Abstract
Background: The success of longitudinal trials depends greatly on using effective strategies to retain participants and ensure internal validity, maintain sufficient statistical power, and provide for the generalizability of study results.
Objective: This paper describes the challenges and specific strategies used to retain participants in a Phase 2B safety and effectiveness study of daily oral and vaginal tenofovir formulations for the prevention of HIV-1 infection in the MTN-003 (VOICE) trial in Kampala, Uganda.
Methods: Once enrolled, participants were seen every 28 days at the research site and their study product was re-filled. Challenges to retention included a mobile population, non-disclosure of study participation to spouse/family, and economic constraints. Strategies used to maintain high participation rates included the use of detailed locator information, a participant tracking database, regular HIV/STI testing, and the formation of close bonds between staff and subjects.
Results: We enrolled 322 women out of the 637 screened. The overall retention rate was 95% over a 3 year follow up period. Only 179 (3%) out of the 6124 expected visits were missed throughout study implementation. Reasons for missed visits included: participants thinking that they did not need frequent visits due to their HIV negative status, time constraints due to commercial sex work, and migration for better employment.
Conclusions: With the implementation of multi-faceted comprehensive follow-up and retention strategies, we achieved very high retention rates in the MTN-003 study. This paper provides a blueprint for effective participant retention strategies for other longitudinal HIV prevention studies in resource-limited settings in Sub-Saharan Africa.
Background
HIV pre-exposure prophylaxis (PrEP) is an HIV prevention method for individuals currently uninfected but at high risk of acquiring HIV.Citation1 MTN-003 was unique in the field of PrEP research as it provided parallel comparisons of oral and topically applied antiretroviral measuresCitation2 to healthy HIV high risk women in Africa followed up monthly for 1–3 years.Citation3 Participant retention is critically important in carrying out this type of longitudinal clinical research trial as participant attrition must be minimized in order to maintain sufficient sample sizes, ensure internal validity and sufficient statistical power, and provide for the generalizability of study results.
The longitudinal study of participants uninfected with HIV presents an increased challenge for the retention of participants. Focus groups conducted in a study of South African women showed that one of the primary barriers to the recruitment and retention of individuals is participants’ assumption that HIV prevention programs are primarily for HIV positive individuals.Citation4 A qualitative study of Ethiopian women found that lack of access to health education, the stigma surrounding discussions of sexuality, and domestic workloads also serve as key barriers to female participation in HIV prevention studies.Citation5 These studies demonstrate that without effective education about HIV prevention and overall sexual health in study communities, such long-term interventional studies will likely not succeed.
Another problem present in HIV prevention work is the young age of some study participants. UNAIDS reported that one in four new HIV infections among women 15–49 years in Uganda occurs in young women aged 15–24 years old.Citation6 As a result, it is necessary to include participants as young as 18 years old in order to properly study preventive measures in a representative population. Legal regulations concerning the vulnerability of individuals under the age of 18 and the legal age of consent in Uganda prevented us from including those in the high-risk age range of 15–18. Younger participants often are highly mobile as they may be moving out of high school to collegeCitation7 or into job markets.Citation8 These frequent relocations serve as an impediment to study retention and as such must be addressed in longitudinal studies.
An acknowledgment of the challenges that hinder retention of female participants in HIV prevention research has spurred the creation of novel retention strategies that enable these crucial public health interventions to be conducted. Community buy-in for studies is imperative, as this will help mitigate misconceptions about the study and decrease stigma around study participation. A multi-site malaria treatment study with 24-month follow-up in Sub-Saharan Africa used an extensive community engagement approach to achieve these aims, which included an initial briefing to the local community followed by repeated progress updates from a community advisory board.Citation9 The relationships developed with these community advisors led to later collaboration to improve implementation of the study since only these community leaders were able to effectively educate the investigators about local customs, assumptions, and beliefs. It is crucial to develop this kind of bilateral cooperative relationship with local communities rather than unilaterally imposing research directives if you want to be able to successfully carry out an effective clinical trial. Additionally, the collection of detailed tracing information is a pillar of successful participant retention programs. A cohort study of inner city populations in the United States found that the number of participants who completed study follow-up increased as the number of contact names given also increased.Citation10 A clinical trial of mothers and infants conducted in The Gambia created a participant tracking database which included mobile phone contact information that helped them achieve a participant retention rate of 94% over a 14 month follow up period.Citation11 A review of the existing literature and the research team’s previous experiences implementing longitudinal clinical trials at the Kampala site helped inform the retention strategies implemented during MTN-003. However, it is necessary to ascertain if these types of strategies are effective in prevention-based studies where participants must actively adhere to treatment protocols, particularly in long-term longitudinal clinical trials. This paper describes the challenges and specific strategies used to retain participants in a Phase 2B safety and effectiveness study of Tenofovir 1% gel, Tenofovir disoproxil fumarate tablet and Emtricitabine/Tenofovir disoproxil fumarate tablet for the prevention of HIV infection in the MTN-003 (VOICE) trial at the Makerere University-Johns Hopkins University (MU-JHU) Research Collaboration in Kampala, Uganda.
Methods
The MTN-003/VOICE study
The MTN-003/VOICE study was a Phase 2B double-blinded, five-arm, multi-site, randomized, placebo-controlled trial in Uganda, South Africa, and Zimbabwe. In Uganda, the study was conducted at the MU-JHU Research Collaboration in Kampala between December 2009 and February 2012. The main objective of the study was to determine the comparative effectiveness and extended safety of daily Tenofovir 1% gel, oral Tenofovir disoproxil fumarate, and oral Emtricitabine/Tenofovir disoproxil fumarate in preventing HIV infection among women at risk for sexually transmitted infections (STIs). Study participants were women aged 18–45 years old at the time of screening who were defined by participant report as sexually active, meaning that they had vaginal intercourse at least once in the three months prior to screening procedures. Potential participants were HIV negative women who were at high risk of acquiring HIV.
Participants were recruited from healthcare units and community-based locations in Kampala, Mukono, Wakiso, Buikwe, Kayunga, Mityana, and Nakaseke districts. These healthcare units included government and private health units such as HIV care sites, STI clinics, family planning clinics, and research centers. Additionally, participants were also recruited from other HIV hot spots identified by the research team such as fishing sites and bars. These sites were identified as appropriate recruitment areas for the study population by the research staff at MU-JHU.
Once enrolled, participants were seen every 28 days for HIV testing and product refill among other procedures. Participants were expected to complete a minimum of 12 months and a maximum of 36 months of study product use. Participants were also expected to complete an additional 8 weeks of follow up post study product discontinuation in order to check for potential delayed seroconversions. The study product regimen consisted of one of five regimens: oral TDF (300 mg) and TDF-FTC placebo, oral TDF-FTC (300 mg of TDF and 200 mg of FTC) and TDF placebo, oral TDF placebo and oral TDF-FTC placebo, vaginal 1% TFV gel, or vaginal placebo gel.Citation2 All participants were scheduled for follow up visits targeted to occur every 28 days following the participant’s study enrollment date. listed in the supplementary materials section details the schedule of visits and evaluations for the study.
Table 1 Demographics of the 322 women enrolled in the VOICE Study in Uganda.
Retention procedures
The MU-JHU site established local standard operating procedures (SOPs) for participant retention in order to minimize loss to follow-up. These strategies were informed by the study protocol, available literature, Microbicide Trials Network (MTN) guidelines, and study site best practices. To maximize retention, the study employed the multi-faceted, comprehensive follow-up and retention strategies listed below:
Community engagement
Community outreach workers serve an important role in community engagement. Prior to study initiation, these outreach workers were trained to find the best ways to get the community involved in the study and gauge whether the community was a good fit for the desired study population. This training involved engaging with the MU-JHU community advisory board and community leaders to see if there was interest in study participation and working through barriers that may hinder the study. The community advisory board consisted of a team of community representatives which included community leaders, media representatives, former study participants, social scientists, people living with HIV/AIDS, and religious leaders. Inclusion of these individuals was based upon the recommendations of the community leaders consulted. The major aim of the community advisory board is to link the community and the research team. The community advisory board met with community leaders multiple times throughout the study. During protocol development, they worked together to figure out the best language to use in the informed consent forms. The community liaison on the community advisory board met with field recruiters throughout the recruitment process to discuss possible solutions to any issues that arose. They reviewed all study protocols before being implemented in order to include the perspective of the community. Without buy-in from the community,Citation12 it is very difficult to carry out a study and can lead to the dissemination of misconceptions and myths about the purpose of the study. These outreach workers were mobilized during the study to complete in-person contact with study participants at their homes and other community locations. These visits involved assisting participants who missed follow-up meetings or were having any other difficulties with the study.
In order to identify potential study participants who are likely to be retained in a longitudinal study, it is crucial to develop ongoing community partnerships. Before the study was initiated, community sensitization sessions were held in order to increase awareness of HIV/AIDS, explain the purpose of HIV prevention research in general, and explain the particular purpose of our study. The community sensitization sessions were conducted by a team of community educators from MU-JHU. The team used flip charts and fact sheets to enhance the community’s understanding of the study. During these sessions, two-way discussions were held about HIV infection, available HIV prevention options, MU-JHU as a research organization, and why the study was being conducted. They also discussed the risks and benefits of study participation, confidentiality of all study procedures, and explained the five different study groups. The emphasis about the five study groups was key so that participants did not later feel that study staff had misled them about the study. These meetings helped address possible misconceptions about HIV/AIDS and the MTN-003/VOICE study. These meetings also provided an avenue for prescreening of individuals who expressed interest in participating in the study. Potential participants were encouraged to discuss study participation with their spouses and other influential family members. Without family knowledge of study participation there is great potential for social harms to result due to the stigma of using HIV medications in many communities. However, there is also potential for social harms to result with family knowledge of study participation due to misconceptions about why individuals may be participating in a HIV prevention study, so these competing interests need to be carefully weighed. These community sessions were continued throughout the duration of the study in order to provide a venue for questions and concerns about the study.
Locator information and use of mapping techniques
Detailed locator information was collected at study enrollment visits, which included addresses for home and work, contact information, names and contact information for next of kin, and home village location. This information was actively reviewed and community outreach workers updated locator information at each subsequent visit. Research staff obtained advance permission from participants to contact them using the variety of options provided by the locator information. The locator information was used to find participants if they missed appointments. Additionally, in the absence of a global positioning system in our setting, mapping techniques were used to establish the exact locations of participant residences and other venues where the participant might be found. These maps were detailed pictures that enabled the health visitors to more easily track participants. Mapping information also included the contact information of community leaders and other relevant persons to the participant. Therefore, when participants were not able to be found in Kampala, the community outreach workers could involve community leaders to help trace them in their home communities. Through the use of locator information and mapping techniques, loss to follow-up was greatly minimized.
Visit reminders
At the point of enrollment, a visit calendar was generated for participants which showed the sequence of visits for the duration of the study. The community outreach workers would call the participants the day before these visits to remind them about their appointments.
Immediate and multifaceted follow-up on missed visits
A database was used which generated a list of the participants who attended their monthly follow-up visits. A separate list of participants who missed their visit was also created and distributed to the community outreach workers. The community outreach workers thus were able to use the locator information to immediately begin tracing these participants and finding out why the participants were not able to attend their visit. As a result, the community outreach workers were able to address problems early on and help the participants best take part in the study without inconveniencing their lives too greatly.
Monthly reports on the percentage of participants completing follow-up visits at each site were generated in addition to the database. These reports helped to identify potential problem sites and the research staff met together to try to address these issues. Furthermore, during weekly research group meetings the community outreach workers had discussions about their difficult cases. The research staff then collaborated to discuss mechanisms to solve these issues and help the participants get back on their regular schedule of follow-up visits.
Results
A total of 637 women were screened and 322 participants were enrolled and followed up between December 2009 and February 2012 at the Kampala site in Uganda. The mean age of the participants was 28.3 years and 25% of the participants were under 25 years old. shows the baseline characteristics of the women enrolled in MTN-003.
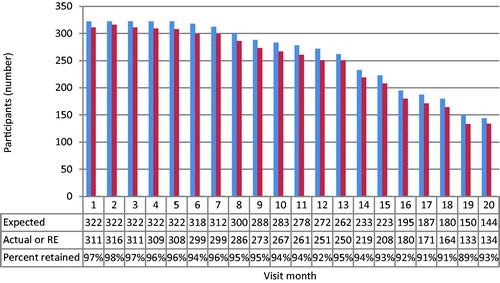
The overall retention rate for the Kampala site was 95% with a range of 89 to 98% as detailed by . The retention rate was measured by comparing the expected number of participants seen at each visit to the actual number of participants seen. Out of a total of 6124 expected visits, only 179 (3%) were missed.
The reasons given by participants for why they missed visits are listed in . Among the reasons listed by participants in the missed visits categorized as other, frequent reasons given include: family member preventing them from attending, husband/partner preventing them from attending, participant travel to home village, and planned missed visits where participants were provided two months of study products. Further conversations with participants who missed visits revealed other main sources for their visit absences, which included: thinking that they did not need frequent visits due to their HIV negative status, finding it difficult to find time for visits due to sex work, and migration out of Kampala for better employment.
Table 2 Participants given reasons for missed visits.
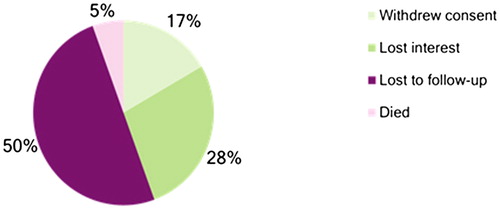
Overall, there were a total of 18 total early terminations at the Kampala sites as detailed by . Three participants withdrew consent because they were not willing to continue with study proceedings, nine were lost to follow up, five lost interest in the study, and one died from a road traffic accident.
Discussion
Having reached the target retention rate of 95% over a 20-month period, this study demonstrates that with effective community engagement, successful participant tracking, visit reminders, and comprehensive missed visit follow-up, it is possible to retain participants in a longitudinal HIV prevention study in a resource limited setting.
Many of the retention strategies used in our study were consistent with other successful HIV prevention participation retention studies. A community-based HIV/STI study conducted in Lima, Peru among marginalized populationsCitation13 also demonstrated success in achieving high retention rates through the collection and regular updating of locator information, field visits in the local community, and the use of technological strategies such as email and internet chat to enable participants to more easily speak with study staff. A similarly high participant retention was achieved in a study of low-income women in the US at high risk for HIV preventionCitation14 through the use of extensive community engagement and participant tracing. These and our study show that the more comprehensive and multi-faceted the retention strategies, the more likely they will be successful.
One of the major differences between our study and most other successful HIV prevention retention strategies was the young age of our participants. The mean age of our participants was 28.3 years with 25% of participants under the age of 25 years old. Studies in UgandaCitation15,Citation16 and the USCitation17 demonstrated that the highest dropout rates were among the youngest age group (ages 13–24).Citation18 Nevertheless, we were still able to achieve high levels of retention (94%) among participants below age 25. This finding is critically important as those in the younger age group are among the most highly mobile of participantsCitation19 and at high risk of HIV infectionCitation20 so it is crucial to be able to retain this population in HIV prevention studies.
The retention strategies implemented in the MTN-003/VOICE study also demonstrate the increasing importance of the use of reminder calls using cell phones in longitudinal research. This technology enabled health visitors to much more easily track down participants than in the past. A 2014 Pew Research Center survey conducted in seven Sub-Saharan countries including Uganda showed that roughly 2/3 of adults possess cell phones and 15% have smart-phones.Citation21 Investigators in Sub-Saharan Africa have already started trying to incorporate mobile phone-based strategies into their studies. These approaches include varying ideas such as using a mobile phone-based case manager approach to prevent mother to child transmission of HIV in South AfricaCitation22 and a text message-based approach to antiretroviral treatment adherence in Kenya.Citation23 Future interventions would be wise to incorporate cell phone-based strategies as an element of their retention approach as cell phone use gets even more widespread in Sub-Saharan Africa.
Although our study was successful at achieving high retention of study participants, the results of VOICE demonstrated low study drug adherence. Over 50% of participants assigned to active study drug sub-groups in a representative sub-cohort showed undetectable levels of TDF in all plasma samples tested, which likely was responsible for the lack of HIV infection protection observed in the larger overall study.Citation24 This paradox of high participant retention and low drug adherence was investigated by a qualitative study follow-up. Participants in the follow-up study explained that they remained in the study even while not regularly taking study products in part due to the access to quality health services, free treatment, and regular HIV testing as well as the education gained from being part of the study and a curiosity of the study results. Participants in this study follow-up suggested implementing real-time product use monitoring and feedback in future PrEP trials in order to improve drug adherence.Citation24
The results of our study are subject to limitations. Retention strategies in MTN-003/VOICE were a secondary analysis of the overall VOICE study and therefore the primary objectives in the study were not designed to assess retention strategies. Due to this, there was no comparison group, so it is not possible to draw causal relationships between the retention strategies used and the retention rate achieved.
The approaches used in the VOICE study were quite labor intensive. They involved members of the community outreach team physically going out into the city and finding participants who missed visits. It may be argued that these measures are not the ideal approach for resource rich settings where labor can be much more expensive. Therefore, it is crucial to adapt retention strategies to the specific location of each study. In studies conducted in wealthier countries, it may be necessary to use more technologically based strategies like call and text reminders due to high labor costs and conduct missed visit meetings via social media applications such as Skype. Nevertheless, we believe that the retention strategies used in VOICE can serve as a model for conducting other longitudinal clinical research studies in resource-limited settings, particularly those in Sub-Saharan Africa.
Conclusion
In a HIV PrEP study conducted at sites in Kampala, Uganda we were able to achieve high levels of participant retention over a 3-year follow-up duration. A multi-faceted retention approach consisting of participant tracking, community engagement, visit reminders, and comprehensive missed visit follow-up was used in order to achieve this retention rate of 95% overall. We believe that the approach used can be successful if applied to future HIV prevention studies, particularly those conducted in other resource-limited areas.
Availability of data and material
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Ethics approval and consent to participate
The VOICE study was approved by the Uganda National HIV and AIDS Research Committee, Uganda National Council of Science and Technology in Uganda, and the Johns Hopkins School of Medicine IRB, United States. Written informed consent for study participation was obtained from all participants.
Notes on contributors
DK, RM, TN, SK, CN, and FMK contributed to data collection. Data analysis and interpretation was done by FMK and MM. JW prepared the manuscript guided by FMK. All authors contributed to the final version of the manuscript; revised the article critically for important intellectual content and approved the final manuscript.
Disclosure statement
This work was presented in part at the HIV Research for Prevention, October 28–31, 2014, Cape Town, South Africa.
Additional information
Funding
References
- CDC. HIV Risk and Prevention: Pre-Exposure Prophylaxis (PrEP) [Internet]. CDC HIV/AIDS. Centers for Disease Control and Prevention; 2016. https://www.cdc.gov/hiv/risk/prep/. Accessed March 17, 2017.
- Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African Women. N Engl J Med. 2015;372(6):509–518.
- MTN-003 Description [Internet]. Microbicide Trials Network. http://www.mtnstopshiv.org/studies/70. Accessed March 10, 2017.
- Onoya D, Sifunda S, Wingood GM, van den Borne B, Ruiter RAC. Barriers to recruitment and retention of HIV-negative Black South African women into behavioral HIV prevention programs. J HIV/AIDS Soc Services. 2011;10(3):248–264.
- Beverley Cummings MM, Negashb W, Bekelec A, Ghileb T. Barriers to and facilitators for female participation in an HIV prevention project in Rural Ethiopia: findings from a qualitative evaluation. Cult Health Sex. 2006;8(3):251–266.
- HIV and AIDS in Uganda [Internet]. AVERT. 2016 https://www.avert.org/professionals/hiv-around-world/sub-saharan-africa/uganda. Accessed March 10, 2017.
- Aseltine RH, Gore S. Mental health and social adaptation following the transition from high school. J Res Adolesc. 1993;3(3):247–270.
- Furstenberg FF, Jr., Rumbaut RC, Settersten RA, Jr. On the frontier of adulthood: emerging themes and new directions. In: Settersten RA, Furstenberg FF, Rumbaut RG, eds. On the frontier of adulthood: Theory, research, and public policy. Chicago: The University of Chicago Press; 2005:3–25.
- Mtove G, Kimani J, Kisinza W, et al. (2018). Multiple-level stakeholder engagement in malaria clinical trials: addressing the challenges of conducting clinical research in resource-limited settings. Trials. 19:190. doi:10.1186/s13063-018-2563-1
- Senturia YD, Mortimer KM, et al. Successful techniques for retention of study participants in an inner-city population. Contr Clin Trials. 1998;19(6):544–554.
- Idoko OT, Owolabi OA, Odutola AA, et al. (2014). Lessons in participant retention in the course of a randomized controlled clinical trial. BMC Res Notes 7:706. doi:10.1186/1756-0500-7-706
- Quinn SC. Ethics in public health research: protecting human subjects: the role of community advisory boards. Am J Public Health. 2004;94(6):918–922.
- Villacorta V, Kegeles S, Galea J, et al. Innovative approaches to cohort retention in a community-based HIV/STI prevention trial for socially marginalized Peruvian young adults. Clin Trial. 2007;4(1):32–41. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2853960/#R10
- Haley D, Lucas J, Golin CE, et al. Retention strategies and factors associated with missed visits among low income women at increased risk of HIV acquisition in the US (HPTN 064). AIDS Patient Care STDs. 2014;28(4):206–217. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3985524/
- Ruzagira E, Wandiembe S, Abaasa A, et al. HIV incidence and risk factors for acquisition in HIV discordant couples in Masaka, Uganda: an HIV vaccine preparedness study. PLoS One. 2011;6:e24037. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0024037#s3
- Kiwanuka N, Ssetaala A, Nalutaaya A, Mpendo J, Wambuzi M, Sewankambo NK. High incidence of HIV-1 infection in a general population of fishing communities around lake Victoria, Uganda. PLoS One. 2014;9:e94932. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4035272/
- Hessol NA, Weber KM, Holman S, et al. Retention and attendance of women enrolled in a large prospective study of HIV-1 in the United States. J Women Health. 2009;18(10):1627–1637. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2825719/
- Abaasa A, Asiki G, Mpendo J, et al. Factors associated with dropout in a long term observational cohort of fishing communities around Lake Victoria, Uganda. BMC Res Notes. 2015;8:815. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4690385/#CR10
- Hessol NA, Schneider M, Greenblatt RM, et al. Retention of women enrolled in a prospective study of human immunodeficiency virus infection: impact of race, unstable housing, and use of human immunodeficiency virus therapy. Am J Epidemiol. 2001;154(6):563–573. https://academic.oup.com/aje/article/154/6/563/75135/Retention-of-Women-Enrolled-in-a-Prospective-Study
- Muula AS. HIV infection and AIDS among young women in South Africa. Croat Med J. 2008;49:423–35. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2443629/
- PRC. Cell Phones in Africa: Communication Lifeline [Internet]. Pew Research Center’s Global Attitudes Project. 2015. http://www.pewglobal.org/2015/04/15/cell-phones-in-africa-communication-lifeline/. Accessed March 17, 2017.
- Schwartz SR, Clouse K, Yende N, et al. Acceptability and feasibility of a mobile phone-based case management intervention to retain mothers and infants from an option B + Program in postpartum HIV care. Matern Child Health J. 2015;19(9):2029–2037.
- Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376(9755):1838–1845. doi:10.1016/S0140-6736(10)61997-6
- Straten AVD, Montgomery ET, Musara P, et al. Disclosure of pharmacokinetic drug results to understand nonadherence. AIDS. 2015;29(16):2161–2171.