Abstract
Background: Dolutegravir (DTG) plus boosted darunavir (bDRV) is a compact, adherence-friendly salvage regimen with the highest genetic barrier to HIV-1 resistance.
Objective: Aim of the present study is to assess the long term (96-week) safety and efficacy of DTG + bDRV in a of multidrug-experienced HIV-1 infected patients, simplifying or building rescue regimens.
Methods: All HIV-1-infected subjects from eleven Italian centers switched to DTG + bDRV between March 2014 and September 2015 were included and followed for minimum 96 weeks.
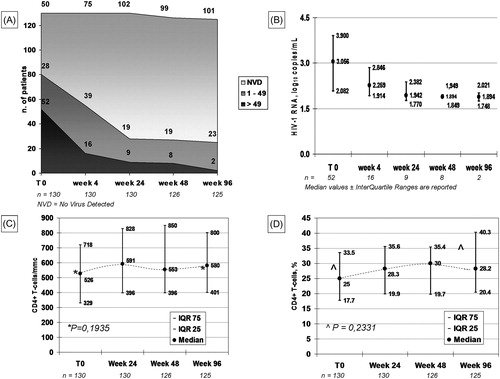
Results: The cohort comprises 130 subjects, switched from 42 different, complex or at least twice-daily regimens, mainly for simplification (44.6%), viral failure (30.0%) or toxicity (16.6%). At baseline 118 had documented resistance to 1–5 antiretroviral classes and 12 lacked genotypic results either for historical reasons or for problems with primer annealing; 52 (40%) had uncontrolled viral replication, three above 500.000 copies/mL. At week 96 two showed ≥50 HIV-1 RNA copies/mL, 23 had 1–49 copies/mL and 101 had no virus detected. The proportion of subjects presenting abnormal values at baseline significantly decreased for serum glucose, creatinine, AST, total cholesterol and triglycerides.
Conclusions: These long-term data confirm the reliability of the two-drug regimen consisting of bDRV plus DTG in salvage settings in HIV-1 infection.
Introduction
Because the introduction of dolutegravir (DTG) the exploration of new two-drug regimens as switch strategies has rapidly developed, initially on the field,Citation1–6 then through the randomized, sponsored clinical trials on cabotegravir plus rilpivirine and dolutegravir plus rilpivirine.Citation7,Citation8 The aim of the present article is to present the first long-term data (96 weeks) on the association of dolutegravir plus boosted darunavir (bDRV) as a salvage strategy or for simplification of effective but complex salvage regimens. Previous data from this cohort have been published.Citation9,Citation10
Materials and methods
General features and Ethics Committees approval
All subjects who had started DTG plus bDRV between 1 March 2014 and 30 September 2015 were included in this observational study. After approval by Ethics Committees (EC) no further enrolment was allowed. After week 48 one centre received a new patient from outside who had been switched in window and was authorized to enroll him by the local EC. For homogeneity no data were collected after week 96 (plus 8 weeks’ window as observational studies cannot dictate strict timelines). Participating subjects signed an informed consent according to local procedures, the study has been approved by the coordinating centre and by all participating centers and conducted according to the Good Clinical Practice Guidelines.
The primary end-point was the proportion of subjects achieving or maintaining viral suppression <50 copies/mL at week 24. Secondary end points were viral suppression at weeks 48 and 96 and safety (proportion of drop-outs for any reason and grade 3–4 adverse events).
Safety data and laboratory standards
The investigators reported to the coordinating center serum glucose, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), high density lipoprotein (HDL-C), low density lipoprotein (LDL-C) and triglycerides (TG) values. The estimated glomerular filtration rate (e-GFR) was calculated according to the Modification of Diet in Renal Disease (MDRD) equation.Citation11 Plasma viral suppression status was classified as harboring ≥50 HIV-1 RNA copies/mL, or detectable viral load below 50 copies/mL, or undetectable (no virus detected, NVD).
HIV-1 plasma RNA was measured locally with either Abbot HIV-1 RT-PCR, threshold 37 copies, COBAS TaqMan HIV-1 test, threshold 20 copies, or Single Copy Assay, threshold 3 copies/mL. TruGene™ was used for resistance testing, validated on the Stanford Algorithm.Citation12 Data were collected from the patients’ case record forms and sent as pre-specified excel files.
For toxicity analyses the Common Terminology Criteria for Adverse Events (CTCAE, Version 4.03, 14 June 2010) was considered.Citation13 Adverse clinical events and deaths were reported to the local Ethics Committees and authorities. The complete dataset is available for queries by the authorities and can be forwarded by email upon formal request.
Statistical analysis
The statistical analysis was limited to median and interquartile range and to the Wilcoxon signed rank test for the comparison between the baseline and 96-week metabolic and immunologic values. The metabolic analyses were performed on an on-treatment basis. The sample size was obtained from the participants’ adhesion.
Results
One hundred and thirty subjects were followed for minimum 96 weeks, median 107 weeks, mean 112 weeks ().
Table 1 Baseline demographic, immunologic, virologic and therapeutic characteristics of the population, reasons for switching therapy and clinical outcomes.
Eighty-one had bDRV in their preswitch regimen and one had DTG. At baseline 118 subjects had documented resistance to 1–5 ARV classes. For 12 patients, viral failure was not accompanied by a genotypic test. Twenty-three subjects (17.7%) had never experienced viral failure but had transmitted drug resistance mutations.
Twenty subjects had reduced baseline sensitivity to DRV, and 12 had reduced sensitivity to INSTIs.
All subjects who were on DRV plus RTV every 24 h switched to the DRV800/Cobi150 mg formulation between week 48 and week 60, further simplifying the regimen to two pills/day.
At week 96 two subjects, both starting from HIV-1 RNA over 5 log10 copies/mL, showed viral replication ≥50 copies/mL (2 and 1.7 log10 copies/mL, respectively), 23 had detectable viremia 1–49 copies/mL and 101 had NVD. The CD4+ T-cell count increased by 54/mm3 and by 3.2% from baseline, statistically not significant ().
Figure 1 Immunologic and virologic data at 96 weeks. (A) viral decay, expressed as number of patients in each of the three viral strata: active replication (≥50 HIV-1 RNA copies/mL), containment <50 copies/mL and no virus detected (NVD); (B) mean ± standard deviation values of viremic patients; (C) median ± interquartile range values for absolute CD4+ T-lymphocyte counts/mmc and (D) for % CD4+ T-lymphocytes. (A) NVD = No Virus Detected; (B), (C) and (D) Median + InterQuartile Range.
The proportion of subjects presenting abnormal values at baseline significantly decreased for serum glucose, creatinine, AST, total cholesterol and triglycerides. Overall 97/234 (41.5%) baseline laboratory alterations returned to normality. The proportion of subjects having MDRD <60 remained stable at 4.6%. Considering the trend for each metabolic parameter overall, none varied significantly ().
Figure 2 Evolution of the main routine metabolic parameters. (A) number of subjects presenting any laboratory abnormality ≥ Grade 1 CTCAE []; (B) median triglyceride values; (C) median values of the other metabolic parameters (D) median Total Cholesterol to HDL ratio. ALT: alanine aminoTransferase; AST: aspartate aminoTransferase; eGFR: estimated glomerular filtration rate; HDL-C: high-density lipoprotein; LDL-C: low-density lipoprotein; TC: total cholesterol; TG: triglycerides.
![Figure 2 Evolution of the main routine metabolic parameters. (A) number of subjects presenting any laboratory abnormality ≥ Grade 1 CTCAE []; (B) median triglyceride values; (C) median values of the other metabolic parameters (D) median Total Cholesterol to HDL ratio. ALT: alanine aminoTransferase; AST: aspartate aminoTransferase; eGFR: estimated glomerular filtration rate; HDL-C: high-density lipoprotein; LDL-C: low-density lipoprotein; TC: total cholesterol; TG: triglycerides.](/cms/asset/3e0976af-4bdf-4d72-bf68-8889e89e90fb/yhct_a_1550290_f0002_b.jpg)
Because our initial publication,Citation9 other experiences have been disclosed, confirming the efficacy of this combination in such a complex setting.Citation14,Citation15 The possibility of a once-daily simple salvage regimen reinforces adherenceCitation16 and this may be the main reason underlying the improvement observed even switching from stable regimens.
Conclusions
Despite the limitations of being an observational study, including a population composed by simplifications and failures, with different drug dosing, the excellent virologic results after 96 weeks of therapy suggest that DTG plus bDRV may be a reliable option in highly treatment-experienced subjects.
Data availability
The data that support the findings of this study are available from the corresponding author, AFC, upon reasonable request.
Notes on contributors
Dr. Amedeo F. Capetti was born in Milano (Italy) on Feb 22, 1964. Capetti has received a degree in Medicine and Surgery, University of Milano Statale; Specialty on Infectious Diseases, University of Milano Statale. Employment and scientific activities – At the ‘Luigi Sacco’ Hospital, ASST Fatebenefratelli – Sacco: Head of the Sexually Transmitted Diseases Unit; Clinical Supervisor of the Phase I Clinical Research Unit; Responsible of the Research and Development branch of the 1st Division of Infectious Diseases. At the University of Milano Statale: Professor of Human Physiology at the University major in Nursing. At the WHO, Geneva, Switzerland: Consultant for the Holy See Delegation. Areas of research: antiretroviral therapy and treatment strategies, immunotherapy, HIV drug resistance, HIV eradication strategies. Reviewer for International Medical Journals: Lancet HIV, AIDS, BMC Infectious Diseases, PLOS One, Open Forum Infectious Diseases, Sexually Transmitted Diseases, Journal of Antimicrobial Agents, HIV/AIDS – Research and Palliative Care, Infection and Drug Resistance. More than 100 scientific publications of which 80 indexed on PubMed Medline.
Dr. Giuseppe V. De Socio received Degree in Medicine and Surgery, University of Perugia; PhD on Infectious diseases, University of Rome “La Sapienza”; Post-graduate specialties in Infectious Diseases, University of Perugia. Employment and scientific activities – He is a medical staff at Department of Internal Medicine2, Infectious Diseases Unit, “Santa Maria della Misericordia” General Hospital Perugia, Italy. His research interests are epidemiological, clinical, and therapeutic research in the field of Infectious Diseases, focusing on HIV infection and comorbidities, specifically cardiovascular diseases and hypertension. Others interest: antimicrobial treatment of bacterial, viral, fungal, and parasites. Member of scientific committee of Italian group CISAI (Italian coordination group for the study of allergies and HIV infection). Reviewer for International Medical Journals: Journal of Acquired Immune Deficiency Syndromes, Journal of Antimicrobial Chemotherapy, Clinical Microbiology and Infection, Journal of Infection, HIV Medicine, PLoS One, Current HIV Research, Journal of the International AIDS Society, International Journal of STD & AIDS, European Journal of Internal Medicine, European Journal of Clinical Nutrition, Metabolism, BMJ Case Reports, Journal of AIDS and HIV Research, Journal of Medicine and Medical Sciences, Future Virology. Associate editor for PLoS One.
Maria V. Cossu, MD received her medical degree from the University of Milan in 2008 and completed her specialization in pharmacology at the same University in 2014. While she completed her residency in pharmacology her main fields of research have been the study of the impact of nitric oxide in the pathophysiology of muscle degenerative disorders and the pharmacology of HIV drugs. She worked in the implementation of a Phase I Unit in the Luigi Sacco to conduct study on healthy volunteers. Currently, since July 1st, 2014 she works as Medical Assistant in the Outpatient Unit of the 1st Division of the infectious Diseases. She is involved in the conduction international clinical trials, which are ongoing at her site, related to HIV, HCV, NASH and antibiotic therapeutic areas. During the last two years, she was involved in an international project, ENDORSE, funded by the EDCTP partnership, with the objective to build capacity in the region of Northern Uganda by training healthcare workers in biosafety and personal protection from infectious agents in both laboratory and patient-care settings. At the beginning of the last year, she was nominated as a member of the Ethics Committee of the ASST FBF SACCO.
Dr. Gaetana Sterrantino graduated in Medicine and Surgery at the University of Florence, Italy. From 1986–88 she worked as Research Associate at the Department of Immunology, University of Manitoba, Manitoba, Canada. She post graduated in the following specialties: at the University of Florence, Italy: 1986 Thisiology and Respiratory Diseases, 1991 Allergology and Immunology; 1999 Tropical and Infectious Diseases; at the University of Tor Vergata, Rome, Italy: 2012 Clinical management of resistance to antiviral drugs. Employment and scientific activities: she is on medical staff at the Azienda Ospedaliero-Universitaria Careggi in Florence, Italy. Her research interest are clinical and therapeutic research in the field of Infectious Diseases focusing on HIV and Pregnancy Infections. She has been Principal Investigator and sub-investigator of Phase II, III and IV clinical trials in the following areas: HIV Infection and infectious diseases. In 2010, 2011 and 2012 she participated in the drafting of the Italian guidelines for HIV. She is reviewer for international medical journals: Plos One, Infection, Future virology, Journal of Antimicrobial Chemotherapy, International Journal of STD & AIDS, Clinical Economics and Outcomes Research, Reviews on Recent Clinical Trials, International Journal of Public Health and Epidemiology.
Giovanni Cenderello He is consultant in Infectious Diseases at Ospedale Galliera in Genoa since 1 October 2009. He got his MD in 1997 and post-graduated in Infectious Diseases in 2001. Since then he worked at ASL-1 Imperiese(Sanremo Hospital) as Junior consultant in Infectious Diseases from September 2002 to September 2009. His research fields are viral infection (HIV and HCV) with a focus on special populations (such as HIC/HCV coinfected patients or HCV people affected by Thalassemja Major). Moreover, he is interested in pharmacoeconomics. He holds post graduated certification in Health Technology assessment. Academic experience 2016–2017 ongoing teaching Infectious Diseases at Nurse students at Genoa University. He is also member of the Italian commission for the application of AIDS National Plan at Ministry of Health. Authored/coauthored of 70 original papers.
Dr. Annamaria Cattelan was born in Treviso (Italy), on Sep 23, 1963. She received Degree in Medicine and Surgery from University of Padova; Specialty on Infectious Diseases, Respiratory Diseases an Tisiology, University of Padova. Employment and scientific activities – At the Azienda Ospedaliera-Universitaria of Padova: Director of the Infectious and Tropical Diseases Unit; Vice-president of the Regional Committee for Health-related Area Vasta Venezia – Rovigo; Member of the Committee for Clinical Research of the province of Rovigo; Member of the HIV/AIDS Italian Expert Panel Areas of research – antiretroviral therapy and treatment strategies, tisiology, respiratory diseases, pharmacoeconomics Scientific publications – 139 scientific publications indexed on PubMed Medline.
Dr. Gian M. Baldin He is graduated at University of Padua, Italy, on 2014 and now Resident in In Infectious and Tropical Diseases at Catholic University of Sacred Heart. His research interests are HIV antiretroviral therapy. He performs a trainee in Ikonda hospital in Tanzania from October to December 2017.
Dr. Alessandro Soria was Born in Milano (Italy) Oct 5, 1976. He received his Degree in Medicine and Surgery, University of Milano Statale; Specialty on Infectious Diseases, University of Milano Statale. Employment and scientific activities – At the ‘San Gerardo’ Hospital, Monza: Physician specialized in Infectious Diseases and the management of HIV infection. Areas of research: antiretroviral therapy and treatment strategies, immunology, tuberculosis, bacterial diseases. 45 scientific publications indexed on PubMed Medline.
Dr. Niccolò Riccardi was born on Jun 5, 1987. He received Degree in Medicine and Surgery, University of Parma; Specialty of Infectious Diseases, University of Genova. He has studied also in Charles University, Prague (Erasmus project), Bali's International Summer School of Travel Medicine and Tropical Disease, University of Brescia, Dakar Bois Sacré and Centre de Santé de Diofior, Fatick Region, Senegal. Employment and scientific activities – At the ‘San Martino’ University Hospital, Genova: Resident in Infectious and Tropical Diseases. Areas of research: antiretroviral therapy and treatment strategies, TB, Parasitic infections in Europe and LMIC, Tropical Medicines, Infections in Pediatric Intensive Care, Infections in pregnancy and MTCT, Infectious complications of malignancies. 49 scientific publications indexed on PubMed Medline.
Dr. Fosca P. Nierowas born in Milano (Italy), on Mar 26, 1965. Niero received Degree in Medicine and Surgery, University of Milano Statale; Specialty on Infectious Diseases, University of Milano Statale. National Diploma of Clinical Ultrasonography. Employment and scientific activities – At the ‘Luigi Sacco’ Hospital, ASST Fatebenefratelli – Sacco: Physician specialized in the management of HIV infection and liver diseases; Ultrasonographist Areas of research: antiretroviral therapy and treatment strategies, post-exposure prophylaxis for HIV, HCV, HBV, Non alcoholic fatty liver disease (NAFLD), cryoglobulinemia. 24 scientific publications indexed on PubMed Medline.
Dr. Benedetto M. Celesiagraduated in Medicine and Surgery at the University of Catania, Italy; received PhD in Infectious Diseases and Immunodeficiency, and is a specialist in Infectious Diseases at the University of Catania, Italy. Employment and scientific activities: He is a medical staff at the unit of infectious diseases ARNAS Garibaldi in Catania, Italy, since 1990. Actually, he is the coordinator of HIV outpatient unit. His main research interest are clinical and therapeutic research in the field of Infectious Diseases focusing on epidemiology, treatment and complications of HIV, HCV, STD, Leishmaniasis. Member of study group as ICONA, CISAI and GEPPO cohort. From 2016 he is teaching infectious diseases at medical school students of university of Catania Principal Investigator and sub-investigator of Phase III and IV clinical trials in the area of HIV Infection and infectious diseases. Reviewer for international medical journals as Plos One, Infection, Journal of AIDS and Clinical Research, BMJ Case Reports, etc. Authored/coauthored 75 peer-reviewed publications.
Dr. Giorgio Barbariniwas born in Voghera, Italy, on August 27, 1951. He graduated with full marks in Medicine and Surgery in September 1976. Postgraduate Specialty in Infectious Tropical Diseases and Gastroenterology Since November 1977 to April, 2018, he was Infectivology and Hepatology Physician in the Clinic of Infectious and Tropical Diseases of Foundation IRCCS Polyclinic San Matteo-University of Pavia, where he was the Chief of Out-patients Structure by 1987. He was visiting scientist in 1993 at the Hepatitis Section of NIAID, NIH, Bethesda, Maryland. The fields of research of Dr Barbarini concern the clinical and therapeutic aspects of HIV, HBV and HCV Infections. He is member of several National and International Scientific Societies and he is author of more than 200 Scientific publications in Domestic and International Journals (his global Impact Factor rate is >550; HI index 33). Since 2012 he is President of CLEO (Club of Italian Hepatologists working in Public Hospitals). Member of “Italian Consulta for AIDS” of Ministry of Health.
Dr. Stefano Rusconiis an Associate Professor in infectious diseases at the University of Milan, Italy, since February 1, 2015. After his M.D. in 1988, he got post-graduate specialties in allergy & clinical immunology and infectious diseases. He is on staff at Luigi Sacco hospital in Milan since February 1994. His research interests are: (i) experimental models of antiretroviral therapy and viral mutants in vitro; (ii) genotypic and phenotypic analysis of resistance to antiretroviral drugs; (iii) design and conduct of clinical trials with antiretroviral drugs. He holds post-graduate certifications in design and conduct of clinical trials and in advanced bioethics and has been the scientific secretary at Milan Area 1 Inter-hospital IRB from July 1999 to January 2017. He has been elected as EACS regional representative in 2012–2016. Academic Years 2010/11-ongoing: teaching Infectious Diseases to Medical School students; 2012/13-ongoing: teaching Infectious Diseases to Prevention Health Sciences students, both at Università degli Studi di Milano, Italy. Since 2012 he is a member of HIV/AIDS ITALIAN EXPERT PANEL for Italian Guidelines. Referee: HIVERA for European Research Projects on HIV/AIDS, International AIDS Conference, World AIDS Conference, Italian Ministry of University and Scientific Research and several medical journals. He has authored/co-authored 187 original peer-reviewed publications.
Giuliano Rizzardini, MD, first began working in Uganda in 1985, shortly after finishing his medical degree. At present is Director of Infectious Diseases Department at Luigi Sacco Hospital in Milan, Italy. His research career began with investigations of HIV in Africa. Later, his primary research focus shifted to clinical trials of antiretroviral drugs. Since 2013 he is a lecturer at School of Clinical Medicine, Faculty of Health Science, University of the Witwatersrand, Johannesburg, South Africa.
Disclosure statement
The authors report no conflict of interest.
References
- Gubavu C, Prazuck T, Niang M, et al. Dolutegravir-based monotherapy or dual therapy maintains a high proportion of viral suppression even in highly experienced HIV-1-infected patients. J Antimicrob Chemother. 2016;71(4):1046–1050.
- Capetti AF, Sterrantino G, Cossu MV, et al. Switch to Dolutegravir plus rilpivirine dual therapy in cART-experienced subjects: an observational cohort. PLoS One 2016;11(10):e0164753.
- Gantner P, Cuzin L, Allavena C, et al. Efficacy and safety of dolutegravir and rilpivirine dual therapy as a simplification strategy: a cohort study. HIV Med. 2017;18(9):704–708.
- Revuelta-Herrero JL, Chamorro-de-Vega E, Rodríguez-González CG, Alonso R, Herranz-Alonso A, Sanjurjo-Sáez M. 2017. Effectiveness, safety, and costs of a treatment switch to dolutegravir plus rilpivirine dual therapy in treatment-experienced HIV patients. Ann Pharmacother. 1060028017728294.
- Palacios R, Mayorga M, González-Domenech CM, et al. Safety and efficacy of dolutegravir plus rilpivirine in treatment-experienced HIV-infected patients: the DORIVIR study. J Int Assoc Provid AIDS Care. 2018;17:2325958218760847.
- Casado JL, Monsalvo M, Rojo AM, Fontecha M, Rodriguez-Sagrado MA. Dolutegravir and rilpivirine for the maintenance treatment of virologically suppressed HIV-1 infection. Expert Rev Clin Pharmacol. 2018;11(6):561–570.
- Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390(10101):1499–1510.
- Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391(10123):839–849.
- Capetti AF, Sterrantino G, Cossu MV, et al. Salvage therapy or simplification of salvage regimens with dolutegravir plus ritonavir-boosted darunavir dual therapy in highly cART-experienced subjects: an Italian cohort. Antivir Ther (Lond). 2017;22(3):257–262.
- Capetti AF, Cossu MV, Orofino G, et al. A dual regimen of ritonavir/darunavir plus dolutegravir for rescue or simplification of rescue therapy: 48 weeks' observational data. BMC Infect Dis. 2017;17:658.
- Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F. Chronic Kidney Disease Epidemiology Collaboration. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–772.
- Stanford University HIV Drug Resistance Database. Available: https://hivdb.stanford.edu/hivdb/by-mutations/report/. Accessed 23 July 2018.
- CTCAE website. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/About.html. Accessed 6 July 2018.
- Stang A, Perry T, Fadul N. Darunavir and dolutegravir combination therapy in ART experienced HIV-infected patients: a preliminary report. OFID. 2017;4(S1):S431.
- Wheeler J, Chan S, Harrigan PR, Becker M, Kasper K, Keynan Y. Dolutegravir with boosted darunavir treatment simplification for the transmitted HIV thymidine analog resistance in Manitoba, Canada. Int J STD AIDS. 2018;29(5):520–522.
- Parienti JJ, Bangsberg DR, Verdon R, Gardner EM. Better adherence with once-daily antiretroviral regimens: a meta-analysis. Clin Infect Dis. 2009;48(4):484–488.
