir Immune Defic Syndr. 2005;38:560–565.
- Decloedt EH, Maartens G. Neuronal toxicity of efavirenz: a systematic review. Expert Opin Drug Saf. 2013;12:841–846.
- Ciccarelli N, Fabbiani M, Di Giambenedetto S, et al. Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology. 2011;76:1403–1409.
- Ma Q, Vaida F, Wong J, et al. Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. J Neurovirol. 2016;22:170–178.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540–545.
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
- Table for Grading the Severity of Adult and Pediatric Adverse Events. November 2014. https://rsc.tech-res.com/docs/default-source/safety/table_for_grading_severity_of_adult_pediatric_adverse_events.pdf.
- Wechsler D. Wechsler Adult Intelligence Scale-Fourth. San Antonio, TX: The Psychological Corporation; 2008.
- Heaton RK, Grant, I, & Matthews, C.G. Comprehensive norms for an expanded Halstead-Reitan battery: demographic corrections, research findings, and clinical applications; with a supplement for the Wechsler Adult Intelligence Scale-Revised (WAIS-R). 1991.
- Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799.
- Mitterling T, Hogl B, Schonwald SV, et al. Sleep and Respiration in 100 Healthy Caucasian Sleepers–A Polysomnographic Study According to American Academy of Sleep Medicine Standards. Sleep. 2015;38:867–875.
- Moyle G, Fletcher C, Brown H, Mandalia S, Gazzard B. Changes in sleep quality and brain wave patterns following initiation of an efavirenz-containing triple antiretroviral regimen. HIV Med. 2006;7:243–247.
- Seay JS, McIntosh R, Fekete EM, et al. Self-reported sleep disturbance is associated with lower CD4 count and 24-h urinary dopamine levels in ethnic minority women living with HIV. Psychoneuroendocrinology. 2013;38:2647–2653.
- Ellis RJ, Badiee J, Vaida F, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25:1747–1751.
- Payne B, Chadwick TJ, Blamire A, et al. Does efavirenz replacement improve neurological function in treated HIV infection? HIV Med. 2017;18:690–695.
- Veasey SC. Piecing together phenotypes of brain injury and dysfunction in obstructive sleep apnea. Front Neurol. 2012;3:139.
- Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165:447–452.
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053.
- Stone KL, Blackwell TL, Ancoli-Israel S, et al. Sleep disordered breathing and risk of stroke in older community-dwelling men. Sleep. 2016;39:531–540.
- Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med. 2009;169:1147–1155.
- Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307.
- Olaithe M, Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36:1297–1305.
- Nishiyama T, Mizuno T, Kojima M, et al. Criterion validity of the Pittsburgh sleep quality index and epworth sleepiness scale for the diagnosis of sleep disorders. Sleep Med. 2014;15:422–429.
- Scarlata S, Pedone C, Curcio G, et al. Pre-polysomnographic assessment using the Pittsburgh sleep quality index questionnaire is not useful in identifying people at higher risk for obstructive sleep apnea. J Med Screen. 2013;20:220–226.
- Kunisaki KM, Akgun KM, Fiellin DA, et al. Prevalence and correlates of obstructive sleep apnoea among patients with and without HIV infection. HIV Med. 2015;16:105–113.
- Gingo MR, Balasubramani GK, Rice TB, et al. Pulmonary symptoms and diagnoses are associated with HIV in the MACS and WIHS cohorts. BMC Pulm Med. 2014;14:75.
- Patil SP, Brown TT, Jacobson LP, et al. Sleep disordered breathing, fatigue, and sleepiness in HIV-infected and uninfected men. PLoS One. 2014;9:e99258.
- Ng AK, Guan C. Impact of obstructive sleep apnea on sleep-wake stage ratio. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:4660–4663.
- Gallego L, Barreiro P, del Rio R, et al. Analyzing sleep abnormalities in HIV-infected patients treated with Efavirenz. Clin Infect Dis. 2004;38:430–432.
- Angarita GA, Emadi N, Hodges S, Morgan PT. Sleep abnormalities associated with alcohol, cannabis, cocaine, and opiate use: a comprehensive review. Addict Sci Clin Pract. 2016;11:9.
- Palagini L, Baglioni C, Ciapparelli A, Gemignani A, Riemann D. REM sleep dysregulation in depression: state of the art. Sleep Med Rev. 2013;17:377–390.
- Kryger M, Roth, T., Dement, W.C. Principles and Practice of Sleep Medicine. 6th ed. Philadelphia, PA: Elsevier, Inc; 2017:229–238.
- Ju YS, Ooms SJ, Sutphen C, et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-beta levels. Brain. 2017;140:2104–2111.
- Brown TT, Patil SP, Jacobson LP, et al. Anthropometry in the prediction of sleep disordered breathing in HIV-positive and HIV-negative men. Antivir Ther. 2010;15:651–659.
- Brigham EP, Patil SP, Jacobson LP, et al. Association between systemic inflammation and obstructive sleep apnea in men with or at risk for HIV infection. Antivir Ther. 2014;19:725–733.
- Darquenne C, Hicks CB, Malhotra A. The ongoing need for good physiological investigation: obstructive sleep apnea in HIV patients as a paradigm. J Appl Physiol. 2015;118:244–246.
- Webster LR, Choi Y, Desai H, Webster L, Grant BJ. Sleep-disordered breathing and chronic opioid therapy. Pain Med. 2008;9:425–432.
- Wetter DW, Young TB, Bidwell TR, Badr MS, Palta M. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med. 1994;154:2219–2224.
- Peppard PE, Austin D, Brown RL. Association of alcohol consumption and sleep disordered breathing in men and women. J Clin Sleep Med. 2007;3:265–270.