Abstract
Background: Raltegravir became the first integrase inhibitor to gain FDA approval; but with limited evidence documenting long-term risks in real world care, especially for major health outcomes of interest.
Objective: Assess raltegravir safety in clinical practice within an integrated health system.
Methods: We conducted a cohort study of HIV-infected adults within Kaiser Permanente California from 2005 to 2013. We compared patients initiating raltegravir during the study period with two groups; a historical cohort (started new antiretroviral regimen [ART] 2005–2007) and a concurrent cohort that did not initiate raltegravir (2007–2013). We used multivariate Cox proportional hazard regression to obtain hazard ratios (HR) for pre-specified incident health outcomes, employing propensity scores to adjust for potential confounding.
Results: The population included 8,219 HIV-infected adults (raltegravir cohort N = 1,757; 4,798 patient-years), with greater years known HIV-infected among raltegravir patients. The raltegravir cohort had increased HR for AIDS-defining (HR 2.69 [1.53–4.71]; HR 1.85 [1.21–2.82]) and non-AIDS-defining malignancies (HR 2.26 [1.29–3.94]; HR 1.88 [1.26–2.78]) relative to both comparison cohorts. Compared to the historical cohort we found no significant difference in all-cause mortality; the raltegravir cohort experienced increased HR for all-cause mortality compared to concurrent (HR 1.53 [1.02–2.31]). Raltegravir appeared protective of lipodystrophy when compared to the historical cohort but associated with increased incidence compared to concurrent. There were no significant differences in the incidence of hepatic, skin, or cardiovascular events.
Conclusions: The potentially elevated risk for malignancy and mortality with raltegravir and residual confounding merits further investigation. We demonstrate the value of observational cohorts for monitoring post-licensure medication safety.
Introduction
Modern antiretroviral therapy (ART) has revolutionized medical treatment for patients with HIV, improving life expectancy and shifting HIV from the realm of terminal illness to that of chronic disease. In this modern era, the goal of treatment is threefold; achieve maximal viral suppression, while limiting adverse effects and keeping ART relatively simple. Raltegravir was the first integrase inhibitor (II) licensed for use as an antiretroviral agent in the United States.Citation1,Citation2 It was approved by the Food and Drug Administration (FDA) for use in treatment-experienced patients in October 2007, and its indication was expanded to include ART-naïve patients and children and adolescents 2–18 years of age in 2009 and 2011, respectively.Citation3–5
The safety and 1- to 5-year effectiveness of raltegravir has been explored in initial drug trials and limited follow-up in phase IV post-marketing studies.Citation3,Citation6–12 Initial studies showed raltegravir to be quite efficacious, with reportedly few adverse effects. Even though the drug required twice-daily administration at time of release, the promising clinical results led raltegravir to become a preferred/recommended agent for first-line therapy.Citation13–15 Another desirable aspect of the drug is that raltegravir can be used in patients with multidrug-resistant HIV-1 and a history of treatment failure.Citation16 Despite its widespread use, due to the relative novelty of raltegravir there is limited empirical evidence that documents the long-term risks related to its use and still greater need for information about its performance in the course of typical “real world” care.
The purpose of this study is to assess the post-licensure safety of raltegravir in routine HIV clinical practice. Kaiser Permanente (KP) is a large integrated healthcare delivery system providing comprehensive medical care to nearly 12 million members, and >25,000 HIV-infected members across the United States. With a longitudinal electronic health record, multidisciplinary HIV care teams, dedicated HIV quality measurement and quality improvement program, and full access to a comprehensive ART formulary, we sought to evaluate our safety experience with raltegravir. Using data from KP, we estimate the 5-year risk of incident pre-specified health outcomes of interest (HOI) in a cohort of HIV-infected adults treated with raltegravir. We also estimate relative rates of the same pre-specified HOI by comparing incidence rates in raltegravir treated individuals to a historical comparison cohort and a concurrent comparison cohort.
Methods
Data source
This observational post-licensure safety study reports results from monitoring the electronic medical records of HIV-infected patients that were treated in the course of HIV clinical practice at Kaiser Permanente Northern California (KPNC) and Kaiser Permanente Southern California (KPSC), both large integrated healthcare systems providing comprehensive care to more than 4 million members each. Study data were gathered using KP HealthConnect, a modified version of the Epic® electronic medical record system. KP HealthConnect data is merged with administrative databases so that all member-level medical, demographic, and health care coverage information can be accessed. Linkages were also made to the SEER-based cancer registries and state- and federal-based mortality files. The institutional review board in both institutions approved this study.
Study population
The study population was identified using the KPNC and KPSC HIV registries. The KPNC HIV registry includes all known HIV/AIDS cases since the early 1980s, with HIV status confirmed by review of medical charts or medical center case lists. Members are initially identified as possibly HIV-infected if they are found to have one or more of the following indicators identified from electronic databases: (1) positive HIV antibody test; (2) detectable HIV viral load; (3) CD4/CD8 ratio <1.0; (4) prescription for any ARV drug; (5) outpatient documentation of HIV infection; (6) HIV/AIDS hospital discharge diagnosis; (7) pathology report for Kaposi sarcoma or Pneumocystis jiroveci pneumonia; and (8) Centers for Disease Control & Prevention AIDS case report forms, infection control nurse notes, and internal physician reporting. The case ascertainment methodology in KPNC is estimated to have >95% sensitivity and >99% specificity based on comparisons of the registry and clinician HIV case lists. A similar methodology is employed in the KPSC registry, although the KPSC registry only dates from 2000.
All HIV-infected patients starting a new ART regimen, antiretroviral naïve or experienced, but containing at least a 30-day supply of raltegravir on or after October 12, 2007 through June 30, 2013 were included in the treatment group, hereafter referred to as the RAL cohort. Two comparison cohorts were used; the historical cohort served as the primary comparison group and the concurrent cohort served as a secondary comparison group. The historical cohort was composed of HIV registry patients that started a new antiretroviral regimen (again, ART experienced or naïve) from January 1, 2005 through the date raltegravir was licensed in the US, October 11, 2007. The concurrent cohort was established to generate comparisons between the RAL cohort and a contemporaneous post-licensure cohort of HIV-infected patients. Patients were eligible for the concurrent cohort if they initiated a new antiretroviral regimen (ART naïve or experienced) that did not contain raltegravir on or after October 12, 2007 through June 30, 2013, and had greater than a 30-day supply of medication. Members in all three cohorts were required to have had at least one year of continuous enrollment with KP prior to index date to allow for the assessment of medical and treatment history.
For all study participants, the date a patient first received a dispensed medication served as the index date for follow-up to start for that patient. For a given HOI, follow-up for a patient treated with raltegravir was censored on the earliest date that the patient experienced the HOI, death, ended membership with KP, or 90 days after the date the patient ended use of raltegravir. Follow-up was calculated separately for each HOI and the occurrence of a given HOI resulted in end of follow-up for that HOI, but not for other HOI. Historical and concurrent controls were eligible to enter the RAL cohort at any time. For patients in either of the comparison cohorts that later entered the RAL cohort, follow-up was ended the day before treatment with raltegravir began.
Outcomes
This study focused on HOI during treatment with raltegravir. Incident events were defined as a documented HOI that was not previously experienced by a patient in at least the 12 months prior to the start of follow-up. HOI were selected based on the existing literature. The primary pre-specified HOI included: (1) hepatic events;Citation17,Citation18 (2) skin events;Citation19,Citation20 (3) cardiovascular events;Citation21 (4) lipodystrophy events;Citation22,Citation23 (5) AIDS-defining malignancies;Citation24–27 (6) non-AIDS defining malignancies;Citation24–27 and (7) muscle events.Citation28,Citation29 In addition, all-cause mortality was assessed. Follow-up was calculated separately for each HOI and the occurrence of a medical event of a particular HOI resulted in censoring for that specific HOI but not for other HOI. Upon discontinuation of raltegravir, HOI occurring up to 90 days post-discontinuation were attributed to raltegravir. For HOI that may recur (e.g. malignancy), additional analyses and algorithms were utilized and incident diagnoses were used; ie, no prior evidence of that malignancy in the EHR. Information on HOI were ascertained using diagnostic (ICD-9 and IDC-03), procedure (CPT), prescription (NDC), and/or laboratory codes, depending on the HOI of interest and were identified from electronic records of outpatient clinic visits, emergency department (ED) visits, hospitalizations, and pharmacy visits based on pre-specified ICD-9, CPT, NDC, and/or laboratory codes. Information from the cancer registry was used to capture malignancies. To assess all-cause mortality, deaths were identified from KP hospital records, as well as from linkages to Social Security Administrative mortality records, and state death certificates.
Statistical methods
Distributions of demographic, behavioral, and clinical characteristics at cohort entry were assessed for each cohort. Cohort-specific HOI incidence rates were calculated as the number of first occurrences of the HOI in the cohort divided by the total at-risk person-time for the given HOI accrued in that cohort. Associated 95% confidence intervals were based on the Poisson distribution.
Multivariable Cox proportional hazards regression was used to provide point and interval estimates of HOI hazard ratios (HR) associated with raltegravir exposure, with cohort exposure treated as time-varying to allow for potential movement from the historical and concurrent cohorts into the RAL cohort. Propensity scores (PS) were utilized to adjust for potential confounders. Using logistic regression with raltegravir exposure as the dependent variable and 30 predictors, two sets of PS were generated, one for each of the two cohort pairs (RAL vs historical, RAL vs concurrent). PS covariates include: age at entry, gender, race/ethnicity, HIV risk behavior, years known HIV infected, HIV RNA at baseline, CD4+ count at baseline, prior AIDS defining diagnosis, years since first ARV medication, prior NRTI use, prior NNRTI use, prior PI use, creatinine at baseline, ALT at baseline, AST at baseline, bilirubin-total at baseline, creatine kinase at baseline, and prior history of Hepatitis B, Hepatitis C, end-stage renal disease, syphilis, diabetes mellitus, hepatic failure, serious rash, AIDS-defining malignancy, non-AIDS-defining malignancy, muscle event, cardiovascular event, lipodystrophy and ART regimen number.Citation30,Citation31 The PS score was categorized into quintiles and was treated as a stratification factor in the Cox regression analyses (i.e. separate strata-specific baseline hazard functions). ART treatment regimen number (1st, 2nd, 3rd+) was also included as a separate covariate in analyses of all outcomes of interest (a priori decision). For the analysis of hepatic events, hepatitis status was included as an additional covariate.
Comparative analyses were only performed when there were more than 20 total events in each patient cohort. In addition to analyses of the overall raltegravir effect in the full study cohorts, separate analyses were also conducted by regimen number (1st, 2nd, and 3rd+), with PS developed in each of the three regimen number subsets for each cohort pair (RAL vs historical, RAL vs concurrent).
Results
Patient characteristics
The study population included 8,219 HIV-infected adults and a total of 4,798 patient-years of raltegravir experience, with 1,044 patients contributing over 2 years each of experience with the medication. The RAL cohort had 1,757 patients, representing more than 30 percent of patients initiating a new regimen in KPSC and KPNC during the study period. presents baseline patient characteristics by cohort.
Table 1 Patient characteristics by cohort
All cohorts were predominantly male, which is representative of the HIV-infected population in KPNC and KPSC. The largest represented racial/ethnic group in all three cohorts was White, and there was greater representation by Latinos than Blacks. Further, HIV risk behavior was majority men having sex with men, reflecting the demographics of the disease within KP California. For each cohort, the vast majority of patients have been KP members for many years, signifying that all patients are generally well known to their providers.
The RAL cohort had a greater percentage of patients with HIV RNA <500 copies/mL at regimen initiation (cutoff due to historical cohort assay limits; p < .001), and a greater percent of patients with CD4 > 200/µL at regimen initiation compared with other cohorts (p < .001). The majority of patients in all cohorts remained virally suppressed throughout, with 75.6% of RAL patients having a HIV RNA <500 copies/mL at time of any HOI, compared with 56.6% among the historical cohort and 56.1% among the concurrent cohort. The RAL cohort was older than the other two cohorts. This age difference was consistent across treatment regimen subgroup comparisons. Consistent with this finding, the RAL cohort had longer KP membership and more years known HIV-infected.
The RAL cohort had statistically greater percent of patients with prior skin events, malignancies (11.7% AIDS-defining and 8.1% non-AIDS-defining malignancies), lipodystrophy diagnoses, and cardiovascular events relative to both the historical and concurrent cohorts. Prior hepatitis B and C diagnosis was statistically greater in the RAL cohort compared with the concurrent cohort. There was also statistically significant heterogeneity of prior ART use by class and regimen number among the cohorts (p < .001 for each ART class). The RAL cohort had a statistically significant higher percentage of triple class exposed patients (p < .001).
Propensity scores
Propensity score diagnostics included examining pairwise differences in the distribution of each PS score covariate (RAL vs historical, RAL vs concurrent) within each PS quintile. In general, there was reasonably good balance on the 30 covariates within each PS quintile. In sensitivity analyses aimed at adjusting for this potential residual confounding, each covariate with more than a 7.5% absolute within-PS stratum difference in covariate prevalence was included in the Cox regression analyses, in addition to PS score and treatment regimen. Inclusion of these additional model covariates had no substantial impact on point and interval hazard ratio (HR) estimates. In addition, results were not sensitive to the approach to PS adjustment, including categorization in deciles and inclusion as a model covariate rather than a stratifying variable in Cox models. Additional sensitivity analyses examined trimming of PS distribution tails where there were only patients from one exposure group represented (n = 42 in the RAL/historical and n = 37 in the RAL/concurrent analyses), with no appreciable impact on regression estimates.
Comparative health outcomes of interest
presents the unadjusted incident rates for all HOI for the three cohorts. For the outcome of “any HOI” (all events aggregated except death) there were no significant differences between the RAL cohort and the concurrent cohort or the historical cohort. One HOI, clinically significant muscle events, did not meet the minimum threshold of absolute events to be included in the comparative analyses.
Table 2 Unadjusted incidence rates for health outcomes of interest (HOI)
In adjusted analyses, mortality results varied by the comparison cohort (, ). Relative to the historical cohort, the RAL cohort had no statistically significant difference in mortality. When stratified by regimen number, there was a trend toward a protective effect with greater regimen number. For either cohort, most events were among males and those ≥50 years old. Compared with the concurrent cohort, the RAL cohort experienced statistically significantly higher incidence for mortality. However, when stratified by regimen number, there was no statistically significant difference between these two cohorts, although in unadjusted analyses the RAL cohort had a larger proportion of deaths among those on their ≥3rd ART regimen ().
Figure 1 Adjusted hazard ratios comparing members of the Raltegravir cohort to each control group for each health outcome of interest.
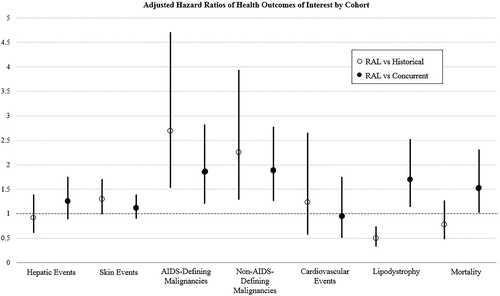
Figure 2 Adjusted hazard ratios comparing members of the Raltegravir cohort to the historical cohort for the health outcomes of interest that had statistically significant differences when stratified by regimen number.
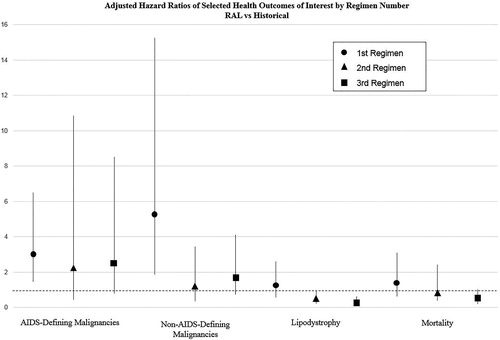
Table 3 Adjusted hazard ratios of health outcomes of interest
There were statistically significant differences among the cohorts for incidence of AIDS-defining malignancies (, ). In adjusted analyses, there was greater risk among the RAL cohort compared to both the historical and concurrent cohorts. Compared to the historical cohort, HR attenuated slightly and was not statistically significant for patients starting raltegravir as their 2nd or ≥3rd regimen (). The majority of the identified cancers were Kaposi sarcoma (66% for the raltegravir cohort, 72% for the historical, and 60% for the concurrent). Non-Hodgkin lymphoma accounted for 40%, 24%, and 40% respectively, with the remainder being invasive cervical cancer. The difference between the RAL and concurrent cohorts was significant among the patients on their 1st ARV, but the point estimate was greatest for patients on their ≥3rd regimen (but not statistically significant); 58% of AIDS-defining malignancies were among the ≥3rd regimen in the RAL cohort, as compared with only six percent in the concurrent cohort.
There were also significant differences in overall incident non-AIDS-defining malignancies between the cohorts (, ). Among these, anal/rectal cancer had a large percent (39% among the raltegravir cohort, 13% in the historical cohort, and 30% in the concurrent cohort). Additionally, the largest other incident non-AIDS-defining malignancies were: Hodgkin lymphoma (7% in the raltegravir cohort); melanoma and other skin cancers (10% in raltegravir, 17% in historical, and 27% in concurrent cohorts); and prostate cancer (11%, 7%, and 14% respectively). There was a significantly greater adjusted HR, 2.26 (1.29–3.94), among the RAL cohort compared to the historical cohort. However, when stratified by regimen number, the HR is statistically significant only among patients on their 1st ART regimen but the HR decreases and loses statistical significance with subsequent regimens (). The RAL cohort also had an increased adjusted HR compared to the concurrent cohort, 1.88 (1.26–2.78). Again, there was an increased risk among those on their 1st ART regimen. There was not a statistically significant difference in the types of non-AIDS-defining malignancies among the RAL and the concurrent cohort.
Compared with the historical cohort, the RAL cohort had a significantly lower adjusted hazard for lipodystrophy, 0.50 (0.33–0.74); this was especially true for patients on their 2nd regimen (HR = 0.47; p < .05) and patients on their 3rd or greater ART regimen (HR = 0.33; p < .001) (). The large majority of lipodystrophy events were lipoatrophy in the RAL cohort, but were unspecified in the historical cohort. Conversely, the RAL cohort experienced a significantly higher adjusted HR of lipodystrophy compared to the concurrent cohort, 1.70 (1.14–2.53). This increased risk appears to be driven by the patients on their 1st ART regimen (HR = 2.40; p < .001). The overall number of lipodystrophy events was less than 5% in both the RAL and concurrent cohorts.
Finally, raltegravir was not associated with a greater likelihood of clinically important hepatic, skin, or cardiovascular events. This null finding was consistent for both comparison cohorts. For the observed hepatic events, the majority occurred in males that were virally suppressed at time of event. In all three cohorts, the most commonly diagnosed clinically important skin event was an unspecified rash or skin eruption. Unlike the RAL cohort, the large majority of skin events in the concurrent and historical cohorts were among those on their 1st antiretroviral regimen (p < .05). Most of the observed cardiovascular events occurred among males and there was significant heterogeneity in the distribution of age at event across the cohorts (p < .05).
Discussion
This study represents almost six years of post-licensure surveillance data of raltegravir, the first integrase inhibitor licensed for use as an antiretroviral medication in the United States. To our knowledge, this is one of the largest studies to examine the association of long-term health outcomes with raltegravir, within the course of ordinary treatment from a single health care system.
In comparison to the outcomes of patients taking other antiretroviral medications, this observational study replicated the results of previous experimental studies in finding that raltegravir appears to be a safe medication in terms of muscle, skin, cardiovascular and hepatic events, with no significant increased risk of clinically adverse events of these types.Citation6–11 We also observed significantly lower hazard risk rates for lipodystrophy in the RAL cohort compared to the historical cohort, suggesting there can be protective effects of this regimen. The different results for lipodystrophy (nearly all lipoatrophy) between the two comparison cohorts may be due to indication bias and years of treatment experience at time of initiation of the ART regimen in question. The RAL cohort had more treatment experienced patients compared to the concurrent cohort. In combination with greater viral suppression, this may have prompted HIV specialists to code this diagnosis and potentially refer for lipodystrophy treatment (injectable poly-L-lactic acid [Sculptra®], a covered benefit in KP). However, we discovered potential harmful associations for raltegravir compared with other ART regimens for AIDS-defining and non-AIDS defining cancers, as well as all-cause mortality, when stratified by comparison and regimen number. Of course, this merits further examination.
We found that the RAL cohort had an overall statistically significantly greater hazard for AIDS-defining malignancies compared to the historical cohort, but this increased risk attenuated slightly and was not statistically significant for patients starting raltegravir as their 2nd or ≥3rd regimen. Similarly, we observed an increased adjusted hazard for non-AIDS-defining in the RAL cohort compared to the historical cohort and the relationship appears to be driven by the increased hazard among the RAL patients on their 1st regimen. It is worth noting the greater numbers of years known HIV-infected among the RAL cohort and the possibility of a screening bias for which may have not been adequately controlled. The data does not permit further biologic explanation of these potential signals. Because we only investigate raltegravir, we are also unable to ascertain if this was a class effect of integrase inhibitors or unique to raltegravir.
Mortality results were dependent on which cohort comparisons were being made. We found no increased risk of mortality comparing the RAL cohort to the historical cohort, and even identified a trend toward protection of death with later ART regimens. There was potential increased relative hazard risk of all-cause mortality when comparing the RAL cohort to the concurrent cohort, however when stratified by regimen number there was no statistically significant difference between the two cohorts. This main effect could be because the patients initially prescribed raltegravir had more advanced HIV disease, although our propensity score methodology attempted to adjust for these differences. As ART regimens increase effectiveness and mortality rates decline generally, differences like these require increased scrutiny.
These findings are interesting in light of a recent 4-year observational study that included an analysis of incident AIDS or death after initiation of raltegravir. In unadjusted analyses, it was observed that patients taking raltegravir had a higher risk of incident AIDS or all-cause death compared to patients taking efavirenz, however this difference was eliminated after accounting for nonrandom sampling, treatment allocation, and dropout.Citation16 The combination of these findings and our results highlight the necessity for vigilance in risk-adjusting observational data and the value of properly powered subgroup analyses. It is also necessary to put these results into the context of the EuroSIDA cohort study, which included a RAL cohort of 1,470 European individuals and focused specifically on incidence of cancer and overall risk of mortality, utilizing a similar methodology to ours. This study concluded that use of RAL was not associated with increased risk of cancer or survival rates, compared to people treated with alternative regimens in routine clinical care.Citation32
There were substantial differences in the clinical histories of the cohorts. The raltegravir cohort, compared to the other cohorts, was older, more years known HIV-infected, and more ART experienced. However, this is not likely to have greatly impacted these results, as we were exploring incident safety concerns, and we adjusted for prior events. While a prior history of cancer may predispose a patient to subsequent cancers, this safety concern would not apply to the other HOI.Citation33,Citation34 As we and others have demonstrated in prior research, worse immune function and older age is associated with increased risk of side effects and cancers.Citation35–37 But in this study, the raltegravir cohort had generally higher CD4 counts than the comparison cohorts.
The current study is not without limitations. The absolute number of events remains low for most outcomes. This is especially true when stratifying by regimen number. Second, as a nonrandomized study, the potential for confounding by indication remains; it is possible that the stratification by quintile propensity score method is not fully capturing the relationship between raltegravir-based and other ART regimens. There might also be unmeasured confounders, which could include varying prescribing practices of raltegravir among providers. For example, physicians who prescribe raltegravir might be in clinics that are also more likely to screen for cancer. While we would have liked to cluster at the practice level, the small number of events and large number of clinics precluded us from doing this analysis. For certain factors such as CD4 count and HIV RNA, we were also only able to control for baseline confounding and were unable to control for time-updated confounding. We acknowledge the limited diversity in sex and race/ethnicity; however, the patient demographics reflect the HIV epidemic in California. The residual heterogeneity potentially impacts the certainty of the results for the multivariate regressions. And while unlikely, misclassification error must always be considered. Finally, the observational nature of this study does not prove causation. The identified associations must be interpreted cautiously because the groups had significant differences in propensity scoring and there are a greater number of years known HIV-infected among the RAL cohort.
This study uses population-based observational data to track the long-term efficacy and safety of antiretroviral therapies. Similarly, one recent study in the US utilized the National Institutes of Health-funded Centers for AIDS Research Network of Integrated Clinical Systems cohort and found that raltegravir and efavirenz-based initial ART have similar 4-year clinical effects, while a study in France used the RACING cohort and concluded that patients treated with raltegravir experienced significant improvements in quality of life over a 2-year period of treatment.Citation16,Citation38 Studies that harness existing clinical data to study the long-term effects of post-licensure drugs are important as further randomized experimental evidence may not be forthcoming. Observational cohort studies that rely upon longitudinal real-world data and rigorous quantitative methods provide a valuable source of information that should be used to corroborate evidence from randomized trials and enhance the external validity of their findings. The findings of this study demonstrate the potential power of electronic health record data and generally support the continued use of raltegravir in everyday practice. For example, while not studied here, there is increasing concern about depression and suicide related to integrase inhibitors.Citation39–41 Large cohorts like this are most amenable to such research.
To our knowledge, this is one of the largest studies to examine the association of long-term health outcomes with raltegravir, the first integrase inhibitor licensed for use as an antiretroviral medication in the US. This study contributes to the literature by using almost six years of post-licensure surveillance data, including subgroup analyses based on regimen experience, and by comparing the clinical cohort in question to a historical cohort and a concurrent cohort from the same study population and health system. As compared to other antiretroviral medications, we found that raltegravir is a relatively safe medication and that it should continue to be used in everyday clinical practice. However, potential signals of increased risk for malignancy and mortality merits further study. This investigation demonstrates the value and necessity of post-licensure safety studies.
Disclosure statement
None of the authors had any personal financial ties or investment with Merck & Co. No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Cocohoba J, Dong BJ. Raltegravir: the first HIV integrase inhibitor. Clin Ther. 2008;30(10):1747–1765.
- Summa V, Petrocchi A, Bonelli F, et al. Discovery of raltegravir, a potent, selective orally bioavailable HIV-integrase inhibitor for the treatment of HIV-AIDS infection. J Med Chem. 2008;51(18):5843–5855.
- Liedtke MD, Tomlin CR, Lockhart SM, Miller MM, Rathbun RC. Long-term efficacy and safety of raltegravir in the management of HIV infection. Infect Drug Resist. 2014;7:73–84.
- US Food and Drug Administration. Drug Approval Package for Isentress (raltegravir). In: Administration UFaD, ed. Washington, DC: US Food and Drug Administration; 2008.
- US Food and Drug Administration. HIV/AIDS historical time line 2000 - 201. In: Administration UFaD, ed. Washington, DC: US Food and Drug Administration; 2014.
- Eron JJ, Cooper DA, Steigbigel RT, et al. Efficacy and safety of raltegravir for treatment of HIV for 5 years in the BENCHMRK studies: final results of two randomised, placebo-controlled trials. Lancet Infect Dis. 2013;13(7):587–596.
- Gotuzzo E, Markowitz M, Ratanasuwan W, et al. Sustained efficacy and safety of raltegravir after 5 years of combination antiretroviral therapy as initial treatment of HIV-1 infection: final results of a randomized, controlled, phase II study (Protocol 004). J Acquir Immune Defic Syndr. 2012;61(1):73–77.
- Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374(9692):796–806.
- Markowitz M, Nguyen BY, Gotuzzo E, et al. Rapid and durable antiretroviral effect of the HIV-1 Integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr. 2007;46(2):125–133.
- Rockstroh JK, DeJesus E, Lennox JL, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr. 2013;63(1):77–85.
- Steigbigel RT, Cooper DA, Teppler H, et al. Long-term efficacy and safety of Raltegravir combined with optimized background therapy in treatment-experienced patients with drug-resistant HIV infection: week 96 results of the BENCHMRK 1 and 2 Phase III trials. Clin Infect Dis. 2010;50(4):605–612.
- Young B, Vanig T, DeJesus E, et al. 96-week results of a pilot study of abacavir/lamivudine and raltegravir in antiretroviral-naive HIV-1-infected patients: the SHIELD trial. HIV Clin Trials. 2011;12(4):228–233.
- Adolescents PoAGfAa. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. In: Services DoHaH, ed. 2016:1–128.
- European AIDS Clinical Society. European AIDS clinical society guidelines version 8.2. In: Society EAC, ed. Vol 8.2: European AIDS Clinical Society; 2017:1–97.
- Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308(4):387–402.
- Cole SR, Edwards JK, Hall HI, et al. Incident AIDS or death after initiation of human immunodeficiency virus treatment regimens including raltegravir or efavirenz among adults in the United States. Clin Infect Dis. 2017;64(11):1591–1596.
- Hurt CB, Napravnik S, Moore RD, Eron JJ, Jr. Hepatic safety and tolerability of raltegravir among HIV patients coinfected with hepatitis B and/or C. Antivir Ther. 2014;19(4):415–422.
- Surgers L, Lacombe K. Hepatoxicity of new antiretrovirals: a systematic review. Clin Res Hepatol Gastroenterol. 2013;37(2):126–133.
- Bonfanti P, Ricci E, Molteni C, et al. Low frequency of skin reactions in a cohort of patients on raltegravir. J Antimicrob Chemother. 2012;67(7):1800–1802.
- Fagard C, Colin C, Charpentier C, et al. Long-term efficacy and safety of raltegravir, etravirine, and darunavir/ritonavir in treatment-experienced patients: week 96 results from the ANRS 139 TRIO trial. J Acquir Immune Defic Syndr. 2012;59(5):489–493.
- Yao AH, Moore CL, Lim PL, et al. Metabolic profiles of individuals switched to second-line antiretroviral therapy after failing standard first-line therapy for treatment of HIV-1 infection in a randomized, controlled trial. Antivir Ther. 2018;23(1):21–32.
- Guaraldi G, Stentarelli C, Zona S, Santoro A. HIV-associated lipodystrophy: impact of antiretroviral therapy. Drugs. 2013;73(13):1431–1450.
- McComsey GA, Moser C, Currier J, et al. Body composition changes after initiation of raltegravir or protease inhibitors: ACTG A5260s. Clin Infect Dis. 2016;62(7):853–862.
- Baker JV, Peng G, Rapkin J, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22(7):841–848.
- Bonnet F, Chene G. Evolving epidemiology of malignancies in HIV. Curr Opin Oncol. 2008;20(5):534–540.
- Engels EA. Non-AIDS-defining malignancies in HIV-infected persons: etiologic puzzles, epidemiologic perils, prevention opportunities. AIDS. 2009;23(8):875–885.
- Teppler H, Brown DD, Leavitt RY, et al. Long-term safety from the raltegravir clinical development program. Curr HIV Res. 2011;9(1):40–53.
- Lee FJ, Amin J, Bloch M, Pett SL, Marriott D, Carr A. Skeletal muscle toxicity associated with raltegravir-based combination antiretroviral therapy in HIV-infected adults. J Acquir Immune Defic Syndr. 2013;62(5):525–533.
- Madeddu G, De Socio GV, Ricci E, et al. Muscle symptoms and creatine phosphokinase elevations in patients receiving raltegravir in clinical practice: Results from the SCOLTA project long-term surveillance. Int J Antimicrob Agents. 2015;45(3):289–294.
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107.
- Schneeweiss S. Developments in post-marketing comparative effectiveness research. Clin Pharmacol Ther. 2007;82(2):143–156.
- Cozzi-Lepri A, Zangerle R, Machala L, et al. Incidence of cancer and overall risk of mortality in individuals treated with raltegravir-based and non-raltegravir-based combination antiretroviral therapy regimens. HIV Med. 2018;19(2):102–117.
- Bradford PT, Michal Freedman D, Goldstein AM, Tucker MA. Increased risk of second primary cancers after a diagnosis of melanoma. Arch Dermatol. 2010;146(3):265–272.
- Engels EA, Yanik EL, Wheeler W, et al. Cancer-attributable mortality among people with treated human immunodeficiency virus infection in North America. Clin Infect Dis. 2017;65(4):636–643.
- Asgari MM, Ray G, Quesenberry CP Jr, Katz KA, Silverberg MJ. Association of multiple primary skin cancers with human immunodeficiency virus infection, cd4 count, and viral load. JAMA Dermatol. 2017;153(9):892–896.
- Silverberg MJ, Chao C, Leyden WA, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(12):2551–2559.
- Silverberg MJ, Leyden W, Horberg MA, et al. Older age and the response to and tolerability of antiretroviral therapy. Arch Intern Med. 2007;167(7):684–691.
- Spire B, Nait-Ighil L, Pugliese P, et al. Quality of life improvement in HIV-1 patients treated with raltegravir in a real-life observational study: RACING. HIV Clin Trials. 2017;18(1):1–16.
- Harris M, Larsen G, Montaner JS. Exacerbation of depression associated with starting raltegravir: a report of four cases. AIDS. 2008;22(14):1890–1892.
- Hill AM, Mitchell N, Hughes S, Pozniak AL. Risks of cardiovascular or central nervous system adverse events and immune reconstitution inflammatory syndrome, for dolutegravir versus other antiretrovirals: meta-analysis of randomized trials. Curr Opin HIV AIDS. 2018;13(2):102–111.
- Cahn P, Rolón MJ, Figueroa MI, Gun A, Patterson P, Sued O. Dolutegravir–lamivudine as initial therapy in HIV-1 infected, ARV-naive patients, 48-week results of the PADDLE (Pilot Antiretroviral Design with Dolutegravir LamivudinE) study. J Int AIDS Soc. 2017;20(1):21678.
