?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Baby diapers are complex products consisting of multiple layers of materials, most of which are not in direct contact with the skin. The safety profile of a diaper is determined by the biological properties of individual components and the extent to which the baby is exposed to each component during use. Rigorous evaluation of the toxicological profile and realistic exposure conditions of each material is important to ensure the overall safety of the diaper under normal and foreseeable use conditions. Quantitative risk assessment (QRA) principles may be applied to the safety assessment of diapers and similar products. Exposure to component materials is determined by (1) considering the conditions of product use, (2) the degree to which individual layers of the product are in contact with the skin during use, and (3) the extent to which some components may be extracted by urine and delivered to skin. This assessment of potential exposure is then combined with data from standard safety assessments of components to determine the margin of safety (MOS). This study examined the application of QRA to the safety evaluation of baby diapers, including risk assessments for some diaper ingredient chemicals for which establishment of acceptable and safe exposure levels were demonstrated.
Disposable baby diapers are widely used in many parts of the developed and developing world. It is estimated that 90% to 95% of diapers used in developed nations are disposable. These products are in contact with the baby's skin almost constantly from birth through approximately the first 3 years of life. Given that diapers are changed, on average, four to five times daily, a baby will be exposed to several thousand disposable diapers before becoming toilet trained.
The widespread use of and constant exposure of babies to disposable diapers mandates the development of robust methods for assessing the safety of these products during use. A generalized risk assessment (RA) paradigm was established by the CitationNational Academy of Sciences in 1983 (CitationNational Academy of Science, 1983) that consists of a four-step process: hazard identification, dose-response assessment, exposure assessment, and risk characterization. Although the application of this process varies and is described using different terminology by different organizations and institutions, the basic principles are the same (CitationInternational Programme on Chemical Safety, 1999; CitationHopper & Oehme, 1989; CitationWilliams & Paustenbach, 2002; CitationOrganization for Economic Cooperation and Development, 2007). This study provides an overview of the application of quantitative risk assessment (QRA) principles to the safety evaluation of disposable diapers.
DESIGN AND USE OF DISPOSABLE DIAPERS: IMPLICATIONS FOR SAFETY ASSESSMENT
Compared with the first disposable diapers that became available in the early 1960s in the United States, the modern diaper benefits from a number of innovations in design and materials. These improvements resulted in products that are highly absorbent and ensure a snug, comfortable fit. These improvements also enable the manufacture of a product that promotes skin hygiene by containing excreta and reducing exposure of the skin to urine and feces.
Diaper Design
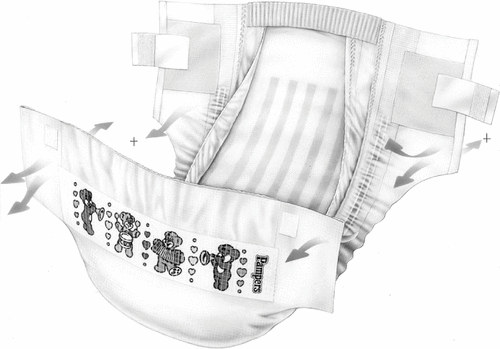
A number of different material layers are employed in the construction of a typical disposable diaper. In general, diapers consist of absorbent layers in the diaper core, contained within an outer structure, or chassis, which ensures proper fit (). Components of a typical diaper include a polypropylene top sheet that is in direct contact with the skin, cellulose pulp and super absorbent polyacrylate granules contained within the diaper core away from direct skin contact, and barrier leg cuffs that ensure fit and prevent leakage (). Additional components include an outer layer of polyethylene film, elastic materials that improve fit, and adhesive or loop tapes for fastening the diaper. Adhesives are used throughout the diaper to hold the layers in proper position. Some diaper variants may also feature lotion on the top sheet, which helps to improve the softness/smoothness properties of diapered skin and reduces the incidences of diaper dermatitis. Further, the top sheet may also be treated with low levels of mild surfactants to overcome the otherwise hydrophobic nature of the polymer top sheet and allow urine to pass through. Most of the diaper weight is made up of polymers of natural or synthetic origin.
2. FIG. 2. Cross section of a typical baby diaper. NW, nonwoven; SAP, superabsorbent polymer; AQL, acquisition layer; BS, back sheet; CBWL, cellulose-based wicking layer.
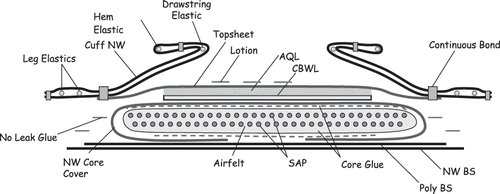
Raw materials used in diaper construction, including the top sheet and superabsorbent materials, have all shown low irritancy potential and no evidence of sensitization in standard skin testing. Because they are solid materials that are biologically inert and have low bioavailability, these polymeric materials enjoy a favorable human safety profile. In addition, diapers also contain small quantities of low-molecular-weight ingredients that are incorporated into the polymeric matrix or present on the polymer surface. These materials may come into contact with the skin, either through direct exposure or as a result of urine extraction, and therefore are relevant to the assessment of diaper safety.
Overall, the safety assessment of a disposable diaper involves the following: identification of constituents of toxicological relevance in individual raw materials (e.g., impurities, manufacturing aids), and their safety evaluation via the quantitative risk assessment (QRA) process. The safety evaluation process involves confirmation of safety for both dermal and systemic endpoints.
Exposure Parameters
Because the diaper is a complex, layered product, with each layer having a different function, the exposure potential for various diaper components ranges from continual direct skin contact for some materials (e.g., the top sheet) to materials with transient or negligible skin contact (e.g., the fasteners). As noted earlier, the majority of chemical components in a diaper are large, inert polymers that are not absorbed through the skin. These materials are of minor concern when evaluating diaper safety. In contrast, low-molecular-weight materials that may be present in the diaper need to be thoroughly assessed. These materials (e.g., monomers, fiber finishes) may be released from the polymer fibers and migrate to the surface of the diaper, and hence become relevant for safety assessment.
Materials in Direct Skin Contact
Diaper raw materials that are in direct contact with the skin include the top sheet, top-sheet lotions, the barrier leg cuff, and the waistband. Skin transfer of chemicals or materials from the diaper onto the skin does not necessarily require solubilization in body fluids (urine) that may be loaded onto the diaper. Chemicals may be deposited or transferred onto the skin directly, or via solubilization in sweat, urine, or sebum. In reality, only a certain amount of top-sheet ingredients are transferred onto the skin. This is due to the integration of the raw materials/chemicals with the polymeric matrix resin of the top sheet. Typically, analytical data on the leachability of the chemical/raw material needs to be obtained, in order to accurately calculate exposure.
Our studies showed that for materials that are intended to transfer to the skin (i.e., top-sheet lotions), less than 7% is actually transferred to the skin (CitationOdio et al., 2000). This percentage transfer, or lotion transfer factor, provides a reasonable and conservative upper bound estimate for transfer of other top-sheet ingredients.
Materials Not in Direct Skin Contact
Diaper components beneath the top sheet are not in direct contact with the skin. These materials include the acquisition layer, the super absorbent polymer, nonwoven core wrap material, core, and chassis glues. Although not in direct contact with skin, these materials may contain constituents with the potential to migrate to the diaper top sheet by a process termed reflux. Exposure to these materials may occur via extraction or solubilization in body fluids, followed by migration to the diaper top sheet.
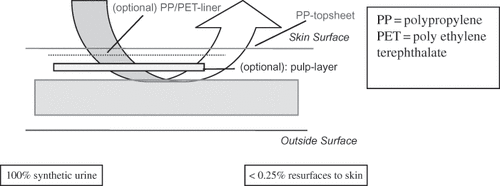
The urine that resurfaces to the top sheet is assumed to be the key carrier for these constituents. In the absence of experimental data from which to estimate the migration of low-molecular-weight materials from the diaper interior to the diaper top sheet, a highly conservative default assumption of 100% extraction into the total urine load may be used for exposure calculations. A refined exposure evaluation would include actual quantification of the extractable ingredient from the fluid matrix.
THE REFLUX APPROACH
Reflux (also termed rewet) is a relevant factor for quantifying exposure to non-skin contact materials, via the QRA method. Reflux is defined as the fraction of total liquid load (i.e., urine) that resurfaces to the top sheet of a diaper and has the potential to come into contact with the baby's skin. Diaper core ingredients that are not in direct skin contact require urine as an aqueous vehicle or carrier to reach the skin. This urine that resurfaces to the top sheet is assumed to be the relevant carrier for ingredients that may be located deep within the diaper. Current diaper risk assessments are based upon the presence and use of highly absorbent core technologies, like superabsorbent polymer (SAP), which have further reduced the reflux level of disposable baby diapers. Reflux values of currently marketed diapers were collected and evaluated and the highest reflux value (0.223%) is chosen for quoting a rounded conservative default reflux of 0.25% (). This reflux value is obtained after testing of diapers that may be worn overnight with a very high urine load, which represents a highly conservative scenario for analysis of reflux.
The reflux factor is measured with varying but common methods, all of which typically use an absorbing filter paper or collagen sheet under pressure on top of a moist diaper. The diaper is typically loaded with synthetic urine or other aqueous solvents. The filter paper/collagen sheet builds on the high capillary forces of cellulose fibers to detect small differences in absorbency between different products.
Reflux values from the filter paper method are in the range of 2–2.5%, while those from the collagen method, however, are more realistic and similar to actual diaper in-use conditions, as collagen closely mimics the re-absorbency characteristics of the upper epidermal layers of the skin. Typically, the PACORM (post acquisition collagen rewet method) method is used to define a default reflux factor.
The PACORM uses a stretched diaper on a fastening plate (CitationHerrlein, 1996). Following diaper loading (4 large gushes of synthetic urine or saline, depending on diaper size), 4 circular collagen sheets (diameter 9 cm each, area 64 cm2 each) are stacked onto the top sheet under pressure (about 236 g/cm2). Reflux is calculated as the weight difference of the collagen sheets before and after exposure to the top sheet. One of the shortcomings of this method is that reflux is measured only under the 64-cm2 collagen sheet area, and not from the entire diaper surface. However, the reflux number obtained is still reasonably conservative, as reflux is measured in the loading area with the highest liquid concentration in the diaper core.
CONSUMER USAGE DATA AND ASSUMPTIONS FOR Quantitative RISK ASSESSMENT
Normal consumer habits and practices for diaper usage are varied across different geographies. As shown in , Procter & Gamble consumer research indicates that the highest number of diapers used per day is observed in Japan, while fewer diapers are used per day in countries like China, India, the Philippines, and Indonesia. Different diaper usage habits too are relevant parameters when determining overall exposure to diaper raw materials and chemicals. For baby diaper exposure assessments, the following parameters are particularly relevant:
The baby body weight changes over the course of the entire diapering time, with an average body weight of approximately 3.5 kg to 4 kg for newborn babies, and an average weight of approximately 10 kg for 12-mo-old babies, and in the range of 18 kg to 25 kg for toddlers. For simplification, an average baby weight of 8 kg is assumed in risk calculations. For worst-case exposure scenarios, the lower baby weight of newborn babies can be assumed.
The number of diapers used per day (diaper change frequency) is higher in newborn babies with approximately 6 diaper changes per day in Japan (compared with 2 to 3 changes per day in India), which goes down to approximately 3 diaper changes per day for toddlers. Some typical diaper use figures for babies of different sizes are provided in . In June 2003, the AHPMA reported an average change frequency of 4.5 diapers a day, accounting for the average change frequency during the entire diapering time (CitationAbsorbent Hygiene Product Manufacturer's Association, 2002). For simplification, a diapering time average change frequency of 5 is assumed in our calculations.
TABLE 2 Typical Diaper Weight Ranges and Change Frequencies (P&G Internal Consumer Usage Data)
The top-sheet transfer factor (7%) is the amount of an ingredient that is transferred from the top sheet of the diaper to the skin of the baby. This is determined by ingredient transfer data generated in support of diaper lotion, which is intended to be transferred to the skin during diaper wear. It is assumed that the transfer of any substance from the top sheet will not be greater than the transfer of an ingredient that is intentionally designed to transfer from the top sheet to the skin (i.e., the top-sheet lotion). A default transfer value of 7% is recommended for exposure calculations of top-sheet ingredients. This value is based upon measurements of a lotion ingredient tracer (stearyl alcohol), which is also representative of other lotion ingredients. This number is considered to be a reasonably conservative default assumption of top-sheet ingredient transfer.
Reflux factor (0.25%). The superabsorbent core of baby diapers is not in direct contact with the skin of the baby and is made up of a polymeric gelling material that quickly helps to soak up urine and contain it within the core. This core technology is specifically engineered to minimize or eliminate any re-exposure of the skin to urine. However, minute amounts of urine might still resurface to the skin via the phenomenon of reflux (). Migration of any ingredients from the lower layers of the diaper, albeit remote, is still possible, and resurfacing urine is assumed to be the carrier of any constituents that might reside in these core lower layer materials that are not in direct skin contact. A default reflux value of 0.25% is recommended by the European Disposables and Nonwovens Association for exposure calculations of these types of raw materials (CitationEuropean Disposables and Nonwovens Association, 2005); this represents the highest reflux observed after analyzing baby diapers using the more biologically relevant PACORM method with extremely high initial synthetic urine loading.
TABLE 1 Habits and Practices—Data for Baby Diaper Usage in Different Countries in Asia: Procter & Gamble Consumer Research Data
MATERIALS WITH NEGLIGIBLE SKIN CONTACT
Several diaper materials used on the outside of the diaper chassis (e.g., outer polypropylene liners, graphic printed surfaces, fastening tapes, disposal tapes) have negligible or very minimal direct skin contact. In terms of exposure assessment, these materials are judged to have negligible exposure, and hence are not considered to contribute toward the overall exposure to materials through diaper wear.
EXPOSURE-BASED RISK ASSESSMENT EXAMPLES
Diapers are mainly constructed of inert, polymeric materials (CitationKosemund et al., 2009). In general, these materials are considered safe and no inherent toxicity issues are anticipated. However, low levels of nonpolymeric components (e.g., monomers, perfume raw materials) may be present in the product. These may be remnants of the diaper raw material manufacturing process, or they might be components of aesthetic materials like perfumes or lotions that are added to the diaper at low levels. The following section provides examples of exposure based risk assessments for materials in baby diapers. Citral, a perfume raw material, and acrylic acid, a residual monomer in superabsorbent polymers that form the core of the diaper, are provided to serve as case studies to demonstrate the principles of QRA.
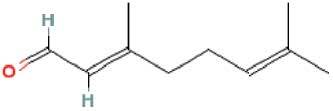
Risk Assessment for Citral
Citral (CAS number 5392-40-5) () is a well-known flavoring agent and is the main constituent of lemon and orange oils. It is commonly used in perfumes and may be a low-level component of the overall complex perfume formula added to a diaper. Perfumes may be added to diapers (typically between the absorbent core and the back sheet) to enhance the overall aesthetics of the diaper. Given the deep-layer application of the perfume in a diaper, it is recommended to use the EDANA reflux level of 0.25% for determining potential exposure. Citral is classified as a weak skin sensitizer and exposure to low levels does not pose a health risk. The identification of such a safe level is achieved through the sensitization quantitative risk assessment (QRA) approach for fragrance ingredients. This approach follows the same four basic steps of the risk assessment paradigm defined by the National Academy of Sciences: hazard identification, dose-response assessment, exposure assessment, and risk characterization (CitationNational Academy of Science, 1983). CitationKosemund et al. (2009) previously described the application of this paradigm for the safety assessment of diapers.
Recently, a commonly recommended methodology was adopted for the QRA of fragrance ingredients by the European Cosmetics, Toiletry and Perfumery Association (COLIPA) Toxicology Advisory Group and the Joint COLIPA/International Association for Soaps, Detergents, and Maintenance Products (AISE)/European Flavor and Fragrance Association (EFFA)/International Fragrance Association (IFRA) Perfume Safety group (CitationQRA Expert Group, 2006). Clear guidance on different elements of the dermal sensitization QRA process (e.g., uncertainty factors, sensitization assessment factors, etc.) is offered in this COLIPA dossier. A weight-of-evidence (WoE) approach was used to determine a no-expected-sensitization-induction level (NESIL), which introduces a more robust approach to allergen potency evaluation for use in risk assessment. In addition, sensitization assessment factors (SAF) within the exposure-based QRA process are based on published data. The SAF take into account three parameters: interindividual variability (the same as in general toxicology), vehicle/product matrix effects, and use considerations (specific for dermal sensitization). A consumer exposure level (CEL) is determined for the perfume and it is essential to express this entity in units of dose per square centimeter of skin. This updated process of QRA for perfume ingredients represents an important step forward in skin sensitization risk assessment.
Once the NESIL, SAF, and CEL for citral are defined, it is possible to proceed to a quantitative risk assessment to confirm safety, and specifically a lack of sensitization hazard. There are two key elements involved in this process of risk characterization:
The establishment of an acceptable exposure level (AEL): The AEL is determined by dividing the WoE NESIL by the SAF, i.e., AEL = WoE NESIL ÷ SAF. Identification of the WoE NESIL is shown in .
Comparison of the AEL to the CEL: This is determined by dividing the AEL by the CEL, which is an indication of the acceptability of the CEL relative to the AEL. The percent concentration of the fragrance ingredient, citral, in a diaper is acceptable if the AEL exceeds the CEL.
TABLE 3 Identification of Weight-of-Evidence No-Expected-Sensitization-Induction-Level (WoE NESIL) for Citral
Assumptions to calculate CEL for citral as a result of diaper wear are:
Amount of perfume / diaper = 5 mg.
Amount of citral in perfume = 0.026%.
Amount of citral/diaper = 0.0013mg.
Five diapers worn/day (based on habits and practices data).
Reflux factor = 0.25% (collagen reflux method, based on maximal loading of 240 ml to 260 ml synthetic urine for all diapers used per day).
Skin contact area = 1186 cm2 (surface area of smallest diaper).
Therefore, CEL for citral = 0.0013 mg × 0.25% × 5 ÷ 1186 cm2 = 0.000000014 mg/cm2/d = 0.000014 μg/cm2/d.
The QRA for citral in baby diapers is summarized in . It is observed that the AEL is greater than the actual CEL by 1,000,000, indicating that use of citral as a perfume ingredient in baby diapers is safe. A margin of safety (MOS) of 1,000,000, while providing overwhelming safety assurance, also indicates that citral may even be safely used at much higher levels in baby diapers, without the risk of skin sensitization.
TABLE 4 Application of QRA for Citral in Perfumes Used in Diaper Products
Exposure to a Diaper Absorbent Core Raw Material—Acrylic Acid
Although the diaper is composed mostly of high-molecular-weight polymeric materials, there may be unreacted, low-molecular-weight monomers or other low-molecular-weight components (e.g., processing aids or impurities) present in these polymeric materials. Exposure to diaper absorbent core raw materials may occur via the reflux phenomenon, and, depending on the material and molecular weight (MW), may also be absorbed through the skin (CitationBos & Meinardi, 2000).
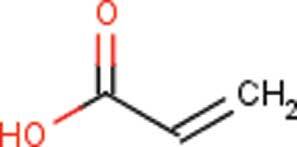
The superabsorbent polymer (SAP) in baby diapers comprises one of two main components that make up the absorbent core of a diaper, with the other being absorbent fluff pulp, which is composed of cellulose fibers. The most important function of SAP is to absorb urine and prevent it from returning to the skin contact layers of the diaper, thus reducing the incidence of diaper rash (P&G internal data). Typically, SAP is manufactured by the polymerization of acrylic acid () in an aqueous solution. During polymerization, the carboxylic acid groups are partially neutralized (75%) with sodium hydroxide. Therefore, a major functional component of SAP is the neutralized, salt form of the carboxylic acid moities in the polymer. Polyacrylates and their sodium salts are generally not a cause for toxicological concern.
However, because polymerization processes are not 100% efficient, low residual amounts of free acrylic acid monomer are expected to be present in SAP. Due to the neutralization of SAP and the physiological conditions that exist in a urine-wetted diaper, most of the free acrylic acid will be present as the salt form, i.e., sodium acrylate. This is an important observation from an exposure perspective because sodium acrylate does not penetrate the skin as easily as does acrylic acid.
A 2002 CIR report on the “Safety of Acrylates Copolymer and 33 Related Cosmetic Ingredients” (CitationFiume, 2002) indicates that linear polymers of acrylic acid may contain unreacted starting materials and catalysts, with residual monomer concentrations typically between 10 ppm and 1000 ppm, with an upper limit of 1500 ppm. (SAP raw material suppliers for baby diapers are currently required to maintain an upper specification limit of 500 ppm for free acrylic acid monomers). Thus, the presence of unreacted acrylic acid monomers in SAP is limited, with the main route of exposure to this residual sodium acrylate occurring dermally via the phenomenon of reflux.
Acrylic Acid—Hazard Characterization
Acrylic acid was evaluated for safety as part of the European Risk Assessment Program (EEC 793/93) (CitationInstitute for Health and Consumer Protection, 2002), and as part of the CIR report on the “Final Report on the Safety Assessment of Acrylates Copolymer and 33 Related Cosmetic Ingredients” (CitationFiume, 2002). It was also evaluated by the U.S. Environmental Protection Agency (CitationU.S. EPA, 1994). The hazard profile of acrylic acid as described in these references is summarized in .
TABLE 5 Hazard Profile of Acrylic Acid
At low levels, acrylic acid is not irritating, as irritation is concentration dependent. Acrylic acid at 1% in acetone shows signs of minimal irritation, but is well tolerated by animals in dermal carcinogenicity studies.
Systemic Risk Assessment for Acrylic Acid
The U.S. EPA Integrated Risk Information System (IRIS) oral reference dose (RfD) for acrylic acid (U.S. EPA, 1994) is estimated as 0.5 mg/kg/d, which is based on a chronic drinking water study in rats, a two-generation reproductive study in rats, developmental studies by the inhalation route in rats and rabbits, and an inhalation/dermal/oral/i.v. bioavailability study in rats and mice. This RfD represents a daily exposure level that is considered to be safe for a lifetime of exposure, including sensitive subpopulations.
A default reflux value of 0.25% (CitationEuropean Disposables and Nonwovens Association, 2005) is recommended for exposure calculations for materials that are not in direct skin contact, and this value may be used for acrylic acid.
Exposure Assessment of Acrylic Acid
The QRA for acrylic acid in diaper cores is summarized in . It is noted that this exposure assessment is highly conservative and assumes that 100% of the ingredient is represented as free acrylic acid in the entire diaper, and that this chemical is available for reflux, and fully penetrates the skin for each of the five diapers used on a daily basis.
TABLE 6 Application of EBRA to Available Acrylic Acid in Diaper Absorbent Cores
Based on the risk assessment and exposure evaluation, and the MOS that is greater than 1, it is concluded that for systemic toxicological endpoints, residual acrylic acid that may be present in the superabsorbent core (SAP) of baby diapers does not present any systemic human safety risk.
THRESHOLD OF TOXICOLOGICAL CONCERN (TTC)
The absence of chemical-specific data for certain diaper ingredients/chemicals may be encountered during the process of safety evaluation. Many of the materials used in the construction of diapers are complex materials that may contain many different chemicals or may have low-level impurities. For example, low levels of unreacted monomers may be present in a high-molecular weight-polymer, or a low level of a solvent may be present in an adhesive. Traditional risk assessment approaches are generally used to confirm safety, based on evaluating the potential for these chemicals to migrate out of the diaper and reach the baby's skin. However, there may be very low residual levels of chemicals/contaminants that do not have a robust safety data set. To confirm safety for such low level residuals, an assessment based on the concept of threshold of toxicological concern (TTC) can be used. This approach is based on the fundamental premise that there is exposure to chemicals below which adverse effects will be negligible or absent. This TTC framework provides a conservative estimate of an acceptable chronic exposure, in the absence of chemical specific data. Although originally developed by the U.S. Food and Drug Administration (CitationU.S. FDA, 1995) to support low-level exposures to indirect food additives (e.g., packaging materials), this framework has since been expanded for application to fragrance materials, and other materials that may be used in normal, personal care products, including baby diapers (CitationBlackburn et al., 2005). The TTC approach has been described in the literature in some detail (CitationKroes et al., 2004). For a chemical that does not have structural alerts for genotoxicity, an acceptable exposure limit is generally accepted to be 1.5 μg/day for an adult (0.025 μg/kg/day, assuming a 60-kg adult body weight).
An example of the application of TTC to a residual level of a chemical contaminant in an adhesive used in the construction of a baby diaper is shown next. Total exposure to the adhesive itself is determined to be 3.6 mg/d.
Assuming a body weight of 8 kg, this exposure is equivalent to 3.6 mg/day divided by 8 kg = 0.45 mg/kg/day (or 450 μg/kg/day). Therefore, an acceptable upper limit on the concentration of the contaminant in this adhesive can be calculated:
In this example, it can be established that a level of up to 56 ppm of a contaminant in the adhesive can be supported using the TTC method. Similarly, if the exact level of the potential adhesive contaminant is known in advance then the TTC method may be used to confirm its safety. In this case, any level below 56 ppm would be considered to be acceptable using this method. It is noted that the TTC method is highly conservative, and if the exposure is determined to be above a TTC-based limit, then more work would generally be done to refine the assessment before determining that it is unacceptably high.
DISCUSSION
The conduct of scientifically sound risk assessments for establishing the safety of raw materials used in the manufacture of personal care products like baby diapers is achieved by using the robust QRA method based upon the conceptual risk assessment framework established by the National Academy of Science (CitationNAS, 1983). QRA is currently used to support the safe introduction of new products and their ingredients into the market for almost all classes of personal care products. CitationKosemund et al. (2009) previously described details of this method, with reference to baby diapers, based on the NAS paradigm.
Being complex, multilayered products, baby diapers are composed of various raw materials that may or may not directly contact the skin. Most of the components of baby diapers are high-molecular-weight, inert polymers that have low bioavailability and are therefore not absorbed through the skin (even though there may be direct skin contact). Nevertheless, exposure evaluation of low-molecular-weight ingredients, like lotion components, perfume ingredients, residual contaminants, residual monomers, or residual process aids, are relevant to ensure overall safety of the raw material and the diaper as a whole.
Exposure to diaper materials not in direct contact with skin may be possible via the phenomenon of reflux, where an aqueous vehicle like urine may help to carry these materials toward the direct skin contact areas of the diaper. This is particularly relevant when diapers are worn overnight with a high urine load. Reflux of a diaper is typically determined by one of two currently existing methods, and these methods provide a conservative, worst-case estimation of the amount of chemical that may be carried toward the skin.
For the purposes of evaluation of exposure to baby diaper raw materials, various parameters like consumer usage habits (number of diapers used per day), surface area of the diaper, top-sheet transfer factor, weight of the baby, and reflux factor are taken into account. These parameters are the foundation for characterizing exposure to a particular diaper raw material. Each of these is evaluated in detail, and depending on the parameter, conservative default assumptions are made or the actual values of the parameter are used for risk assessment. Default assumptions may be replaced with actual values, a process that helps to refine the overall exposure assessment.
Two examples are used to demonstrate the principles of QRA: Citral, a common ingredient in perfumes, is evaluated for its sensitizing potential, and acrylic acid, a constituent ingredient of the super absorbent polymer (SAP), is evaluated for its systemic toxicity potential. An assessment of the skin sensitization potential of citral was demonstrated using the QRA methodology recommended by the COLIPA toxicology advisory group. The large margin of society (MOS >1,000,000) demonstrated by comparing the AEL of citral to the calculated CEL of citral through diaper usage provides overwhelming safety assurance that presence of citral in a diaper product is indeed safe and does not pose an increased risk for skin sensitization when used as a perfume constituent. Similarly, a QRA for acrylic acid shows that this residual monomer present in SAP enjoys a wide MOS (42) with respect to systemic toxicity potential.
The safety of low residual levels of chemicals or contaminants whose toxicological profiles are not known can be confirmed by using the concept of threshold of toxicological concern (TTC). Use of the TTC is based on the fundamental premise that there is an exposure to contaminants or chemicals below which adverse effects will be negligible or absent. The TTC method may be used to drive the establishment of safe levels of residual constituents or contaminants that may be present in diaper raw materials.
Despite variations in diaper usage habits and practices across various countries (and tiers of consumers), and despite the diversity of different diaper raw materials used to construct a diaper, the consistent use of the QRA method for the evaluation of diaper raw materials demonstrated the robustness of the method. The use of QRA helps to ensure that all raw materials used in baby diapers globally are safe for direct skin and indirect skin exposure. The safe introduction of new materials and cutting-edge technologies in baby diapers is ensured by this process. The use of QRA methodology allows consumer goods companies like P&G to continue to innovate with newer and emerging materials, and to safely provide the latest diaper designs and technologies to consumers around the world.
This work was funded by The Procter & Gamble Company. The authors thank Bill Broening, PhD, DABT, for critical review of this article, and Lisa Bosch for help with initial formatting. P. Rai, E. Krause, D. Marsman, and S. Felter are employees of The Procter & Gamble Company.
REFERENCES
- www.nappyinformationservice.co.uk/AHPMA_press_statement_04mar04.htm Absorbent Hygiene Product Manufacturers Association, 2002. [Mintel Market Intelligence Report: Waste Management]
- BlackburnK. L.StickneyJ. A.Carlson-LynchH. L.McGinnisP. M.ChappellL.FelterS. P.Application of the threshold of toxicological concern approach to ingredients in personal and household care productsRegul. Toxicol. Pharmacol.200543249259
- BosJ. D.MeinardiM. H. M.The 500-dalton rule for the skin penetration of chemical compounds and drugsExp. Dermatol.20009165169
- www.edana.org/story.cfm?section=|edana_nonwovens&story=testmethods.xml European Disposables and Nonwovens Association. 2005. Worldwide Strategic Partners: Standard test methods for the nonwoven industry
- FiumeM. Z.Final report on the safety of assessment of acrylates copolymer and 33 related cosmetic ingredientsInt. J. Toxicol.200221S3150
- HerrleinM. K.European Patent EP0797967 A11996
- HopperL. D.OehmeF. W.Chemical risk assessment: A reviewVet. Hum. Toxicol.198931543554
- Institute for Health and Consumer Protection, European Chemicals BureauAcrylic acidOffice for Official Publications of the European CommunitiesLuxembourg2002 European Union risk assessment report, 28: EUR 19836
- www.ifraorg.org/Home/Publications/Download-the-IFRA-Amendments/page.aspx/134 International Fragrance Association. 2006. IFRA standard, 40th Amendment: Citral
- www.inchem.org/documents/ehc/ehc/ehc210.htm International Programme on Chemical Safety. 1999. Environmental Health Criteria 210: Principles for the assessment of risks to human health from exposure to chemicals
- KosemundK.SchlatterH.OchsenhirtJ.KrauseE.MarsmanD.ErasalaG.Safety evaluation of superabsorbent baby diapersRegul. Toxicol. Pharmacol.2009538189
- KroesR.RenwickA.G.CheesemanM.KleinerJ.MangelsdorfI.PiersmaA.SchlatterJ.van SchothorstF.VosJ. G.WurtzenG.Structure-based thresholds of toxicological concern (TTC): Guidance for application to substances present at low levels in the dietFood Chem. Toxicol.2004426583
- LalkoJ.ApiA. M.Citral: Identifying a threshold for induction of dermal sensitizationRegul. Toxicol. Pharmacol.2008516273
- National Academy of ScienceRisk assessment in the federal governmentNational Academy PressWashingtonDC1983 National Research Council
- OdioM.O'ConnorR.SarbaughF.BaldwinS.Continuous topical administration of a petrolatum formulation by a novel disposable diaper. 2. Effect on skin conditionDermatology2000200238243
- www.oecd.org/about/0,2337,en_2649_34373_1_1_1_1_1,00.html Organization for Economic Cooperation and Development. 2007. About chemicals hazard/risk assessment
- www.rifm.org/doc/QRA_Technical%20Dossier%20FINAL%20REV%202006%206%2022_1.pdf QRA Expert Group (A. M. Api, D. A. Basketter, P. A. Cadby, M.-F. Cano, G. Ellis, G. F. Gerberick, P. Griem, P. M. McNamee, C. A. Ryan, and R. Safford). 2006. Dermal sensitization quantitative risk assessment (QRA) for fragrance ingredients, Technical dossier
- Scientific Committee on Cosmetic Products and Non-Food Products Intended for ConsumersOpinion concerning fragrance allergy in consumers.1999 SCCNFP / 0017/98 Final
- cfpub.epa.gov/ncea/iris/index.cfm?fuseaction=iris.showQuickView&substance_nmbr=0002 U.S. Environmental Protection Agency. 1994. Acrylic acid. Integrated Risk Information Systems (IRIS)
- U.S. Food and Drug AdministrationFood additives: Threshold of regulation of substances used in food-contact articles: Final ruleFed. Reg.1995603658236596
- WilliamsP. R. D.PaustenbachD. J.Risk characterization: Principles and practiceJ. Toxicol. Environ. Health B20025337406