Abstract
The acute effects and the time course of fine particulate pollution (PM2.5) on atrial fibrillation/flutter (AF) predictors, including P-wave duration, PR interval duration, and P-wave complexity, were investigated in a community-dwelling sample of 106 nonsmokers. Individual-level 24-h beat-to-beat electrocardiogram (ECG) data were visually examined. After identifying and removing artifacts and arrhythmic beats, the 30-min averages of the AF predictors were calculated. A personal PM2.5 monitor was used to measure individual-level, real-time PM2.5 exposures during the same 24-h period, and corresponding 30-min average PM2.5 concentration were calculated. Under a linear mixed-effects modeling framework, distributed lag models were used to estimate regression coefficients (βs) associating PM2.5 with AF predictors. Most of the adverse effects on AF predictors occurred within 1.5–2 h after PM2.5 exposure. The multivariable adjusted βs per 10-μg/m3 rise in PM 2.5 at lag 1 and lag 2 were significantly associated with P-wave complexity. PM2.5 exposure was also significantly associated with prolonged PR duration at lag 3 and lag 4. Higher PM2.5 was found to be associated with increases in P-wave complexity and PR duration. Maximal effects were observed within 2 h. These findings suggest that PM2.5 adversely affects AF predictors; thus, PM2.5 may be indicative of greater susceptibility to AF.
Several studies suggested an association between ambient air pollution and atrial fibrillation/flutter (AF) (CitationRich et al., 2006), stroke, and cardiovascular disease (CVD) (CitationMiller et al., 2007; CitationPope et al., 2004), but the impact of air pollution on AF risk may have been underestimated (CitationWhitsel and Avery, 2010). Approximately 4% of U.S. adults aged 60 yr or more have been diagnosed with AF (CitationGo et al., 2001). Moreover, AF is a well-established risk factor of ischemic stroke (CitationAtrial Fibrillation Investigators, 1994). AF is also a potent risk factor for heart failure (CitationRoy et al., 2009), and CVD (CitationAtrial Fibrillation Investigators, 1994). However, it is well recognized that a large proportion of AF cases are asymptomatic and intermittent. Thus, it is difficult to investigate AF, either as a consequence of other exposures such as air pollution exposure, or as the cause of stroke and CVD in a healthy population (CitationSoliman et al., 2009). Meanwhile, several studies identified electrocardiogram (ECG) P-wave based measures as predictors of AF, including paroxysmal forms of this arrhythmia (CitationAndrikopoulos et al., 2000; CitationBudeus et al., 2007; CitationDilaveris et al., 1998; CitationGorenek et al., 2007; CitationMaterazzo et al., 2007; CitationMehta et al., 2000; CitationOzdemir et al., 2007; CitationPassman et al., 2001; CitationRaitt et al., 2004; CitationStafford et al., 1997). Thus, these P-wave-based measures are termed AF predictors. Most importantly, CitationSoliman and coworkers (2009) reported significant prospective associations between the AF predictors and the incidence of ischemic stroke in a large middle-aged population-based sample. Therefore, in order to investigate AF pathway as one of the potential mechanisms that link PM exposure and stroke, one can investigate the air pollution effects on the P-wave based AF predictors. Thus, this study was undertaken to examine the acute effect and time course of individual-level exposures to fine PM, defined as particles with aerodynamic diameter ≤2.5 μm (PM2.5), on the AF predictors in a community-dwelling sample of healthy individuals.
MATERIALS AND METHODS
Population
For this report, data collected for the Air Pollution and Cardiac Risk and its Time Course (APACR) study were used; this study was designed to investigate the mechanisms and the time course of the adverse effects of PM2.5 on cardiac electrophysiology, blood coagulation, and systemic inflammation. Recruitment methods and examination procedures for the APACR study were published elsewhere (CitationHe et al., 2010; CitationLiao et al., 2010). Briefly, all study participants were recruited from communities in central Pennsylvania, primarily from the Harrisburg metropolitan area, in which the air pollution level was similar to, if not higher than, other major metropolitan areas on the East Coast of the United States. All participants gave written informed consent prior to their participation in the study. The inclusion criteria for the study included nonsmoking adults ≥45 yr old who had not been diagnosed with severe cardiac problems (defined as diagnosed valvular heart disease, congenital heart disease, acute myocardial infarction or stroke within 6 mo, or congestive heart failure). Approximately 75% of the individuals who were contacted and who met inclusion criteria were enrolled in the APACR study. Our targeted sample size was 100 individuals, and 106 individuals were enrolled and examined for the APACR study.
Study participants were examined at the Pennsylvania State University General Clinical Research Center (GCRC) in the morning between 8 and 10 a.m. on day 1. All participants fasted for at least 8 h prior to the clinical examination. After completing a health history questionnaire, a trained research nurse measured seated blood pressure thrice, height, and weight, and drew 50 ml of blood for biomarker assays according to the blood sample preparation protocols. A trained investigator connected the PM2.5 and Holter ECG recorders. Participants were given an hourly activity log to record special events that occurred over the next 24 h, including outdoor activities, exposure to traffic on the street, traveling in an automobile, and any physical activities. The entire clinical examination session lasted approximately 1 h. Participants were then released to proceed with their usual daily routines. The next morning, the participants returned to the GCRC to remove the PM2.5 and Holter monitors, deliver the completed activity log, have another 50 ml of blood drawn, and provide a urine sample. Then an exercise echocardiography was performed on each participant according to a standardized protocol to measure the participant's ventricular function and structure. The entire day-2 session lasted for about 1 h and 45 min. Penn State University College of Medicine Institutional Review Board approved the study protocol. Each participant received $50, a certificate for breakfast in the hospital cafeteria, and compensation for the mileage associated with travel to and from the GCRC.
Personal PM2.5 Exposures
The APACR study used a personal PM2.5 DataRam (pDR, model 1200, Thermo Scientific, Boston) for real-time 24-h personal PM2.5 exposure assessment. Details of the exposure assessment (CitationHe et al., 2010; CitationLiao et al., 2010) and the instrument's performance were reported elsewhere (CitationHoward-Reed et al., 2000; CitationRea et al., 2001; CitationWallace et al., 2006; CitationWilliams et al., 2009). Real-time PM2.5 concentrations were initially recorded at 1-min intervals. For each participant, 30-min, segment-specific averages were calculated on the whole and half hour, as the PM2.5 exposure variables. The PM2.5 exposure variables therefore consisted of 48 repeated measures on each participant.
Continuous Ambulatory ECG and AF Predictors
A high-fidelity (sampling frequency 1,000 Hz) 12-lead HScribe Holter System (Mortara Instrument, Inc., Milwaukee, WI) was used to collect the 24-h Holter beat-to-beat ECG data. The high-fidelity ECG significantly increases the resolution and enhances the accuracy of various waveform measurements. The details of the Holter ECG data collection and reading were published by CitationHe et al. (2010) and CitationLiao et al. (2010). In brief, the Holter ECG data were scanned to a designated computer for offline processing by an experienced investigator using a modified version of the Holter System software and the SuperECG software (also developed by Mortara Instrument, Inc.). The main objectives of the offline processing were to (1) verify the Holter-identified ECG waves, (2) identify and label additional electronic artifacts and arrhythmic beats in the ECG recording, and (3) perform beat-to-beat ECG analysis used to estimate various ECG waveform parameters. Relevant to this study, the following three P-wave-related measures were calculated as AF predictors:
-
P-wave duration (ms), as the time between the first “onset” and last “offset” deflection from the isoelectric line.
-
PR duration (ms), as the time between the first onset defection of the P and R wave (the latter representing the onset of ventricular depolarization), with longer PR duration indicating higher risk of AF.
-
P-wave complexity (unitless), as the average ratio of the second to the first eigenvalue, the value of which reflects the relative complexity of atrial depolarization (CitationPriori et al., 1997), with more complexity in the atrial depolarization indicating the higher risk of AF.
Heart-Rate Variability (HRV) Variables
Time- and frequency-domain heart-rate variability (HRV) analyses were performed on the ECG recording, after removing artifacts with standardized visual inspection and statistical filters. HRV indices were calculated from 30-min segment-specific recordings, after removing artifacts and ectopic beats, using HRV Analysis System (CitationDepartment of Applied Physics, University of Kuopio, Finland) according to current recommendations (CitationTask Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996), and focused on the following HRV indices as measures of cardiac autonomic modulation (CAM): standard deviation of all normal RR intervals (SDNN, ms), square root of the mean of the sum of the squared differences between adjacent normal RR intervals (RMSSD, ms), power in the low frequency range (0.04–0.15 Hz, LF), power in the high frequency range (0.15–0.4 Hz, HF), and the ratio of LF to HF (LF/HF).
Weather Variables
Real-time temperature and relative humidity were obtained using the HOBO H8 logger (Onset Computer Corporation, Bourne, MA). The real-time temperature and relative humidity were recorded at 1-min intervals initially. For each participant, 30-min segment-specific averages were calculated, corresponding to the PM2.5 and Holter measures. Each weather covariable also consisted of 48 repeated measures on every participant.
Other Participant-Level Covariables
A standardized questionnaire administered on day 1 of the study was used to collect the following individual-level information: (a) demographic variables, including age, race, gender, and highest education level; (b) use of medication, including anti-anginal, antihypertensive, and antidiabetic agents; and (c) physician-diagnosed chronic disease history of CVD (including revascularization procedures and myocardial infarction), hypertension, and diabetes. The averages of the second and third measures of seated systolic and diastolic blood pressures on day 1 were used to represent a participant's blood pressure levels. Day 1 fasting glucose was measured by the Penn State GCRC central laboratory. CVD was defined by anti-anginal medication use or a history of CVD. Hypertension was defined by antihypertensive medication use, physician-diagnosed hypertension, systolic blood pressure ≥140 mm Hg, or diastolic blood pressure ≥90 mm Hg. Diabetes was defined by antidiabetic medication use, physician-diagnosed diabetes, or fasting glucose ≥126 mg/dl. Body mass index (BMI) was defined as the ratio of weight to height squared (BMI, kg/m2).
Statistical Analysis
Distributed lag models (CitationAlmon, 1965; CitationPope & Schwartz, 1996; CitationSchwartz, 2000) were used under a linear mixed-effects models framework (CitationLaird & Ware, 1982) with a first-order autoregressive covariance structure to (1) model the correlation between observations from the same participant and (2) estimate the regression coefficient between 30-min PM2.5 and the AF predictors (the outcome variables). All independent variables were treated as fixed effects, and time, broken into 30-min segments, was included as a categorical variable. This general specification places no specific functional form of time. Residual diagnostics were used to assess the appropriateness of modeling assumption, and no sizeable departures were detected. In these models, one lag indicates a 30-min separation between the exposure and outcome. Thus, lag 0 indicates the spontaneous relationships between PM2.5 and the AF predictors, and lag 1 indicates 30 min between the PM2.5 and AF predictors, and so on. A constrained distributed lag model, the polynomial distributed lag model, was selected to reduce the potential collinearity of PM2.5 between individual lags using a second-degree polynomial. The linear mixed-effects model framework was selected because it allows us to explicitly model the expected correlation between measurements taken from the same participant. As the measurements are equally spaced, the natural choice for correlation structure, autoregressive order 1, was used throughout the analyses to account for the potential autocorrelation. A second-degree polynomial for the distributed lag model was used because the amount of variability in the outcomes of interest explained by the air pollution measures is not large, so that the degree of polynomial needs to be selected parsimoniously. In our experience, second-degree polynomials perform adequately, which is supported by Schwarz (2000). Another advantage of the distributed lag model is its ability to facilitate interpretation of the cumulative effects of the lags included in the model, as well as individual lag effects. Because the PM2.5 and ECG variables were assessed in parallel over 48 lags (24 h), the cut off was decided a priori to model no more than 10 lags was decided, which allowed us to fit the distributed lag model using at least 75% of the data. Using P-wave complexity as the primary outcome variable, models were initially fitted containing the largest number of lags (lag 0–10) determined a priori, and reduction in the total number of individual lags was made by back-eliminating the longest (e.g., lag 10) in succession until a significant cumulative effect (p < .05) was identified (lag 0 to lag 3 for P-wave complexity in this report). This model was then used as our final model for the other two AF predictors (PR interval and P durations). Because the cumulative effects including lag 0 to lag 3 in the model were not significant for these two AF predictors, one more lag (lag 4) was forced into the model to avoid potential underascertainment of significant cumulative effect on these two AF predictors. All results are expressed per 10-μg/m3 increase in PM2.5. In the final models, all time-dependent covariables, such as weather and HRV variables, were entered in the models using the same distributed lag structure as the PM2.5 variable. SAS version 9 (SAS Institute, Inc., Cary, NC) was used for all analyses. The criterion for significance was set at p < .05.
RESULTS
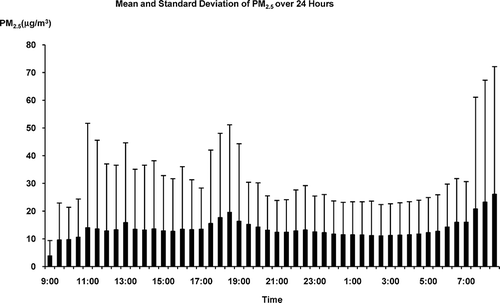
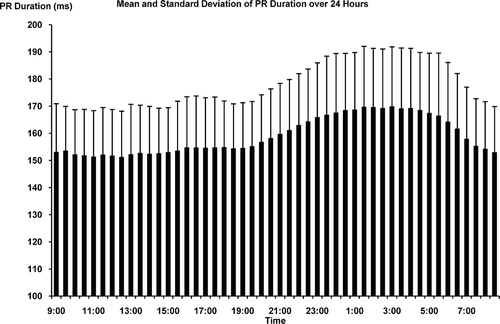
The demographic and CVD risk profiles of the study population are presented in . The mean age of the participants was 56 yr, with 74% non-Hispanic white, 26% minorities (including Blacks, Hispanics, and Chinese), 59% female, and 43% having chronic diseases (mostly hypertension). At the population level, the distributions of both the PM2.5 exposure and AF predictors were approximately normal. The temporal distributions of the PM2.5 and PR duration are presented in and , each of which plots means and standard deviations versus time of day. Both the PM2.5 and AF predictor exhibited substantial variation within 24 h, and within 30-min averaging periods between participants. Excluding 48 (0.94%) of the 30-min segments in which the proportion of artifacts and arrhythmic beats exceeded 20% of recording time left 5040 pairs of 30-min PM2.5 exposure and AF predictor data points collected from 106 individuals over a 24-h period.
TABLE 1. Demographic Characteristics and Health Status of the Study Population
The cumulative effects and individual lag effects of PM2.5 on each of the AF predictors are summarized in as multivariable adjusted regression coefficients β (SE, p value) associated with PM2.5 exposure (per 10-μg/m3 increment in PM2.5). In summary, elevated PM2.5 was positively associated with P-wave complexity and PR duration. A significant cumulative effect was observed for P-wave complexity and a quantitative cumulative effect on PR duration. PM2.5 was not significantly associated with P-wave duration. Examining the individual lag effects, the multivariable adjusted regression coefficients for a 10-μg/m3 rise in PM2.5 were significant for lag 1 and lag 2 (approximately 0.5–1 h after the elevation of PM2.5) for P-wave complexity, and lag 3 and lag 4 (approximately 1.5–2 h after PM2.5 increase) for PR duration, respectively. In summary, both individual lag effects and cumulative effects from the data presented in suggest an acute response of these AF predictors to PM2.5 exposures. Additional adjustment for chronic disease (model 2 in ) did not change the pattern of association from the models adjusted only for major demographic and weather-related variables (model 1 in ).
TABLE 2. Regression Coefficient of AF Predictors Associated With a 10-μg/m3 Increment of PM2.5 Concentration
To elucidate the role of CAM on the relationship between PM2.5 exposure and AF predictors, model 2 in was repeated for P-wave complexity and PR duration outcomes after adjusting for HRV and heart rate (HR) measures, using one HRV or HR variable at a time. The HRV and HR adjusted regression coefficients are presented in . In summary, the overall patterns of associations between PM2.5 and these two AF predictors were greatly attenuated, but mostly remained statistically significant with additional adjustment for HRV and HR variables as measures of CAM.
TABLE 3. Multivariable Adjusted Regression Coefficient (SE, p Value) of AF Predictors Associated With a 10-μg/m3 Increment of PM2.5 With Additional Adjustment for HRV Variables
The interaction terms between PM2.5 and CVD-related chronic conditions were tested; none were statistically significant (data not shown). Therefore, the effects of PM2.5 on P-wave-related variables did not differ depending on whether an individual had previous CVD-related conditions. Stratified analysis according to chronic disease status revealed similar associations among participants with and without CVD-related conditions (data not shown).
DISCUSSION
Although epidemiologic studies consistently showed an association between exposure to increased PM air pollution and risk of CVD (CitationDominici et al., 2005; CitationFranklin et al., 2007, Citation2008; CitationKrewski et al., 2005; CitationOstro et al., 2006; CitationYang et al., 2004; CitationZanobetti & Schwartz, 2009) and stroke (CitationMiller et al., 2007; CitationPope et al., 2004), the mechanisms responsible for such an association have not been fully identified. Since AF is a well-established risk factor for stroke, it is biologically plausible that PM and stroke relationship may be mediated in part by PM-related increases in AF risk. This potential mechanism is supported by data from CitationRich and coworkers (2006), who reported an elevated risk of paroxysmal AF after an acute gaseous air pollution exposure in patients wearing implantable cardioverter-defibrillators (ICDs). The association, however, may not be generalizable to a healthy population. Indeed, there has been no publication of such a relationship in healthy community-based samples. Given the asymptomatic and intermittent nature of AF in healthy individuals and the established relationship between AF predictors and future AF and the risk of incident stroke, this study was conducted to examine the temporal relationship and between PM2.5 exposures and AF predictors in a healthy community-dwelling sample of nonsmokers, and through such association to elucidate the PM2.5 effects on susceptibility to AF and stroke. The operational hypothesis is that acute exposures to PM2.5 are associated with predictors of AF. If the association is confirmed, this would suggest that exposure to PM2.5 is also associated with higher risk of AF development and thereby stroke.
In this study, acute exposure to PM2.5 significantly affected P-wave complexity and PR duration, but not P-wave duration. Overall, these findings support our a priori hypothesis. Specifically, data suggested that elevated PM2.5 was associated with more complex P-wave morphology and longer PR duration, and both are indicative of disturbed atrial depolarization. Similar to our previous findings, in the same population, of PM2.5 effects on HRV (CitationHe et al., 2010) or QT intervals (CitationLiao et al., 2010), the effect size of PM2.5 on AF predictors is relatively small. From the etiologic perspective, the small effect indicates a weak influence of PM2.5 on risk of AF. Therefore, the changes of AF predictors due to PM exposure in this population-based healthy sample may not be clinically relevant. However, considering the fact that the entire U.S. population is exposed to ambient, indoor, and personal sources of PM2.5, on a continuous, daily basis, minor PM2.5 elevation may possess a greater impact on the cardiovascular health of the public than is generally appreciated. Moreover, the effects on AF predictors in this study were measured in generally healthy individuals. It is possible that PM2.5 effects on AF predictors may be greater among those with underlying structural heart disease, ischemic heart disease, or heart failure, although the lower power tests of such interactions in this context did not support this possibility. Future studies could target clinical subgroups likely to be more PM-susceptible, especially those rendered more vulnerable by residential proximity to important PM2.5 sources, e.g., highways.
Although several animal-based studies showed that PM (CitationGordon et al., 2000), diesel exhaust (CitationCampen et al., 2003), cigarette smoking (CitationRai et al., 1983), and highly toxic fly ash PM (CitationHazari et al., 2009) exposure were associated with PR prolongation, the mechanisms responsible for PM induced changes in P-wave based ECG measures are not fully understood. The results presented in indicate that the magnitude of association between PM2.5 and P-complexity and PR duration were greatly attenuated but mostly remained statistically significant after adjusting for cardiac autonomic modulation as potential intermediating factors. To our knowledge, this is the first study to demonstrate the above summarized findings in a healthy community-dwelling sample. If such an attenuation effect can be confirmed by other human- and animal-based studies, this would suggest that PM exposure first produces an acute imbalance of autonomic modulation, characteristic of increased sympathetic and decreased parasympathetic modulations. Through the autonomic modulation mechanism, PM might lead to disturbances of P-wave-based ECG parameters that are indicative of higher susceptibility to AF. Other potential mechanisms may include, first, PM induced coronary artery constriction, which is supported by the observation by CitationBrook and coworkers (2002), who reported a sudden conduit vasoconstriction after a short-term PM2.5 and O3 exposure in a sample of healthy volunteers. CitationMills and coworkers (2005) also demonstrated similar patterns. Second, PM may also decrease the oxygen–carrying capacity of the blood, as supported by studies reported by several investigators (CitationClarke et al., 2000; CitationSavage et al., 2002; CitationSeaton et al., 1999). Lastly, it is also plausible that PM exerts a direct adverse effect on cardiac electrophysiological parameters, such as AF predictors examined in this study. More mechanistic studies are needed to investigate the exact pathways from PM exposure to the adverse cardiac electrophysiological effects. Considering the large attenuation of PM effects on P-wave complexity and PR duration, after adjustment of HRV and HR, cardiac autonomic modulation was considered as the predominant mechanism.
The strengths of our study include that both the PM2.5 exposure and the AF predictors were measured from individual levels on a real-time basis over 24 h. The exposure and the outcome data were treated as 30-min repeated measures, and thus benefited from increased statistical power in associating the exposure and outcomes. This type of data also enabled us to control for many individual-level unmeasured factors that may influence the AF predictors. Although the results generated from this study are compelling, there are still several limitations. First, smokers and people who had severe cardiac events in the past 6 mo were excluded. Therefore, one may not be able to generalize the association seen in these data to individuals with more severe CVD. It is possible that PM2.5 effects on AF predictors might be greater in individuals with underlying structural heart disease, ischemic heart disease, or heart failure. Second, most of the participants reported that they stayed indoors the majority of the time during the 24-h study period, except when they had to travel by automobile. This behavior pattern is reflected in the relatively low levels of exposure to PM2.5. In general, our participants had limited indoor exposures, e.g., secondhand smoke. Thus, it was not possible to assess whether exposures at much higher levels would exhibit similar associations. However, the personal monitors and real-time Holter system were purposely used to collect the true individual-level exposure and routine ECG data, respectively. The associations observed in these individuals may be more reflective of their routine exposure and outcome associations. Third, it is well known that HR increases during physical activity, which could be related to higher PM2.5 exposure if it occurs outdoors. It is also well known that PR duration is HR dependent. Thus it is possible that the PM2.5 and AF predictors association in this study is confounded by physical activity that occurred during some segment of the 24-h study period. However, since most of participants reported that they stay indoors for the vast majority of the time during 24-h period, it is unlikely that the association is solely due to physical activity outside of their homes, which is also supported by the findings of significant associations between PM2.5 and AF predictors after adjustment of lag-specific HR in . Fourth, the Holter ECG data were not collected in a controlled setting or the conventional supine position. Thus, the short-term variation of other factors that may impact the ECG measures can not be fully accounted for. However, it is not feasible to keep a healthy participant in a supine indoor position for 24 h. Even if this were achieved, the results from such a study design would likely have limited variation in PM2.5 exposure levels, and would have limited utility for generalization to a real world situation. In contrast, our study captures the range of activities occurring in real life, including time spent outdoors, time spent commuting in an automobile, and various other activities undertaken by typical community-dwelling individuals free of chronic disease. Fifth, the sample size of this study is small, and individuals with chronic conditions consisted mostly of well-controlled hypertensives. As a result, the statistical power was limited to detect significant effect modification by chronic disease status. Lastly, pDR estimated PM2.5 concentrations over 24 h were used in this study as an estimation of personal exposures. This nephelometric device responds to the optical as opposed to the true gravimetric properties of the particles it encounters. It should be recognized that the optical properties (calibrated using Arizona road dust) might not be highly representative of the actual PM2.5 aerosol the study participants encountered. Previous studies reported that the Arizona road dust calibrated pDR, identical to the one used in this study, provides mass concentration estimates in reliable agreement with that from gravimetric mass-based measures (correlation coefficients >0.8), but 10–50% higher than those from gravimetric mass-based measures (CitationHoward-Reed et al., 2000; CitationRea et al., 2001; CitationWilliams et al., 2009). Based on these validation studies, the personal exposures used in this study might systematically overestimate the true environmental conditions that existed. However, this systematic overestimation of the true exposures to PM2.5 should not have biased the pattern of the observed associations. Under the assumption that the pDR systematically overestimates the true exposure by 10–50%, it can be argued that the reported effect sizes per 10-μg/m3 increase in the pDR estimated PM2.5 reported here actually represents effects per 6.67–9 μg/m3 increase in the true PM2.5 exposure. By extension, this implies that the reported effect in this study may be systematically underestimated.
In summary, acute exposure to PM2.5 at the individual level is directly associated with longer PR duration and more complexity of atrial depolarization, and the time to such an effect is approximately 1.5–2 h. The adverse effect of PM2.5 on these AF predictors is independent of major confounding factors and cannot be attributed solely to its autonomic effects. Overall, these findings support the possibility that PM2.5 may affect vulnerability to atrial fibrillation/flutter, and partly through such a mechanism, higher exposures to PM2.5 increase the risk of AF and ischemic stroke.
Acknowledgments
This study is funded by NIEHS (1 R01 ES014010). The authors thank David Mortara of Mortara Instrument, Inc., for providing the SuperECG software for the analysis of the electrocardiographic data.
REFERENCES
- Almon , S. 1965 . The distributed lag between capital appropriations and expenditures . Econometrica , 33 : 178 – 196 .
- Andrikopoulos , G. K. , Dilaveris , P. E. , Richter , D. J. , Gialafos , E. J. , Synetos , A. G. and Gialafos , J. E. 2000 . Increased variance of P wave duration on the electrocardiogram distinguishes patients with idiopathic paroxysmal atrial fibrillation . Pacing Clin. Electrophysiol. , 23 : 1127 – 1132 .
- Atrial Fibrillation Investigators . 1994 . Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials . Arch. Intern. Med. , 154 : 1449 – 1457 .
- Brook , R. D. , Brook , J. R. , Urch , B. , Vincent , R. , Rajagopalan , S. and Silverman , F. 2002 . Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults . Circulation , 105 : 1534 – 1536 .
- Budeus , M. , Felix , O. , Hennersdorf , M. , Wieneke , H. , Erbel , R. and Sack , S. 2007 . Prediction of conversion from paroxysmal to permanent atrial fibrillation . Pacing Clin. Electrophysiol. , 30 : 243 – 252 .
- Campen , M. J. , McDonald , J. D. , Gigliotti , A. P. , Seilkop , S. K. , Reed , M. D. and Benson , J. M. 2003 . Cardiovascular effects of inhaled diesel exhaust in spontaneously hypertensive rats . Cardiovasc. Toxicol. , 3 : 353 – 361 .
- Clarke , R. W. , Coull , B. , Reinisch , U. , Catalano , P. , Killingsworth , C. R. , Koutrakis , P. , Kavouras , I. , Murthy , G. G. , Lawrence , J. , Lovett , E. , Wolfson , J. M. , Verrier , R. L. and Godleski , J. J. 2000 . Inhaled concentrated ambient particles are associated with hematologic and bronchoalveolar lavage changes in canines . Environ. Health Perspect. , 108 : 1179 – 1187 .
- Department of Applied Physics, University of Kuopio, Finland. The HRV Analysis Software. http://venda.uku.fi/research/biosignal (http://venda.uku.fi/research/biosignal) (Accessed: 10 January 2009 ).
- Dilaveris , P. E. , Gialafos , E. J. , Sideris , S. K. , Theopistou , A. M. , Andrikopoulos , G. K. , Kyriakidis , M. , Gialafos , J. E. and Toutouzas , P. K. 1998 . Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation . Am. Heart J. , 135 : 733 – 738 .
- Dominici , F. , McDermott , A. , Daniels , M. , Zeger , S. L. and Samet , J. M. 2005 . Revised analysis of the National Morbidity, Motality, and Air Pollution Study: Mortality among residents of 90 cities . J. Toxicol. Environ. Health A , 68 : 1071 – 1092 .
- Franklin , M. , Zeka , A. and Schwartz , J. 2007 . Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities . J. Expos. Sci. Environ. Epidemiol. , 17 : 279 – 287 .
- Franklin , M. , Koutrakis , P. and Schwartz , P. 2008 . The role of particle composition on the association between PM2.5 and mortality . Epidemiology , 19 : 680 – 689 .
- Go , A. S. , Hylek , E. M. , Phillips , K. A. , Chang , Y. , Henault , L. E. , Selby , J. V. and Singer , D. E. 2001 . Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study . J. Am. Med. Assoc. , 285 : 2370 – 2375 .
- Gordon , T. , Nadziejko , C. , Chen , L. C. and Schlesinger , R. 2000 . Effects of concentrated ambient particles in rats and hamsters: An exploratory study . Res. Rep. Health Effects Inst. , 93 : 5 – 34 . discussion 35–42
- Gorenek , B. , Parspur , A. , Timuralp , B. , Birdane , A. , Ata , N. , Cavusoglu , Y. and Unalir , A. 2007 . Atrial fibrillation after percutaneous coronary intervention: Predictive importance of clinical, angiographic features and P-wave dispersion . Cardiology , 107 : 203 – 208 .
- Hazari , M. S. , Haykal-Coates , N. , Winsett , D. W. , Costa , D. L. and Farraj , A. K. 2009 . A single exposure to particulate or gaseous air pollution increases the risk of aconitine-induced cardiac arrhythmia in hypertensive rats . Toxicol. Sci. , 112 : 532 – 542 .
- He , F. , Shaffer , M. , Li , X. , Rodriguez-Colon , S. , Wolbrette , D. , Williams , R. , Cascio , W. and Liao , D. 2010 . Individual-level PM(2.5) exposure and the time course of impaired heart rate variability: The APACR study . J. Expos. Sci. Environ. Epidemiol , 21 : 65 – 73 .
- Howard-Reed , C. , Rea , A. W. , Zufall , M. J. , Burke , J. M. , Williams , R. W. , Suggs , J. C. , Sheldon , L. S. , Walsh , D. and Kwok , R. 2000 . Use of a continuous nephelometer to measure personal exposure to particles during the U.S. Environmental Protection Agency Baltimore and Fresno Panel studies . J. Air Waste Manage. Assoc. , 50 : 1125 – 1132 .
- Krewski , D. , Burnett , R. , Jerrett , M. , Pope , C. A. 3rd , Rainham , D. , Calle , E. , Thurston , G. and Thun , M. 2005 . Mortality and long-term exposure to ambient air pollution: Ongoing analyses based on the American Caner Society cohort . J. Toxicol. Environ. Health A , 68 : 1093 – 1109 .
- Laird , N. M. and Ware , J. H. 1982 . Random-effects models for longitudinal data . Biometrics , 38 : 963 – 974 .
- Liao , D. , Shaffer , M. L. , Rodriguez-Colon , S. , He , F. , Li , X. , Wolbrette , D. L. , Yanosky , J. and Cascio , W. E. 2010 . Acute adverse effects of fine particulate air pollution on ventricular repolarization . Environ. Health Perspect. , 118 : 1010 – 1015 .
- Materazzo , C. , Piotti , P. , Mantovani , C. , Miceli , R. and Villani , F. 2007 . Atrial fibrillation after non-cardiac surgery: P-wave characteristics and Holter monitoring in risk assessment . Eur. J. Cardiothorac. Surg. , 31 : 812 – 816 .
- Mehta , A. , Jain , A. C. , Mehta , M. C. and Billie , M. 2000 . Usefulness of left atrial abnormality for predicting left ventricular hypertrophy in the presence of left bundle branch block . Am. J. Cardiol. , 85 : 354 – 359 .
- Miller , K. A. , Siscovick , D. S. , Sheppard , L. , Shepherd , K. , Sullivan , J. H. , Anderson , G. L. and Kaufman , J. D. 2007 . Long-term exposure to air pollution and incidence of cardiovascular events in women . N. Engl. J. Med. , 356 : 447 – 458 .
- Mills , N. L. , Tornqvist , H. , Robinson , S. D. , Gonzalez , M. , Darnley , K. , MacNee , W. , Boon , N. A. , Donaldson , K. , Blomberg , A. , Sandstrom , T. and Newby , D. E. 2005 . Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis . Circulation , 112 : 3930 – 3936 .
- Ostro , B. , Broadwin , R. , Green , S. , Feng , W. and Lipsett , M. 2006 . Fine particulate air pollution and mortality in nine California counties: Results from CALFINE . Environ. Health Perspect. , 114 : 29 – 33 .
- Ozdemir , O. , Soylu , M. , Demir , A. D. , Topaloglu , S. , Alyan , O. , Geyik , B. and Kutuk , E. 2007 . P-wave durations in patients experiencing atrial fibrillation during exercise testing . Angiology , 58 : 97 – 101 .
- Passman , R. , Beshai , J. , Pavri , B. and Kimmel , S. 2001 . Predicting post-coronary bypass surgery atrial arrhythmias from the preoperative electrocardiogram . Am. Heart J. , 142 : 806 – 810 .
- Pope , C. A. 3rd and Schwartz , J. 1996 . Time series for the analysis of pulmonary health data . Am. J. Respir. Crit. Care Med. , 154 : S229 – S233 .
- Pope , C. A. 3rd , Burnett , R. T. , Thurston , G. D. , Thun , M. J. , Calle , E. E. , Krewski , D. and Godleski , J. J. 2004 . Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease . Circulation , 109 : 71 – 77 .
- Priori , S. G. , Mortara , D. W. , Napolitano , C. , Diehl , L. , Paganini , V. , Cantu , F. , Cantu , G. and Schwartz , P. J. 1997 . Evaluation of the spatial aspects of T-wave complexity in the long-QT syndrome . Circulation , 96 : 3006 – 3012 .
- Rai , U. C. , Mohan , M. , Deswal , K. and Srinivasan , V. 1983 . Effect of cowdung smoke inhalation on E.C.G. of rats . Indian J. Physiol. Pharmacol. , 27 : 146 – 150 .
- Raitt , M. H. , Kusumoto , W. , Giraud , G. D. and McAnulty , J. H. 2004 . Electrophysiologic predictors of the recurrence of persistent atrial fibrillation within 30 days of cardioversion . Am. J. Cardiol. , 93 : 107 – 110 .
- Rea , A. W. , Zufall , M. J. , Williams , R. W. , Sheldon , L. and Howard-Reed , C. 2001 . The influence of human activity patterns on personal PM exposure: A comparative analysis of filter-based and continuous particle measurements . J. Air Waste Manage. Assoc. , 51 : 1271 – 1279 .
- Rich , D. Q. , Mittleman , M. A. , Link , M. S. , Schwartz , J. , Luttmann-Gibson , H. , Catalano , P. J. , Speizer , F. E. , Gold , D. R. and Dockery , D. W. 2006 . Increased risk of paroxysmal atrial fibrillation episodes associated with acute increases in ambient air pollution . Environ. Health Perspect. , 114 : 120 – 123 .
- Roy , D. , Talajic , M. , Dubuc , M. , Thibault , B. , Guerra , P. , Macle , L. and Khairy , P. 2009 . Atrial fibrillation and congestive heart failure . Curr. Opin. Cardiol. , 24 : 29 – 34 .
- Savage , S. T. , Lawrence , J. E. , Coull , B. A. , Okabe , K. , Wolde-Mariam , W. and Wellenius , G. A. 2002 . Hematologic response to inhalation of concentrated air particles (CAPs) and transient myocardial ischemia (MI) . Am. J. Respir. Crit. Care Med. , 165 : A304
- Schwartz , J. 2000 . The distributed lag between air pollution and daily deaths . Epidemiology , 11 : 320 – 326 .
- Seaton , A. , Soutar , A. , Crawford , V. , Elton , R. , McNerlan , S. , Cherrie , J. , Watt , M. , Agius , R. and Stout , R. 1999 . Particulate air pollution and the blood . Thorax , 54 : 1027 – 1032 .
- Soliman , E. Z. , Prineas , R. J. , Case , L. D. , Zhang , Z. and Goff , D. C. J. 2009 . Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study . Stroke , 40 : 1204 – 1211 .
- Stafford , P. J. , Cooper , J. , Fothergill , J. , Schlindwein , F. , deBono , D. P. and Garratt , C. J. 1997 . Reproducibility of the signal averaged P wave: Time and frequency domain analysis . Heart , 77 : 412 – 416 .
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . 1996 . Heart rate variability: Standards of measurement, physiological interpretation and clinical use . Circulation , 93 : 1043 – 1065 .
- Wallace , L. , Williams , R. , Rea , A. and Corghan , C. 2006 . Continuous weeklong measurements of personal exposures and indoor concentrations of fine particles for 37 health-impaired North Carolina residents for up to four seasons . Atmos. Environ. , 40 : 399 – 414 .
- Whitsel , E. A. and Avery , C. L. 2010 . The environmental epidemiology of atrial arrhythmogenesis . J. Epidemiol. Commun. Health , 64 : 587 – 590 .
- Williams , R. , Rea , A. , Vette , A. , Croghan , C. , Whitaker , D. , Stevens , C. , McDow , S. , Fortmann , R. , Sheldon , L. , Wilson , H. , Thornburg , J. , Phillips , M. , Lawless , P. , Rodes , C. and Daughtrey , H. 2009 . The design and field implementation of the Detroit Exposure and Aerosol Research Study . J. Expos. Sci. Environ. Epidemiol. , 19 : 643 – 659 .
- Yang , C. , Chen , Y. , Yang , C. and Ho , S. 2004 . Relationship between ambient air pollution and hospital admissions for cardiovascular disease in Kaohsiung, Taiwan . J. Toxicol. Environ. Health A , 67 : 483 – 493 .
- Zanobetti , A. and Schwartz , J. 2009 . The effect of fine and coarse particulate air pollution on mortality: A national analysis . Environ. Health Perspect. , 117 : 898 – 903 .