Abstract
Background: IMPAACT PROMISE 1077BF/FF was a randomized study of antiretroviral therapy (ART) strategies for pregnant and postpartum women with high CD4+ T-cell counts. We describe postpartum outcomes for women in the study who were randomized to continue or discontinue ART after delivery.
Methods: Women with pre-ART CD4+ cell counts ≥350 cells/mm3 who started ART during pregnancy were randomized postpartum to continue or discontinue treatment. Women were enrolled from India, Malawi, South Africa, Tanzania, Uganda, Zambia, and Zimbabwe. The primary outcome was a composite of progression to AIDS-defining illness or death. Log-rank tests and Cox regression models assessed treatment effects. Incidence rates were calculated per 100 person-years. A post hoc analysis evaluated WHO Stage 2/3 events. All analyses were intent-to-treat.
Findings: 1611 women were enrolled (June 2011–October 2014) and 95% were breastfeeding. Median age at entry was 27 years, CD4+ count 728 cells/mm3 and the majority of women were Black African (97%). After a median follow-up of 1.6 years, progression to AIDS-defining illness or death was rare and there was no significant difference between arms (HR: 0·55; 95%CI 0·14, 2·08, p = 0.37). WHO Stage 2/3 events were reduced with continued ART (HR: 0·60; 95%CI 0·39, 0·90, p = 0.01). The arms did not differ with respect to the rate of grade 2, 3, or 4 safety events (p = 0.61).
Interpretation: Serious clinical events were rare among predominately breastfeeding women with high CD4+ cell counts over 18 months after delivery. ART had significant benefit in reducing WHO 2/3 events in this population.
Introduction
There are mixed data about the risk of morbidity and mortality in HIV-infected postpartum women. The literature reports a 2- to 10-fold increase in the risk of dying during pregnancy and the postpartum period for HIV-infected versus uninfected women.Citation1–4 However, the data supporting this risk come from older studies in HIV-infected women with low CD4+ T-cell counts, in an era when short course antiretrovirals were used for prevention of mother-to-child transmission (MTCT) of HIV and thresholds for antiretroviral therapy (ART) were in the range of 200–350 cells/mm3.Citation5,Citation6 A recent randomized study of pregnant women in Botswana with CD4+ T-cell counts >200 cells/mm3 who received either triple nucleoside or protease-inhibitor (PI) based therapy through the breastfeeding period showed a concerning number of maternal deaths after cessation of ART.Citation7 However, in the PROMISE 1077HS study, which randomized formula feeding postpartum women with high CD4+ counts (>400 cells/mm3) to continue or discontinue ART after delivery, morbidity and mortality were extremely low.Citation8
The International Maternal Pediatric Adolescents AIDS Clinical Trials Network (IMPAACT) Promoting Maternal and Infant Survival Everywhere Breastfeeding/Formula-Feeding (PROMISE 1077BF/FF) study was a randomized clinical trial (ClinicalTrials.gov Identifier: NCT01061151) that included long-term follow-up of women beyond the time their infants were at risk of MTCT and allowed several important maternal health questions to be answered using randomized comparison groups. The trial was performed at a time when World Health Organization (WHO) recommendations included prophylaxis with either zidovudine monotherapy or three-drug ART for prevention of MTCT and when adult treatment criteria included CD4+ T-cell counts of <350 cells/mm3. In this analysis we characterize postpartum health outcomes for women in PROMISE 1077BF/FF who were predominately breastfeeding and randomized to stop or continue ART after delivery, with reinstitution of ART for clinical disease progression or when CD4+ cell counts declined to <350 cells/mm3. We also compare clinical outcomes among predominately breastfeeding women in this cohort, to those previously published from formula-feeding women in PROMISE 1077HS.
Methods
Study design
The PROMISE BF/FF study included a series of open-label, parallel randomization components to address key questions in the management of HIV-infected women with high CD4+ T-cell counts and their infants. The antepartum randomization of PROMISE 1077BF/FF compared the efficacy and safety of maternal triple ART prophylaxis versus dual maternal plus infant ARV prophylaxis regimens for prevention of perinatal transmission among women with baseline CD4+ counts ≥350 cells/mm3 who did not meet clinical guidelines for treatment initiation at the time of the study.Citation9 A second, postpartum randomization compared the effects on maternal health of continuing or discontinuing the use of maternal ART postpartum. A third, post-breastfeeding randomization compared the effects on maternal health of continuing versus discontinuing ART at cessation of breastfeeding. The trial was performed in settings where breastfeeding was common, but allowed enrollment of both breastfeeding and formula feeding mothers. Data presented here include all women randomized postpartum (second randomization) and the subset of women randomized to continued ART after the cessation of breastfeeding (third randomization) ().
Figure 1 Description of the PROMISE 1077 BF/FF women included in the analysis. This analysis includes all women on three-drug ART during pregnancy who were randomized postpartum to continue or discontinue, as well as all women randomized to continue ART at cessation of breastfeeding (black boxes).
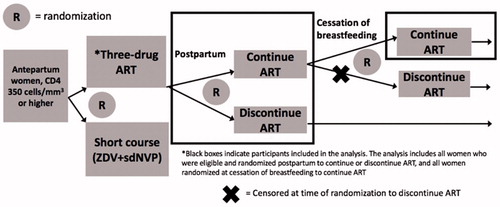
PROMISE was planned prior to the results of studies such as START and TEMPRANO, large, randomized clinical trials in men and non-pregnant women illustrating the benefit of ART regardless of CD4+ count. PROMISE 1077BF/FF was the first, along with its partner study PROMISE 1077HSCitation8 (done in predominately formula feeding settings where triple ART was the standard of care for prevention of MTCT) to evaluate the question of continued ART among women of reproductive age. The analysis presented here is the primary a priori planned analysis of the PROMISE 1077BF/FF postpartum randomization to compare the effects on maternal health of continuing versus discontinuing maternal ART postpartum.
Participants
The PROMISE 1077BF/FF antepartum component enrolled HIV-infected pregnant women, antiretroviral-naïve except for prior prophylaxis in pregnancy, without other indications for ART based on local guidelines. Women were enrolled from 15 sites in India, Malawi, South Africa, Tanzania, Uganda, Zambia, and Zimbabwe between June 2011 and October 2014. Pregnant women ≥18 years of age or who had attained the minimum age of independent consent as defined by the local institutional review board were eligible to enroll if they had documentation of a CD4+ T-cell count ≥350 cells/mm3 within 30 days prior to enrollment. Participants could not have a clinical indication for ART, including any WHO clinical stage 3 or 4 condition, or any clinically significant illness within 30 days prior to entry. Participants who were randomized to antepartum triple ART and still met the initial study eligibility criteria after delivery were eligible for the 1077BF/FF postpartum randomization to assess effects on maternal health of continuing versus discontinuing ART. The study was approved by the institutional review board or ethics committee at each participating site and written informed consent was obtained from all participants.
Randomization
The PROMISE 1077BF/FF postpartum randomization was an open-label, parallel, randomized clinical trial to evaluate two strategies for the management of ART among postpartum women: continuing ART (CTART) or discontinuing ART (DCART) and restarting when clinically indicated. Participants were randomized within 28 days after delivery in a 1:1 ratio to either CTART or DCART by a web-based, central computer randomization system using permuted block allocation with stratification by country. Participants randomized to DCART re-started if they met one of the following criteria; (1) developed an AIDS-defining/WHO Clinical Stage 4 illness, (2) had a confirmed CD4+ T-cell count <350 cells/mm3, (3) developed a clinical condition considered an indication for ART by country-specific guidelines or (4) otherwise required ART as determined in consultation with the study clinical management committee. Detailed study methods for PROMISE 1077BF/FF have been published with primary antepartum outcome data.Citation9
Procedures
The primary preferred study ART regimen was tenofovir, emtricitabine or lamivudine, and lopinavir/ritonavir. This regimen was chosen because it was the recommended regimen for use by the United States Department of Health and Human Services HIV treatment guidelines at the time the study was designed. Women randomized to zidovudine, emtricitabine or lamivudine, and lopinavir/ritonavir in the antepartum component could continue on zidovudine at the discretion of the clinician. Regimens not provided by the study were allowed if they included three or more agents from two or more classes of ART.
All participants were to be followed until 96 weeks after the last delivery in the PROMISE Antepartum Component. Participants were seen for clinical and safety evaluations 4 weeks after delivery and at 12 weeks, and then every 12 weeks thereafter. HIV-1 RNA was measured at 12-week intervals to maximize the benefits of ART and to determine when treatment should be changed. Virologic failure was defined as two successive measurements of plasma HIV-1 RNA >1000 copies/mL, with the first measurement taken at or after at least 24 weeks on ART. Women receiving ART who had a plasma HIV-1 RNA level >1000 copies/mL at or after 24 weeks were to return (if possible within 4 weeks) for confirmatory plasma HIV-1 RNA.
Outcomes
The primary efficacy outcome was a composite of progression to AIDS-defining illness (WHO Clinical Stage 4 event) or death from any cause. All potential primary outcomes were reviewed, blinded to arm assignment, by an independent four-member committee (Maternal Endpoint Review Group). Three prespecified secondary outcomes were analyzed: (1) HIV/AIDS related events or WHO Clinical Stage 2/3 events, (2) HIV/AIDS-related events or death, and (3) a safety outcome that included selected Grade 2 laboratory abnormalities (renal, hepatic, and hematologic) and all Grade 3 or higher laboratory values and signs and symptoms. A post hoc analysis evaluated WHO Clinical Stage 2/3 events.
For the study, an HIV/AIDS-related event was defined as WHO Clinical Stage 4 illnesses, pulmonary tuberculosis (TB) and other serious bacterial infections, including single episode bacterial pneumonia or any bacterial infection that satisfies one of the following conditions: (1) grade 4 event, (2) resulted in unscheduled hospitalization within three days of the bacterial infection, or (3) caused death. For the safety outcome, the DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, 2004 Version 1·0 (clarification August 2009) was used to grade adverse events.Citation10 If a participant had more than one qualifying event in a given category, only the highest grade event was counted.
Statistical analysis
The sample size was determined by the number of women randomized to the relevant arms of the PROMISE 1077BF/FF Antepartum Component to address the perinatal HIV transmission objectives. It was anticipated that approximately 1734 evaluable breastfeeding women and 510 evaluable formula feeding women would be randomized to triple ART in the Antepartum Component and agree to the postpartum randomization to CTART or DCART, and would be followed for an average of 3 years after the postpartum randomization. Power calculations indicated that this sample size would provide 90% power to detect a reduction in the annualized primary outcome rate from 3.33% in the DCART arm to 2.03% in the CTART arm, based on a two-sided Type I error of 5% and assuming a 5% annual loss-to-follow-up rate, and that data from 50% of the breastfeeding women in the CTART arm would be censored, for the purposes of this analysis, at approximately 1 year postpartum due to discontinuing ART at cessation of breastfeeding.
In July 2014, because of slow accrual, the sponsor (NIAID) decided to stop all PROMISE 1077BF/FF randomizations when the Antepartum Component reached its accrual target for breastfeeding women or on 1 October 2014, whichever came first. In July 2015, after release of the START study results,Citation10 all PROMISE participants were informed about START and offered ART. Therefore, the primary analysis is based on data collected from visits before 7 July 2015.
The study was reviewed by an independent National Institute of Allergy and Infectious Diseases (NIAID)-sponsored Data Safety and Monitoring Board (DSMB). The DSMB reviewed annual interim analyses of safety, study logistics, and an assessment of the accuracy of the assumed annualized primary outcome rate, and performed two interim analyses of efficacy and futility.
Analyses used the principle of intention-to-treat and included all women randomized in the Postpartum Component. Since the PROMISE 1077BF/FF cohort included only a small number of women who formula fed, a sensitivity analysis was performed excluding these women from the analysis of clinical and safety outcomes. As noted above, the follow-up data for women in the CTART arm who stopped ART after breastfeeding cessation were censored at the time of the post-breastfeeding randomization if (1) they were randomized to discontinue ART in the PROMISE post-breastfeeding randomization, or (2) did not participate in the post-breastfeeding randomization. To assess whether this censoring may have introduced bias, we compared the characteristics of CTART arm women who were randomized in the post-breastfeeding randomization to those who were ineligible for the randomization, and no clinically significant differences were found (Supplementary Table 1).
Comparisons for categorical outcomes used Fisher’s exact test. Comparisons between randomization arms with a survival outcome used the log-rank test and Cox regression models for estimation of treatment effect. The time-to-event distributions were summarized using Kaplan–Meier estimators. Incidence rates were estimated using a quasi-Poisson model with time as an offset. Incidence rates are displayed per 100 person-years (py). A two-sided p-value <0.05 was considered statistically significant. Statistical analyses were performed using Statistical Analysis System (SAS) software version 9.4 (SAS Institute, Cary, NC).
Results
Study patients
A total of 1994 women were randomized to a triple ART regimen in the Antepartum Component of PROMISE, and of these, 1612 met eligibility for postpartum randomization. Reasons for non-enrollment are included in . Of the 1611 women included in the analysis, 57 (3.5%) discontinued follow-up prematurely, 23 in the CTART arm and 34 in the DCART arm.
Figure 2 CONSORT flow diagram for women included in the analysis (non-enrollment reasons were reported for both the mother and the infant if the randomization occurred for the postpartum component).
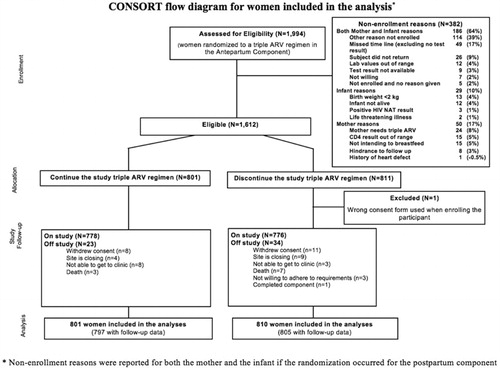
Ninety-five percent of women were breastfeeding. The two groups were well balanced at entry (). Median age at entry was 27 years, CD4+ T-cell count 728 cells/mm3 and the majority of women were Black African (97%) and enrolled from South Africa (32%), Malawi (28%), and Zimbabwe (19%). Most women were WHO Clinical Stage 1 (97%) and the majority (89%) had CD4+ T-cell counts ≥500 cells/mm3. The median follow-up time was 84 weeks.
Table 1 Baseline characteristics of women in the Maternal Health Cohort of PROMISE 1077 BF/FF
Adherence to randomization strategy
Nine women (1%) randomized to DCART started ART before reaching a protocol-defined indication for treatment (seven due to clinician decision and two due to participant request). In contrast, 29 women (4%) of those randomized to CTART prematurely discontinued ART during follow-up (15 due to participant request and 14 due to non-adherence to ART and/or study visits).
Primary outcome results
Eleven participants experienced a primary composite outcome event: three in the CTART arm (0.24 per 100 py) and eight in the DCART arm (0.49 per 100 py). The estimated hazard ratio (HR) for time to first AIDS-defining illness or death comparing the two randomized arms was 0.55 (95%CI 0.14, 2.08, p = 0.37). The events in the CTART arm included three deaths: one extrapulmonary TB, one suicide, and one woman with a ruptured ectopic pregnancy. In the DCART arm, the events included one individual with extrapulmonary TB who recovered, and seven deaths (three bacterial infections, one pulmonary TB, one pulmonary hypertension, one diabetic ketoacidosis, and one fulminant hepatitis of unknown etiology). The estimated rate of death was lower in the CTART arm, however not statistically different from the DCART arm (0.24 per 100 py compared to 0.43 per 100 py; HR 0.65, 95%CI 0.17, 2.53). The CD4+ T-cell count closest to the time of event was above 500 cells/mm3 for all women with events, with the exception of the participant with diabetic ketoacidosis, who had a CD4+ T-cell count of 277 cells/mm3.
Secondary outcome results
In the analysis of HIV/AIDS-related or WHO Clinical Stage 2 or 3 events, the estimated HR was 0.63 (95%CI 0.43, 0.91, p = 0.01). This difference was driven by the WHO Clinical Stage 2 and 3 events, with 33 events in the CTART arm (2.70 per 100 py) and 72 in the DCART arm (4.66 per 100 py) yielding a HR =0.60 (95%CI 0.39, 0.90, p = 0.01). The majority of events were moderate weight loss, herpes zoster, and fungal nail infections (). There were 10 participants with pulmonary TB, with seven confirmed by isolation of the organism from culture. These cases were equally distributed between arms (HR =1.44; 95%CI 0.41, 5.05, p = 0.56). Of note, 183 women were on isoniazid preventive therapy for a median of 72 weeks (87 in CTART and 96 in DCART). Approximately half the women in the study were on cotrimoxazole prophylaxis (median 72 weeks, 397 in CTART and 439 in DCART).
Table 2 World Health Organization Clinical Stage 2 and 3 events by arm
Safety results
The safety outcome occurred in 160 women in the CTART arm (15.3 per 100 py) and in 189 women in the DCART arm (13.9 per 100 py), yielding a HR of 0.95 (95%CI 0.76,1.17, p = 0.61). Grade 2 or higher renal events occurred in less than 0.5% of women in each arm and grade 2 or higher elevations in alanine aminotransferase (ALT) in 2% of women in the CTART arm and 4% in the DCART arm. Grade 3 and 4 adverse event rates were similar across arms (6.0 per 100 py in the CTART arm versus 6.2 per 100 py in the DCART arm; HR 1.0, 95% CI 0.74, 1.38, p = 0.96) ().
Table 3 Laboratory adverse eventsTable Footnotea
CD4+ T-cell count trajectories, virologic failure, and summary of key outcomes
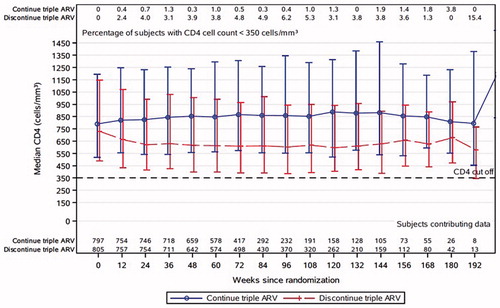
Trajectories of CD4+ T-cell counts over time are shown in . The median CD4+ T-cell count was higher among women in the CTART arm at all observed visits. During follow-up, 33% (N = 267) of women in the DCART arm started ART for a protocol defined clinical indication. Of these, 11% re-started ART for a CD4+ T-cell decline to less than 350 cells/mm3 (median CD4+ T-cell count at initiation 316 cells/mm3). One hundred thirty-five (19%) women in the CTART arm experienced a virologic failure during study follow-up. Genotyping was not performed in real time to determine whether virologic failure was due to resistance.
Figure 3 CD4+ T-cell counts over time (median, 10th and 90th percentile, and proportion <350 cells/mm3). The median CD4+ T-cell counts in the continue (blue) and discontinue (red) arms are shown at each follow-up visit. The proportion with CD4+ T-cells below 350 cells/mm3 are shown across the top of the figure at each time point.
A complete summary of outcomes by arm are described in . displays the survival curves for the primary composite outcome, the safety outcome, and the post-hoc WHO Clinical Stage 2 and 3 analysis. In the sensitivity analysis excluding women who did not breastfeed (n = 81), findings did not change with respect to clinical or safety outcomes.
Figure 4 Survival curves for key study findings (A) primary composite endpoint; (B) WHO 2 and 3 events; (C) safety endpoint. A: Primary Composite Endpoint (HR 0.55, p = 0.37). B: WHO 2 and 3 events (HR 0.63, p = 0.01). C: Safety Endpoint (HR 0.95, p = 0.61).
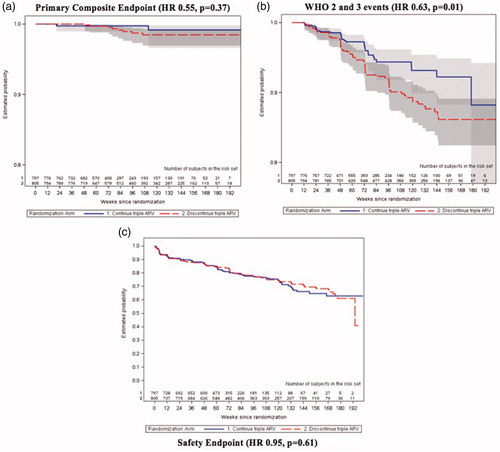
Table 4 Clinical endpoints in women in the Maternal Health Cohort of PROMISE 1077 BF/FF
Discussion
Morbidity and mortality in postpartum HIV-infected women
During an average of 1.6 years of postpartum follow-up in PROMISE, serious events occurred at a much lower than expected rate across the entire study population. At the time the study was designed, based on published data, we assumed an annualized event rate for AIDS defining illness or death of 3.33% for women in the DCART arm. The actual event rate was <1%. Primary events were more common in the DCART arm and the ability to detect significant differences was limited by the small number of events and relatively small sample size. ART did reduce the rate of WHO Clinical Stage 2/3 events, particularly moderate weight loss and herpes zoster. Reductions in WHO 2/3 events have been observed in other randomized studies of women with higher CD4+ counts who stop ART postpartum.Citation11
While the absolute rates of disease progression in our study are lower than other studies, the magnitude of benefit of ART was similar. TEMPRANO, a large, randomized trial from the Ivory Coast (approximately three quarters female), compared immediate versus deferred ART (until participants met WHO criteria for restarting therapy) in individuals with CD4+ T-cell counts >500 cells/mm3, and showed a significant reduction in the rate of death or severe HIV-related illness in the immediate arm, with a HR almost identical to that found in our study (HR 0.56 in TEMPRANO versus 0.55 in PROMISE).Citation12 However, the TEMPRANO event rate was higher than seen in PROMISE (3.8 events per 100 py versus 0.49 per 100 py).Citation13 The median age in TEMPRANO was 35 years compared to 27 years in our study. Our study enrolled from more diverse settings compared to TEMPRANO (71% low income, 3% lower-middle income, and 26% upper-middle income). In the deferred arm of START, the event rate was lower than TEMPRANO, but higher than PROMISE (1.38 per 100 py).Citation10 START was also an older population (median age 36 years) and largely male (∼70%), with only one-fifth of participants enrolled from African settings, limiting generalizability. Despite the low rate of serious clinical events in PROMISE, we observed 10 deaths during follow-up, all in women with CD4+ T-cell counts above 500 cells/mm3. These events reflect the need for improved care for this vulnerable population.
Several publications have reported low rates of disease progression in HIV-infected postpartum women with high CD4+ T-cell counts, similar to our data from PROMISE. Data from Haiti showed slow CD4+ T-cell decline among postpartum women with >500 cells/mm3 at delivery (on average taking 5–7 years to fall <200 cells/mm3),Citation13 and in the Kesho Boro study, among women with CD4+ T-cell counts ≥350 cells/mm3 who were followed for 18 months postpartum after stopping ARVs, <5% experienced HIV progression (defined by death, a WHO clinical stage 3 or 4 event, or a CD4+ T-cell decline to <350 cells/mm3).Citation14 In HIV Prevention Trials Network 046, a study of infant nevirapine for prevention of MTCT through breastmilk, <2% of African postpartum women with CD4+ T-cell counts ≥550 cells/mm3 at delivery experienced HIV disease progression up to 12 months postpartum.Citation15
The role of breastfeeding in postpartum maternal morbidity and mortality
For the last several decades there has been debate over whether breastfeeding increases risk for adverse maternal health outcomes among HIV-infected women. A randomized study from the pre-ART era showed increased mortality in HIV-infected Kenyan breastfeeding women regardless of CD4+ T-cell count, with the authors hypothesizing that an increased metabolic demand from breastfeeding has a detrimental effect on health.Citation16 Three additional African studies from the era of short-course antiretrovirals for prevention of MTCT refuted this finding, showing no evidence for an increase in mortality among HIV-infected breastfeeding women up to 24 months after delivery.Citation17–19 These studies were followed by a meta-analysis of over 4000 women with a median CD4+ T-cell count in the mid-400 cell/mm3 range, showing no difference in mortality up to 18 months after delivery.Citation20
Perhaps the best comparison to breastfeeding women in PROMISE 1077BF/FF are women who were concurrently enrolled in PROMISE 1077HS, which was a study of continuing or discontinuing ART postpartum and carried out in countries where formula feeding was the standard of care. PROMISE 1077HS was a contemporary cohort performed in 52 sites in eight countries evaluating the same primary and secondary maternal health outcomes as 1077BF/FF. Primary 1077HS data have been published elsewhere.Citation8 In comparing the results of these two cohorts, rates of the primary outcome, the safety outcome, and WHO 2 and 3 events were similar (). These data reinforce the low rate of serious adverse clinical events in postpartum women with high CD4+ T-cell counts, and suggest that HIV-infected women who breastfeed are not at increased risk compared to women who formula-feed.
Table 5 Comparison of maternal health outcomes in PROMISE BF/FF compared to PROMISE HS
TB risk in postpartum women
Evidence suggests that postpartum women may be at 2-fold higher risk of developing active TB as compared to non-pregnant/postpartum women.Citation21 However, most available TB burden data on pregnant/postpartum women are from retrospective cohorts, cross-sectional studies, or prospective cohorts in the pre-ART era. The literature suggests that while ART decreases the risk of active TB by 67%,Citation22 the risk remains significantly elevated despite CD4 recovery on ART,Citation23 even among those initiating ART at CD4+ T-cell counts >350 cells/mm3.Citation24 Our data contrast with these findings and have several strengths, including the randomization of women, and the fact that participants were seen every 12 weeks and evaluated, when ill, with input from clinical experts (the clinical management committee) to help guide the evaluation of those suspected of having TB. Our TB rates (0.4 per 100 py in both arms) are similar to those recently reported from a randomized trial of isoniazid started in pregnancy versus deferred to 12 weeks postpartum. In this study, the median CD4+ T-cell count at enrollment was ∼500 cells/mm3 and the rates of TB were also low (0.6 per 100 py in both arms).Citation25 Taken together, these data support low rates of TB in postpartum women with high CD4+ counts.
Safety of ART in postpartum women
Rates of adverse events as defined by the composite safety outcome were low in our cohort and did not differ significantly between women who continued versus discontinued ART postpartum. This was observed despite frequent use of lopinavir/ritonavir, which is known to cause high rates of gastrointestinal side effects.Citation26,Citation27 Among PROMISE 1077BF/FF women, grade 3 and 4 gastrointestinal events occurred in <1% in each arm. In a study of pregnant women in Botswana randomized to abacavir/lamivudine/zidovudine or lopinavir/ritonavir with zidovudine/lamivudine and followed for 6 months postpartum, grade 3 and 4 laboratory events were similar in the two groups (12% versus 15%), and there was no difference in grade 3 or 4 signs/symptoms (6% in each group).Citation28 Most studies of lopinavir/ritonavir during pregnancy and postpartum for prevention of MTCT have not followed participants for extended postpartum durations, often completing follow-up by 6 weeks. In PROMISE 1077BF/FF, women were followed for a median of 1.6 years after delivery and during this prolonged follow-up, ART was not only well-tolerated, but also did not increase signs and symptoms beyond rates seen in women who were not on ART. In the modern era, lopinavir/ritonavir and efavirenz are being replaced by newer antiretrovirals, including integrase inhibitors. Data are needed on ART regimens that are safe and efficacious for women of reproductive age, including for conception, during pregnancy, and postpartum.
The strengths of this study include randomization, conduct in multiple countries, rigorous assessments of safety and efficacy, and rigorous assessments of clinical outcomes. The analyses were limited by the lower than expected event rate and relatively short follow-up, reducing the ability to draw conclusions about the longer-term health of reproductive-age women. In addition, the main study regimen of lopinavir/ritonavir is no longer commonly used, limiting generalizability.
Conclusion
In this large, multi-site, randomized trial evaluating continuation versus discontinuation of postpartum ART, serious clinical events were rare among women with high CD4+ cell counts over 1.6 years postpartum, regardless of whether or not they received postpartum ART. Outcomes appear similar between this PROMISE cohort of predominately breastfeeding women compared to a contemporary PROMISE cohort of formula feeding women. In combination, these two PROMISE trials represent over 3000 women from 15 countries and provide reassuring data on the robust health of HIV-infected reproductive aged women with high CD4+ T-cell counts. In both studies, use of ART led to a reduction in clinical disease, consistent with current guidelines for ART for all, regardless of CD4+ T-cell count. Longer-term data are needed on clinical outcomes and strategies to optimize the health of HIV-infected women through their reproductive years and beyond.
Trial registration
ClinicalTrials.gov Identifier: NCT01061151.
Supplemental Material
Download MS Word (14.4 KB)Acknowledgments
The PROMISE protocol team gratefully acknowledges the dedication and commitment of the more than 3500 mother–infant pairs without whom this study would not have been possible. We wish to acknowledge the following site investigators for their hard work and dedication to the PROMISE study: Kilimanjaro Christian Medical Centre (KCMC) - Blandina T. Mmbaga, MD; Pendo Mlay, MD; Boniface Njau, MPH; Wits RHI Shandukani Research Centre CRS - Masebole Masenya, MD; Janet Grab, BPharm; Soweto IMPAACT CRS - Nasreen Abrahams, MBA, Mandisa Nyati, MBChB, Sylvia Dittmer, MBChB; FAM-CRU CRS - Magdel E Rossouw, MBChB, Lindie Rossouw, MBChB; Malawi CRS - Francis Martinson, MBChB; Ezylia Makina, RNM, Beteniko Milala, BAE; Durban Paediatric HIV CRS - Raziya Bobat, Nozibusiso Rejoice Skosana, BN, Sajeeda Mawlana, MBChB; George CRS - Martin Mwalukanga, Diploma in Clinical Medicine, Felistus Mbewe, Mwangelwa Mubiana-Mbewe, MBChB, MMed, MBA; MU-JHU Research Collaboration (MUJHU CARE LTD) CRS - Maxie Owor, Dorothy Sebikari, MBChB, Patience Atuhaire, MBChB; Umlazi CRS - Daya Moodley, Vani Chetty, BScHon, Megeshinee Naidoo, MBChB, Alicia Catherine Desmond, MPharm; Blantyre CRS - Bonus Makanani, Sufia Dadabhai, Salome Kunje, BSc; Alex Siyasiya, Certificate in Microbiology, Mervis Maulidi, Certificate in Nursing and Midwifery; St Mary’s CRS - Patricia Mandima, MBChB, Jean Dimairo, Bpharm; Seke North CRS - Lynda Stranix-Chibanda, MBChB, Teacler Nematadzira, MBChB, Gift Chareka, MSc; Byramjee Jeejeebhoy Medical College (BJMC) CRS - Ramesh Bhosale, Sandesh Patil, MBBS, Ramesh Bhosale, MD, Neetal Nevrekar, MD; Harare Family Care CRS - Tapiwa Mbengeranwa, MBChB, Tichaona Vhembo, MBChB, Nyasha Mufukari, Bpharm.
We also want to express our deepest gratitude to FHI360: Central Operations Center - Katie McCarthy, MPH; Kathleen George, MPH, Megan Valentine, MPA; Laboratory Center - Amy James Loftis, Susan Fiscus; Statistical and Data Analysis Center - Camlin Tierney, PhD, Patricia DeMarrais, PhD, Jane Lindsey, ScD; Data Management Center - Barb Heckman, Michael Basar, BS, Amanda Zadkilka, BS, Barbara Heckman, BS.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Risa M. Hoffman
Dr. Risa M. Hoffman MD, MPH is an Associate Clinical Professor in the Division of Infectious Diseases at UCLA. Dr. Hoffman’s research focuses on HIV co-morbidities as well as on the implementation of HIV service delivery in resource-limited settings, and she has performed collaborative research with teams in Malawi, Zambia, South Africa, and Brazil. She has been a member of the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT), participating as an investigator on randomized clinical trials focused on improving clinical treatment strategies for HIV-infected pregnant and postpartum women globally. She served as a PROMISE site investigator and was a member of the Clinical Management Committee. For this manuscript she worked closely with the biostatistics team on the analysis plan, and was responsible for writing the first draft and leading revisions.
Konstantia Nadia Angelidou
Konstantia Nadia Angelidou has an MSc in biostatistics and an MBA and was a biostatistician at the Harvard School of Public Health during implementation of the PROMISE study. She now works for Director of Analytics at Point Right, Inc. For the manuscript, she performed the analysis and helped with writing and editing.
Sean S. Brummel
Dr. Sean S. Brummel is a Research Scientist for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT), the Pediatric HIV and AIDS Cohort Study (PHACS), and the PROMISE Ongoing Treatment Evaluation (PROMOTE) cohort study. He has worked with IMPAACT and PHACS since 2010; and serves as the PROMOTE PI for the Center for Biostatistics in AIDS Research. Dr. Brummel has extensive training in statistical methods for clinical trials and has worked in Epidemiology since 2006. He has taken the statistical lead on numerous epidemiologic studies with topics that include adolescent substance use, executive functioning, adherence, epigenetics, and genetic epidemiology. As Senior Statistician on the PROMISE randomized clinical trial, he has taken the statistical lead on studies involving maternal health, repeat pregnancy outcomes, antiretroviral uptake, data management, and database creation. Dr. Brummel is the Senior Statistician for the Maternal Health component of PROMISE, and was responsible for presenting the Maternal Health interim results to the Data and Safety Monitoring Board, for signing off on the analysis reports and analysis programs, and was responsible for statistical issues that arose during the follow-up of the study. Dr. Brummel contributed to the review, editing, and final signoff of the manuscript.
Friday Saidi
Friday Saidi, MBBS is an obstetrician/gynecologist affiliated with the University of Malawi College of Medicine. He was a site investigator for the PROMISE study and participated in manuscript writing and editing.
Avy Violari
Avy Violari MBBS Dr. Violari is a paediatrician who is the head of the Perinatal HIV Research Unit (PHRU) paediatric division. She was the lead investigator of the CHER trial at the PHRU. Dr. Avy Violari led one of the largest and most successful prevention of mother-to-child transmission of HIV (PMTCT) programmes in Africa. She has extensive clinical trials experience in paediatric and PMTCT research with a special interest in HIV/AIDS in children, especially infants with early infection. Dr. Violari has been involved in 24 HIV/AIDS trials, acting as Principal Investigator for 6 of them. She was the Principal Investigator of the PROMISE trial at the PHRU. She assisted with manuscript writing and editing.
Dingase Dula
Dingase Dula MBBS is a medical doctor who served as the PROMISE site investigator at the Johns Hopkins Research Project in Blantyre Malawi. Dr. Dula helped with manuscript writing and editing.
Vidya Mave
Vidya Mave, MD is an assistant professor of medicine at the Johns Hopkins University School of Medicine. Her research focuses on infectious diseases, particularly HIV and tuberculosis.Dr. Mave serves as director and clinical research site (CRS) leader of the NIH-funded Baltimore-Washington-India HIV and Infectious Diseases Clinical Trials Unit (BWI-CTU), a collaborative research partnership in Pune, India that is part of the world’s largest HIV/TB therapeutic trials conducted by the AIDS Clinical Trials Group (ACTG) and the International Maternal Pediatric Adolescent AIDS Trials Network (IMPAACT). She was a site investigator for PROMISE and participated in manuscript writing and editing.
Lee Fairlie
Dr Lee Fairlie is the Director of Maternal and Child Health at Wits RHI, qualified as a Paediatrician in 2005, and has significant clinical and research experience in paediatric, adolescent and maternal health, including HIV and co-morbidities, specifically TB and vaccine preventable diseases. She has worked in the HIV and infectious diseases field since 2006. She leads the Maternal and Child Team at Wits RHI and the team conducts clinical trials, implementation science research and clinical work, providing technical assistance. She was site CRS leader, IoR overseeing all conduct of study including clinical work with participants, also assisted with writing and review of the manuscript.
Gerhard Theron
Gerhard Theron MBBS Prof Gerhard Theron was the head of the Department of Obstetrics and Gynaecology, Tygerberg Hospital and Faculty of Medicine and Health Sciences, Stellenbosch University. As emeritus professor he is involved in post- and undergraduate training as well as research. The focus of his research is perinatal HIV and the use of a novel uterine balloon tamponade device to manage postpartum haemorrhage. He is the author or co-author of 84 publications in peer reviewed scientific journals and has presented papers at 28 international and 65 national congresses. Outside the university he is involved in outreach programmes and serves on various committees and has won numerous awards including the Albert Strating prize for Preventative Medicine. He serves on the FIGO Committee for Safe Motherhood and Newborn Health, with dedicated goals to reduce global maternal and perinatal mortality rates. Dr. Theron was a site investigator overseeing all aspects of the trial at our unit. Reading and editing the paper prior to submission for publication.
Moreen Kamateeka
Moreen Kamateeka; MBChB, MPH is a public health specialist and clinical researcher with special interest in maternal and child health. She holds a Bachelor of Medicine and bachelor Surgery degree (MBChB) from Mbarara University of Science and Technology, Uganda, and a Masters degree in Public Health (MPH) from the University of Manchester, United Kingdom. She is currently working with the Field Epidemiology Network (AFENET), Nigeria country office, as a Coordinator on HIV programmes and also works part time with the Makerere University-Johns Hopkins University Research Collaboration (MUJHU) Research collaboration as a consultant clinical researcher. She has over 10 years’ experience in HIV clinical research particularly in the area of Prevention of Mother To Child HIV Transmission from her work at MUJHU. She also serves as a member of the HIV/AIDS Research and Treatment Open Journal Editorial Board. On this PROMISE study, she served as a Study Coordinator and then later a site Co-Investigator for the MUJHU, Kampala site. She was charged with the broad responsibility of providing technical oversight of implementation of the clinical, laboratory, data and regulatory components of study as well as supervising the study staff. She has participated in reviewing this manuscript.
Tsungai Chipato
Tsungai Chipato FRCOG is a Professor of Obstetrics and Gynecology at the University of Zimbabwe with more than 16 years of experience in clinical trials, including pharmacokinetic studies and Phase III placebo controlled trials. Since 2007, he has been CRS Leader for St. Mary’s CRS, where in 1998 he was instrumental in implementing the first PMTCT studies in Zimbabwe. Dr. Chipato is a member of the Medical Research Council of Zimbabwe Technical Committee and is the current Chair of the Board of the Zimbabwe National Family Council. He has 39 publications that include peer-reviewed articles. Dr. Chipato participated in manuscript writing and editing.
Benjamin H. Chi
Benjamin H. Chi MD Dr. Chi holds appointments in the Department of Obstetrics and Gynecology and Department of Epidemiology at the University of North Carolina at Chapel Hill. He lived in Lusaka, Zambia, from 2003 to 2015, where he developed an extensive research portfolio focused on the prevention of mother-to-child transmission (PMTCT), HIV care and treatment, and maternal-child health. He has served as PI for numerous grants funded by the National Institutes of Health, Centers for Disease Control and Prevention, Doris Duke Charitable Foundation, and Elizabeth Glaser Pediatric AIDS Foundation. Although he moved back to Chapel Hill in June 2015, he retains strong collaborative ties with the University of Zambia, the Zambian Ministry of Health, and other local partners. Alongside his research, Dr. Chi leads several training programs designed to foster U.S. collaborations abroad, including the UNC Global Women’s Health Fellowship and the UJMT Fogarty Global Health Fellows Consortium. He is also recipient of a K24 award from NIH, which provides dedicated effort to mentor students, postdoctoral fellows, and junior faculty members in patient-oriented research. Dr. Chi participated in writing and editing the manuscript.
Lynda Stranix-Chibanda
Lynda Stranix-Chibanda is a Zimbabwean researcher with 18 years of clinical trial experience and advocacy for HIV prevention. A Paediatrician by training, Lynda lectures at the University of Zimbabwe College of Health Sciences and mentors postgraduate students. She is the Site Leader for Seke North Clinical Research Site in Chitungwiza, Zimbabwe directing NIH-funded clinical trials related to the prevention and treatment of HIV in women, adolescents and children. She is Chair of the IMPAACT network’s Prevention Scientific Committee, contributing to the HIV prevention research agenda and participating in clinical trials from protocol concept stage through close-out. Lynda provides technical assistance to the national PMTCT programme and has contributed to policy development for pre-exposure prophylaxis use. She serves as Vice-Chair of the National Health Research and Development Committee, the local Institutional Review Board, and is versed in the ethical considerations for conducting clinical trials in resource poor settings and among vulnerable populations. As PROMISE Protocol Team Member, Lynda participated in protocol development, manuscript concept formulation and preparation. As CRS Leader, she led a team of site personnel in implementing the protocol, collecting data and overseeing participant safety.
Teacler Nematadzira
Teacler Nematadzira MBChB is a Research Medical Officer /Investigator at the University of Zimbabwe-Clinical Trials Research Centre. He was a PROMISE site investigator and participated in manuscript writing and editing.
Dhayendre Moodley
Dhayendre Moodley PhD is an Associate Professor at the University of KwaZulu-Natal. She served as the PROMISE site investigator for the CAPRISA-Umlazi Clinical Research Site and participated in manuscript writing and editing.
Debika Bhattacharya
Debika Bhattacharya MD Dr. Bhattacharya is an Associate Clinical Professor at UCLA, specializing in the management of HIV and viral hepatitis coinfection. Her NIH funded research includes studies which evaluate the predictors of clinical outcomes in HIV/HBV coinfected patients, including pregnant HIV/HBV coinfected women. Her research involves identifying molecular and clinical predictors of viral hepatitis outcomes in HIV coinfection. She also conducts clinical research in viral hepatitis infection at the UCLA CARE Center. She has clinics at the UCLA CARE Center and at the West Los Angeles Veteran’s Affair Medical Center. She is a member of the writing panels for the CDC/NIH/HIVMA/IDSA/AAP Guidelines for the Prevention and Treatment of Opportunistic Infections Among HIV-Exposed and HIV-Infected Children and for the NIH-CDC-HIVMA/IDSA Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents.She is also a member of the AASLD/IDSA HCV Guidance Panel. Role: Member of the clinical monitoring committee and co-chair of substudy of PROMISE, contributed to management and adjudication of clinical events during the protocol, writing, and editing manuscript draft.
Amita Gupta
Amita Gupta MD Dr. Gupta is Deputy Director of the Johns Hopkins (JH) Center for Clinical Global Health Education (CCGHE), and Associate Professor of Infectious Diseases at the Johns Hopkins School of Medicine, with a joint appointment in International Health at the Johns Hopkins Bloomberg School of Public Health. Board certified by the American Board of Internal Medicine in infectious diseases, Dr. Gupta specializes in international public health, clinical research, and education in infectious diseases, HIV/AIDS, and tuberculosis (TB). Since 2002, her work has been focused primarily on India, where she leads several Indo-JHU research collaborations. Dr. Gupta is Co-principal Investigator of the NIH-funded Baltimore-Washington-India HIV Clinical Trials Unit (BWI-CTU), and she is an active clinical investigator in multi-country HIV/TB trials conducted by the AIDS Clinical Trials Group (ACTG) and the International Maternal Pediatric Adolescent AIDS Trials Network (IMPAACT). Dr. Gupta assisted in the writing and editing and served on the clinical management committee of the PROMISE trial.
Anne Coletti
Anne Coletti MSc, is a Senior Scientist at FHI 360 who has had leadership roles in numerous clinical trials related to women’s health, PMTCT, and HIV prevention. She participated in the design and implementation of PROMISE and assisted with writing and editing the manuscript.
James A. McIntyre
James A. McIntyreFRCOG is the CEO of Anova, Honorary Professor in the School of Public Health & Family Medicine at the University of Cape Town, Honorary Senior Lecturer in the Mailman School of Public Health at Columbia University and Vice-Chair of the US NIH-funded International Maternal Paediatric and Adolescent AIDS Clinical Trials (IMPAACT) Network. He previously worked for 25 years at the Chris Hani Baragwanath Hospital in Soweto, South Africa. He was an early member of the PROMISE team, helping with study design and implementation. He participated in manuscript writing and editing.
Karin L. Klingman
Karin L. Klingman MD is an infectious diseases specialist and Medical Officer at the Division of AIDS, NIAID. She participated in the PROMISE trial design, supported implementation, and participated in the maternal health manuscript writing and editing.
Nahida Chakhtoura
Nahida Chakhtoura MD is a Medical Officer at NICHD. She is an obstetrician/gynecologist and prior to working at NICHD she had leadership roles at the University of Miami, including Chief of Women’s Health Services. Dr. Chakhtoura provided leadership during implementation of PROMISE and was involved with manuscript writing and editing.
David E. Shapiro
Dr. David E. Shapiro PhD is a Principal Research Scientist in the Center for Biostatistics in AIDS Research at the Harvard T. H. Chan School of Public Health and the Director of the NIH-funded Statistical and Data Management Center (SDMC) for the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network. Dr. Shapiro has worked as a senior statistician for perinatal and pediatric HIV research studies for over 20 years, as a member of the SDMC for IMPAACT and its predecessor the Pediatric AIDS Clinical Trials Group (PACTG).He served as the SDMC’s Perinatal Section Head from 1995 through 2006, its Associate Director from 2003 through 2010, and has been the SDMC Director since 2010.Dr. Shapiro has taken the lead statistical role in 24 perinatal and pediatric HIV clinical trials, including Phase I, II, and III trials and observational studies in the U.S. and internationally, and has served as a member or chair of the independent data and safety monitoring committee for three clinical trials. He has authored or co-authored over 60 peer-reviewed publications. Dr. Shapiro’s statistical research interests and publications focus on methodology for assessing the accuracy of diagnostic and screening tests. Dr. Shapiro was the senior biostatistician for the PROMISE study.
Judith S. Currier
Judith S. Currier, MD, MSc Dr. Currier is Professor of Medicine and Chief of the Division of Infectious Diseases and Co-Director of the Center for AIDS Research and Education Center (CARE) in the Department of Medicine at University of California, Los Angeles (UCLA). She is Chair of the NIH sponsored AIDS Clinical Trials Group and serves on the leadership team for the Expanding Quality Improvement for HIV/AIDS in Malawi (EQUIP) Project. Her research has focused on HIV therapeutics and long-term complications of HIV disease with an emphasis on sex differences and antiretroviral therapy, cardiovascular disease, and women’s health.
References
- Calvert C, Ronsmans C. The contribution of HIV to pregnancy-related mortality: a systematic review and meta-analysis. AIDS 2013;27(10):1631–1639.
- Wandabwa JN, Doyle P, Longo-Mbenza B, et al. Human immunodeficiency virus and AIDS and other important predictors of maternal mortality in Mulago Hospital Complex Kampala Uganda. BMC Public Health. 2011;11:565.
- Moran NF, Moodley J. The effect of HIV infection on maternal health and mortality. Int J Gynaecol Obstet. 2012;119(Suppl 1):S26–S29.
- Zash RM, Souda S, Leidner J, et al. High proportion of deaths attributable to HIV among postpartum women in Botswana despite widespread uptake of antiretroviral therapy. AIDS Patient Care STDS. 2017;31(1):14–19.
- Lathrop E, Jamieson DJ, Danel I. HIV and maternal mortality. Int J Gynaecol Obstet. 2014;127(2):213–215.
- Holtz SA, Thetard R, Konopka SN, Albertini J, Amzel A, Fogg KP. A systematic review of interventions to reduce maternal mortality among HIV-infected pregnant and postpartum women. Int J MCH AIDS. 2015;4(2):11–24.
- Shapiro RL, Kitch D, Ogwu A, et al. HIV transmission and 24-month survival in a randomized trial of HAART to prevent MTCT during pregnancy and breastfeeding in Botswana. AIDS 2013;27(12):1911–1920.
- Currier JS, Britto P, Hoffman RM, et al. Randomized trial of stopping or continuing ART among postpartum women with pre-ART CD4 ≥ 400 cells/mm3. PLoS One 2017;12(5):e0176009.
- Fowler MG, Qin M, Fiscus SA, et al. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med. 2016;375(18):1726–1737.
- Group ISS, Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807.
- Pilotto JH, Velasque LS, Friedman RK, et al. Maternal outcomes after HAART for the prevention of mother-to-child transmission in HIV-infected women in Brazil. Antivir Ther. 2011;16(3):349–356.
- Group TAS, Danel C, Moh R, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–822.
- Coria A, Noel F, Bonhomme J, et al. Consideration of postpartum management in HIV-positive Haitian women: an analysis of CD4 decline, mortality, and follow-up after delivery. J Acquir Immune Defic Syndr. 2012;61(5):636–643.
- Kesho Bora Study Group. Maternal HIV-1 disease progression 18-24 months postdelivery according to antiretroviral prophylaxis regimen (triple-antiretroviral prophylaxis during pregnancy and breastfeeding vs zidovudine/single-dose nevirapine prophylaxis): the Kesho Bora randomized controlled trial. Clin Infect Dis. 2012;55(3):449–460.
- Watts DH, Brown ER, Maldonado Y, et al. HIV disease progression in the first year after delivery among African women followed in the HPTN 046 clinical trial. J Acquir Immune Defic Syndr. 2013;64(3):299–306.
- Nduati R, Richardson BA, John G, et al. Effect of breastfeeding on mortality among HIV-1 infected women: a randomised trial. Lancet. 2001;357(9269):1651–1655.
- Sedgh G, Spiegelman D, Larsen U, Msamanga G, Fawzi WW. Breastfeeding and maternal HIV-1 disease progression and mortality. AIDS. 2004;18(7):1043–1049.
- Kuhn L, Kasonde P, Sinkala M, et al. Prolonged breast-feeding and mortality up to two years post-partum among HIV-positive women in Zambia. AIDS. 2005;19(15):1677–1681.
- Taha TE, Kumwenda NI, Hoover DR, et al. The impact of breastfeeding on the health of HIV-positive mothers and their children in sub-Saharan Africa. Bull World Health Organ. 2006;84(7):546–554.
- Breastfeeding and HIVITS Group. Mortality among HIV-1-infected women according to children's feeding modality: an individual patient data meta-analysis. J Acquir Immune Defic Syndr. 2005;39(4):430–438.
- Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis. 2012;55(11):1532–1549.
- Lawn SD, Wood R, De Cock KM, Kranzer K, Lewis JJ, Churchyard GJ. Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. Lancet Infect Dis. 2010;10(7):489–498.
- Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One. 2012;7(3):e34156.
- Kufa T, Mabuto T, Muchiri E, et al. Incidence of HIV-associated tuberculosis among individuals taking combination antiretroviral therapy: a systematic review and meta-analysis. PLoS One. 2014;9(11):e111209.
- Gupta A, Montepiedra, G, Aaron, L, et al. Randomized trial of safety of isoniazid preventive therapy during or after pregnancy. Presented at the Conference on Retroviruses and Opportunistic Infections, March 4-7, Boston, Massachusetts, USA. Abstract 142LB. Available at http://www.croiconference.org/sessions/randomized-trial-safety-isoniazid-preventive-therapy-during-or-after-pregnancy. Accessed March 13, 2018.
- Orkin C, DeJesus E, Khanlou H, et al. Final 192-week efficacy and safety of once-daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV-1-infected treatment-naive patients in the ARTEMIS trial. HIV Med. 2013;14(1):49–59.
- Malan N, Su J, Mancini M, et al. Gastrointestinal tolerability and quality of life in antiretroviral-naive HIV-1-infected patients: data from the CASTLE study. AIDS Care. 2010;22(6):677–686.
- Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362(24):2282–2294.
