ABSTRACT
Safety issues regarding consumer products contaminated with trace amounts of chemicals are of great concern to consumers, with the degree of concern occasionally escalating to the psychological syndrome, chemophobia, i.e., the fear of chemicals. Hazardous substances frequently implicated in safety concerns include heavy metals (arsenic, mercury, cadmium, and lead), volatile organic compounds (VOC) such as benzene and o-toluidine, pesticides, carcinogens, radioactive substances, and endocrine disrupting chemicals (EDC) such as bisphenol A and phthalates. To improve communication of risk to society, members of academia, government, consumer organizations, and industry participated in this workshop to discuss and exchange perspectives on trace chemical safety. From the perspective of academia, integrated risk assessments need to be implemented to encompass various exposure sources and routes. The identification and investigation of new exposure-related biomarkers are also recommended to verify direct causal relationships between specific chemical exposure and effects on human health. As for regulation, governments need to establish and maintain acceptable limits for trace chemicals in products. In addition, harmonized efforts need to be undertaken among government agencies to share regulatory limits and effectively control trace chemicals in consumer products. Manufacturers need to faithfully abide by Good Manufacturing Practice (GMP) guidelines, monitor sources of contamination, and minimize these for consumer safety. To effectively resolve safety issues arising from trace chemicals exposure, collaborative efforts are needed involving academia, government, consumer organizations, and industry. Further, scientific evidence-based risk assessment is a critical approach to effectively manage trace chemical safety issues.
Introduction
Household items which are used are quite diverse and include cosmetics, detergents, bath supplies, toys, diapers, and clothing contain or are synthesized from chemicals. Although consumers demand safe household goods, safety issues regarding chemicals present in these products may arise. Trace amounts of chemicals that produce these safety issues in consumer products include heavy metals, carcinogens, endocrine-disrupting chemicals (EDC), pesticides, and radioactive substances (Koo and Lee Citation2004; Lim et al. Citation2018a, Citation2018b).
Consumer concerns regarding the dangers of harmful substances have been growing over time, and consumers have developed a vague sense of anxiety termed “chemophobia” (Hartings and Fahy Citation2011; Francl Citation2013) with respect to impurities and/or trace quantities of chemicals that are unintentionally present in daily necessities. Trace chemicals are defined as substances unavoidably present in natural or synthetic materials in minuscule amounts, from parts per million (ppm) to parts per billion (ppb) or parts per trillion (ppt) levels (Zenobio et al. Citation2015). Trace elements or metals are categorized into three groups by the World Health Organization (WHO Citation1973): essential, probably essential, and potentially toxic. Toxic elements are of concern in terms of public health and there is public demand for effective regulation. However, it is not always simple in practice to regulate trace chemicals unlike other hazardous chemicals because these substances are not intentionally added and often arise unintentionally as contaminants during manufacturing processes or from unknown sources. This indicates that trace chemicals may occur ubiquitously from both identified and unidentified sources and detected levels of these substances are generally within or similar to typical background levels in consumer products (Lim et al. Citation2018b, Citation2018c).
Although chemical substances that are harmful to humans need to be removed from daily necessities, the presence of trace chemicals in household items due to various pollutants resulting from human activities might be completely unavoidable.
Various harmful compounds have been detected in human blood and urine, including heavy metals, volatile organic compounds (VOC), metabolites of polycyclic aromatic hydrocarbons (PAH), phthalates, phenolic substances, and pesticides (Choi et al. Citation2017; Tohon et al. Citation2018; Tsai et al. Citation2018; Wallace, Kormos, and Pleil Citation2016). These findings indicate that the individuals are continuously exposed to a variety of harmful substances (Serrazina et al. Citation2018; Harmon et al. Citation2018). However, because of the diverse potential exposure sources and range of blood levels detected of these substances, it is difficult to determine how direct exposure to these harmful substances is related to the use of household products. In addition, it should be noted that advances in analytical technologies have enabled the detection of these trace chemicals at previously undetectable infinitesimal levels.
The purpose of this review was to analyze the perspectives of experts in academia, regulatory agencies, consumer organizations, and industry regarding the handling of trace chemicals that may exist in consumer goods, as well as attempt to resolve the problems related to trace chemical safety by discussing how to efficiently manage the associated risks through mutual risk communication.
Common trace chemical safety issues in consumer products
The safety issues of chemicals in household goods have been raised for a variety of products. Levels of harmful chemical substances in cosmetics are regulated by individual governments, although the regulations differ from country to country. The European Union (EU) banned completely more than 1,300 chemicals, known or suspected to cause cancer, genetic mutations, reproductive harm, or birth defects, attributed to use in cosmetics. The list of banned chemicals is presented in Annex II and the restricted substances are listed in Annex III of the EU Cosmetics Regulation (EU Citation2018). More than 300 substances are allowed for use with restrictions (Regulation (EC) NO 1223/2009). The U.S. FDA (Citation2018a) does not require cosmetic ingredients and products to be approved before they go onto the market under the Federal Food, Drug, and Cosmetic Act (FD&C Act), except for color additives not intended for employment as coal tar hair dyes. Therefore, manufacturers have a legal responsibility to manage the safety of their products and ingredients. Some chemicals banned in the EU are still commonly utilized in products in the United States. In Canada, Health Canada regulates cosmetic ingredients through the Cosmetic Regulations, under the Food and Drug Act, and a “hotlist” containing more than 500 substances prohibited or restricted for cosmetic use (Health Canada Citation2018).
In South Korea, the Safety Standard for Cosmetics banned 1,065 substances in cosmetics and allows restricted use of 75 substances, 59 preservatives, 126 colorants, 30 sunblock agents, and 45 hair dyes (MFDS Citation2017). In China, more than 1,200 substances have been banned and utilization of 51 preservatives, 157 colorants, 27 sunblock agents, and 75 dyes is restricted in cosmetics (SFDA Citation2015).
The following are examples of trace chemicals and harmful substances that have often been detected in products and led to safety issues and consequent public backlash.
Heavy metals
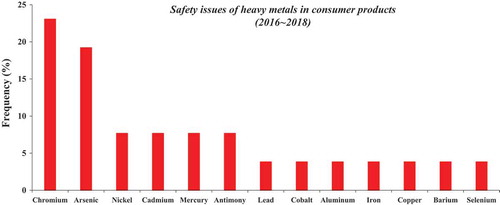
The detection of heavy metals in cosmetics and associated safety issues have frequently appeared in public discourse (). In the past, mercury (Hg), lead (Pb), and arsenic (As) were used as skin whiteners but their trace presence in cosmetics is now strictly regulated. However, cases of unintentional inclusion of heavy metals such as As, Pb, Hg, cadmium (Cd), cobalt (Co), nickel (Ni), antimony (Sb), and chromium (Cr) were found in cosmetics (Bocca et al. Citation2014; Roy et al. Citation2018). Therefore, public awareness of potential safety problems related to heavy metals has been raised, and consumers have developed anxiety regarding the use of such products (Asia Times Citation2018; Lim et al. Citation2018a). Some heavy metals are carcinogenic while others induce skin sensitization. Aluminum (Al) is a light metal that was also detected in various cosmetic products, including color cosmetics, face and body care products, hair cosmetics, and herbal cosmetics (Borowska and Brzóska Citation2015). Higher levels of metals are more frequently present in color cosmetics (lipsticks, lip glosses, eyeshadows, compact powders, and foundation creams) than other types of cosmetics (Kaličanin and Velimirović Citation2016; Lim et al. Citation2018a). Heavy metals tend to accumulate in the body and may upon chronic exposure exert adverse effects in a variety of organs. Therefore, it was suggested that heavy metal levels in cosmetics available on the market need to be routinely monitored to ensure these metal concentrations are within acceptable limits. In the U.S., Hg is not allowed in cosmetic products, except at less than 1 ppm, and only if its presence is unavoidable under Good Manufacturing Practice (GMP) (CFR Citation2018a). The maximum level of Hg was established at 65 ppm when used as a preservative in a cosmetic only intended for use in the eye area, and when no effective and safe non-mercurial substitute preservative is available. The level of As, Pb, and Hg should not exceed 3 ppm, 10 ppm, and 1 ppm, respectively, in the color additives §74.2052 D&C Black No. 2 and §74.2705 FD&C Yellow No. 5 used in cosmetics under GMP (CFR Citation2018a). In China, the maximum allowable amounts of Hg, Pb, and As in cosmetics were established at 1 mg/kg, 10 mg/kg, and 2 mg/kg, respectively, while Cd should not exceed 5 mg/kg (SFDA Citation2015).
Carcinogens
Potential carcinogens which might contaminate cosmetics include dioxins, nitrosamines, 1,4- dioxane, benzo[a]pyrene, asbestos, and acrylamide. The International Agency for Research on Cancer (IARC) has classified such chemicals as human carcinogens (Group 1), probable human carcinogens (Group 2A), and possible human carcinogens (Group 2B) based upon human or animal evidence (IARC Citation2018). In January 2017, the French magazine 60 Millions de Consommateurs (60 Million Consumers) reported that dioxins and pesticides were detected in disposable diapers (The Local Citation2017). In addition to diapers, cases of dioxins detected in other household goods such as tampons were reported (DeVito and Schecter Citation2002; Shin, Ahn, and Seo Citation2005). 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is an EDC classified as a human carcinogen (Group 1) by IARC. Therefore, public anxiety regarding TCDD presence in diapers may be of greater concern than detection of other instances of trace chemicals in household products as this compound is recognized to exert toxic effects for babies in developmental stages (WHO Citation2011).
Nitrosamines, such as N-nitrosodimethylamine (NDMA, Group 2A), N-nitrosodiethylamine (NDEA, Group 2A), and N-nitrosodiethanolamine (NDELA, Group 2B), are often detected in consumer products and classified as probable (Group 2A) or possible (Group 2B) human carcinogens by IARC (Lim et al. Citation2018b; IARC Citation2018). The Scientific Committee on Consumer Safety (SCCS) suggested in 2012 that a limit of 50 µg/kg for nitrosamines needs to be applied to both raw materials and finished products (SCCS Citation2012). In 2018, the anti-hypertensive drug valsartan was recalled from market in many countries including Asia (South Korea, China, Taiwan, Japan, etc.), Europe (Germany, Italy, the Netherlands, Austria, etc.), and North America (Canada, USA).
1,4-Dioxane (Group 2B) is not intentionally added to consumer products but may be generated as a byproduct during the manufacturing process due to the ethoxylation of surfactants employed in shampoos, detergents, and cosmetics (SCCS Citation2015). Acceptable limits vary by country: the SCCS suggests that a trace quantity of 1,4-dioxane ≤10 ppm, representing a lifetime cancer risk (LCR) ≤10−5, in cosmetic products is considered safe (SCCS Citation2015). In contrast, 1,4-dioxane was restricted to less than 30 mg/kg in China (SFDA Citation2015). However, most of the 1,4-dioxane may be removed from ethoxylated compounds by means of vacuum stripping at the end of the polymerization process (ATSDR Citation2012).
Benzo[a]pyrene (Group 1), a prototype of polycyclic aromatic hydrocarbons (PAH), results in contamination from the use of petrolatum in cosmetic products. Therefore, to reduce the level of benzo[a]pyrene (BaP), application of highly purified petrolatum may be critical for manufacturing cosmetics. In specifications of 21CFR 74-Subpart C-cosmetics, the concentration of BaP should not exceed 0.005 mg/kg (5 ppb) and total PAH should not exceed 0.5 mg/kg for §74.2052 D&C Black No. 2. (CFR Citation2018b).
Acrylamide (Group 2A) may be found as a contaminant arising from the utilization of polyacrylamide as an anti-static, film-forming or binding agent in cosmetics. The presence of acrylamide is prohibited since polyacrylamides are used in leave-on cosmetics: lotions, creams, moisturizers, sunblock, and hair products (CIR Citation2005). The SCCS (Citation1999) suggested that the content of acrylamide in polyacrylamide used should be less than 0.1 ppm in body care leave-on products and less than 0.5 ppm in other cosmetic products.
Radioactive substances
Contamination of consumer products manufactured in Japan, by radioactive materials, has been a serious global safety issue since the nuclear accident at Fukushima Daiichi Nuclear Power Plant (Anzai et al. Citation2012). Consumers may be reluctant to use cosmetics manufactured in Japan because of potential contamination by radioactive substances. However, manufacturers claim that the possibility of atmospheric radioactive contamination during the production process is extremely low because cosmetics are produced in highly controlled indoor environments to avoid contamination from foreign substances and radioactive materials in the atmosphere. In addition, the radioactive material present in the water used for cosmetics constitutes an infinitesimal amount that does not pose any apparent potential risk to consumers. To further ensure safety, manufacturers routinely monitor levels of radioactive materials in cosmetics such that consumers may trust manufacturer claims. Potential sources of radioactivity include rocks, mud, and water that may have been contaminated by nuclear waste from power plants. Another potential source of radioactive contamination is gamma irradiation, which is employed for antimicrobial purposes in packaging materials and sealed packages that contain consumer products (Haji-Saeid, Sampaa, and Chmielewski Citation2007; Sivri et al. Citation2006). However, 60Co gamma radiation used is considered safe because the method does not leave any residues in cosmetic products when Good Manufacturing Practice (GMP) guidelines for manufacturing cosmetics are strictly adhered to.
Volatile organic chemicals (VOC)
Volatile organic chemicals have been detected in disposable sanitary pads, and many women questioned and displayed anxiety regarding the safety of sanitary pads (Korea Herald Citation2017). Sanitary pads were found to contain carcinogenic VOC such as benzene (Group 1), ortho-toluidine (Group 1), ethylbenzene (Group 2B), and chloroform (Group 2B). However, an assessment of human health risks from these detected VOCs indicated that exposure levels were very low, and the Ministry of Food and Drug Safety subsequently concluded that the possibility of these VOCs inducing adverse reactions in humans was also potentially very low (Korea Pharma Today Citation2017). For VOC and highly volatile organic compounds (HVOCs), 40 CFR 59 Subpart C sets limits for their levels in consumer products such as antiperspirants, deodorants, hairsprays, shaving cream, and nail polish removers (CFR Citation2015). Methanol is a VOC employed as a solvent to dissolve other chemicals. Methanol is prohibited in cosmetics in Japan, and a maximal limit of methanol in cosmetics is 2 g/kg in China (SFDA Citation2015). In Denmark, methanol must not be used in cosmetic products but may be utilized as a denaturant for ethanol and isopropyl alcohol at a maximal allowed concentration (in the final product) of 5%, calculated as % ethyl and isopropyl alcohol (DMOE Citation2013).
Perspectives from academia
Individuals are exposed to a wide variety of “unintentional” pollutants in our daily lives. However, it does not follow that chemophobia will consequently develop. By assessing the toxicological properties such as point of departure (POD) and exposure levels to these unintentional pollutants, human health risk assessments can be conducted. Thus, the role of toxicologists is to carry out reliable risk assessments for unintentional trace chemicals to which exposure is unavoidable, using the best available scientific methods.
The following summarizes several perspectives required for experimentation to improve the safety of household goods.
Development/improvement of trace chemical analysis methods
The use of appropriate analytical instruments, reliable technological methods by skilled analysts, and accurate measurements of concentrations are essential in the identification of trace chemicals in household goods. If the inappropriate instrument is used or the analyst lacks capability, problems may occur in the reliability of the trace chemical analysis. In cases where trace chemical levels are determined in blood or urine of consumers to establish a causal relationship with the use of particular consumer products, analytical methods need to be considered that best reflect possible routes of human exposure such as skin, oral or inhalation following the utilization of these products (Norberg and Norberg Citation2016).
Limitations and uncertainties in animal toxicity data
Toxicity data derived from animal toxicity testing have been of great value to predict human safety. However, data on the toxicity of trace chemicals obtained in animal experiments are affected by several factors. The results of toxicity tests may vary greatly with age, gender, animal species/strain, route of administration, use of vehicle, physicochemical properties, dose, duration of exposure, and diet. Therefore, the interpretation of animal data is limited as when applied to humans results need to be extrapolated while concomitantly considering uncertainty factors (UF). In addition, alternatives to animal testing need to be used due to measures that restrict animal toxicity testing of raw materials to be used in cosmetics. Therefore, methods for evaluating safety employing chemical toxicity information, such as quantitative structure–activity relationships (QSAR) and read-across, have been proposed as alternatives. In addition, since exposure to trace chemicals in household goods is characterized by long-term in vivo exposure to low concentrations, both animal and in vitro models need to be developed that mimic this exposure. Finally, the development of a toxicity test using the adverse outcome pathway (AOP) also deserves consideration (Clewell et al. Citation2018; Fukushima et al. Citation2018).
Application of integrated risk assessment
The safety profiles of harmful trace chemicals that may be present in household items may be judged and managed through risk assessment methodology. Threshold of toxicological concern (TTC) was proposed as a measure of risk assessment for primary screening in the event of exposure to low doses of chemicals including trace amounts (Kroes, Kleiner, and Renwick Citation2005). Although this method has certain limitations, it may be employed in the risk assessment of various trace chemicals for which toxicological data are extremely limited. Humans are exposed to trace chemicals cumulatively over long periods of time, not only through various household goods but also through environmental exposure, particularly in the case of heavy metals. Therefore, since humans are exposed to trace chemicals from differing sources, integrated risk assessment needs to include exposure assessment. When assessing the risk of trace chemicals, uncertainties need to be considered. In particular, additional consideration is required for the application of UF when studying vulnerable groups such as pregnant women, children, infants, and elderly people (Choi et al. Citation2018). Choi et al. (Citation2017) noted that blood concentrations of heavy metals were found to be higher in elderly subjects compared to children. Therefore, when exposed to the same heavy metal sources, elderly population exposure needs to be assessed more conservatively due to higher uncertainty. In addition, to determine the effects of unintentionally present trace chemicals in household goods on humans, correlations between the effects of chronic exposure and toxicological modes of action (MOA) need to be investigated. However, assessing the influence of such extremely small quantities of chemicals on humans is realistically difficult.
Accurate assessment of human exposure to, and safety profiles of, trace chemicals requires not only analysis of these substances, but also the identification of more specific biomarkers for exposure to trace chemicals (Pleil et al. Citation2018; Kim et al. Citation2018; Serrazina et al. Citation2018). Thus, the selection and use of biomarkers need to be validated to enable optimal use in human health risk assessment.
Perspectives from regulatory agencies
In managing public perception and chemophobia, the role of government is to secure consumer safety through laws and regulations that aim to prevent trace chemicals from entering or maximally reducing their levels in consumer products prior to reaching the consumer. To this end, identifying the sources of trace chemicals might help in the development of plans to diminish their presence in commercial products.
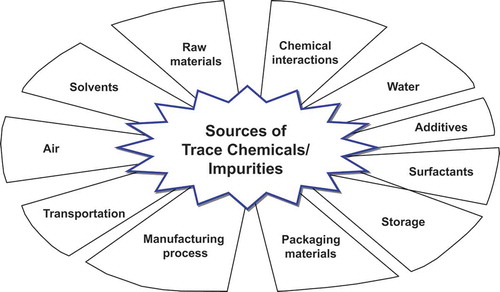
The unintentional presence of trace chemicals in household goods might occur as a result of the following ():
trace chemicals exist as impurities because the raw materials used in the product are not of sufficiently high purity.
the raw material (monomer) of a polymerized packaging container elutes from the packaging material at a concentration greater than the migration limit.
trace chemicals remain on the surface of the packaging container and become mixed into the product.
trace chemicals are generated as unwanted byproducts in the manufacturing process.
trace chemicals become mixed into the product at various stages of manufacturing processes, such as migration from a machine used in production, unsanitary manufacturing environments, and problems in the quality management system.
exposure during storage of packaged goods or secondary goods.
trace chemicals become mixed into the product during transportation of packaged products to a storage or sales site.
Examples of raw materials used for manufacturing may include purified water, ingredients, surfactants, emulsifiers, additives, and solvents. In order to minimize exposure to trace chemicals and understand their effects, the raw materials employed in the products need to be thoroughly examined for trace chemicals contaminants. In the case of cosmetics, the government requires manufacturers to use raw materials that have been evaluated for safety. Further, manufacturers need to ensure that their raw materials are not banned and do not exceed the limits specified for restricted materials. However, there is no official international guideline or regulation for the purity of raw materials (Siedel Citation2013). In the case of hazardous substances that may unintentionally contaminate cosmetic raw materials, levels deemed safe are determined through risk assessments. However, since the substances used as raw materials in cosmetics are diverse, the possibility for new, unknown harmful substances to contaminate cosmetics still exists. Therefore, governments need to constantly collect information on potential new harmful contaminants through both domestic and global research data, sharing of information with foreign specialized organizations, and information monitoring to accurately assess possible hazards.
Trace chemical safety can be managed using risk assessment. Governments conduct risk assessments using internationally accepted methods and procedures set forth in the “Regulations Regarding Risk Assessment Methods and Procedures” (Notification of the Ministry of Food and Drug Safety) and the “Cosmetics Hazard Assessment Guidelines.” The assessment methods are briefly outlined as follows.
Assess the risk of a substance by reviewing its physicochemical properties and relevant toxicity data including acute toxicity, repeated dose toxicity, organ toxicity, reproductive, and developmental toxicity.
Identify the POD or allowable threshold of toxicity of the target substance.
Calculate the daily exposure of the human body to chemicals due to the use of cosmetics.
Determine whether daily exposure of humans exceeds the threshold or compare it with the POD value to assess potential human health risk by the target substance.
In addition to cosmetics, governments are constantly studying the validity of standards through risk assessments of substances that may unintentionally contaminate other household goods. In the case of unintentionally introduced harmful substances, governments: 1) assess whether the quantities present in products are harmful to humans by collecting and inspecting products in circulation; 2) set and update standards when necessary by conducting risk assessments and reviewing the validity of limit criteria already in place; and 3) support industry in development of manufacturing processes for decreasing levels of unintentional harmful substances through research and development projects and providing guidelines for reduction management. The U.S. FDA believes manufacturers can minimize impurities in their products through GMP and suggests that manufacturers need to test both ingredients and finished products to ensure that certain manufacturer specifications are met (U.S. FDA Citation2018b)
Requirement of impurity profiles
Manufacturers are required to disclose an impurity profile containing the levels of chemicals of general concern listed in , which is not exhaustive (PCC Citation2018). Examples of these chemicals include heavy metals, carcinogens, VOC, and phthalates, which may be generated by catalysts, residual monomers, solvents, and other unreacted starting materials or contaminants.
Table 1. Examples of chemicals of concern to be included in impurity profiles.
Perspectives from consumer organizations
Consumers generally expect that all consumer products available on the market are safe based upon the notion that the government only allows manufacturers to sell products that are approved for safety. In addition, consumers consider the use of safe products to be a consumer’s right because individuals pay the price for products, including taxes. When purchased products turn out to be unsafe for humans due to chemical toxicities or other causes, consumers exhibit concern with respect to safety and consequently become emotionally affected.
Origin of chemophobia and risk perception
Consumers’ fear of chemicals began to rise with the detection of pesticides in food products in the early 1980s. There have been cases where high levels of pesticides were detected in fruits and vegetables (U.S. FDA Citation2015). Similar concerns have been raised regarding the safety of cosmetics.
Since experiencing these safety issues, consumers have been suspicious regarding the roles of regulatory agencies and manufacturers and whether these entities perform their duties properly in ensuring consumer safety. In 2011, hundreds of babies in South Korea died from exposure to humidifier disinfectants, and more than 1,000 people who were exposed suffered from infectious respiratory diseases (Lee, Kim, and Kwon Citation2012). This type of incident may contribute to the development of chemophobia in consumers.
Jon Entine (Citation2011), in the book Scared to Death: How Chemophobia Threatens Public Health, describes how the public misunderstands chemicals and chemical risk due to a variety of factors:
Advances in analytical chemistry that enable detection of ever smaller amounts of substances
Evolution of the internet and social media
The work of environmental advocacy organizations by committed activists, but often few scientists
Biased reports regarding consumer claims that suggest that synthetic chemicals are inherently risky
Consumer campaigns against industrial products
Governments inclined to respond to consumer claims in politically safe but scientifically unsound ways
Public distrust of authority, including government, industry, and the scientific community.
A recent survey on chemical perception reported that 15 out of 100 consumers suffer from chemophobia, which is considered a type of psychological disease and not merely a social phenomenon triggered by the media (Yoo Citation2018). Approximately 50% of 1,541 participants (age 19–65) in this survey indicated they were concerned about chemicals and tried to avoid them as much as possible. The level of consumer safety awareness seen does not have clear basis nor judgmental criteria. A lack of comprehensive knowledge and information leads to unsubstantiated anxiety.
Request for systematic safety management by government
In South Korea, cosmetics, potentially harmful products, sanitary products, and industrial products for self-safety checks are managed separately predominantly by four departments: the Ministry of Food and Drug Safety, the Ministry of Environment, the Ministry of Health and Welfare, and the Ministry of Trade, Industry and Energy. Substance registration and labeling of ingredients and safety for consumers vary with laws. Consumer chemical products deemed “products that may be harmful” are managed by the Ministry of Environment through categorization of products, risk assessments, safety labeling standards, safety investigations, and action orders, but there are still limitations in the effectiveness, applicability, and responsiveness to market changes.
In the U.S., the Consumer Product Safety Act (CPSA) and Consumer Product Safety Improvement Act (CPSIA) are enforced by the U.S. Consumer Product Safety Commission (CPSC) on all consumer chemical products (CPSC Citation2018). In Japan, the Ministry of Economy, Trade and Industry’s Consumer Affairs Agency setup organic management systems through the Consumer Household Products Safety Act, the Household Goods Quality Labeling Act, and the Framework Act for Consumer Protection in order to implement comprehensive quality control, labeling standards, and consumer protections for market products (MHLW Citation2018a). Under the Product Liability Act, a “product defect” is defined as a lack of safety that the product should ordinarily provide. Cosmetics, medical products, and medical devices are regulated by the Law on Securing Quality, Efficacy and Safety of Products, under the Pharmaceutical Affairs Law (MHLW Citation2018b; Inomata Citation2014).
Due to lack of cooperative systems between departments managing consumer chemical products, and integrated management systems covering related laws, consumers still believe that there are blind spots in safety management, and which managing body is responsible may not be clear when a safety issue occurs. In addition, the absence of a product ingredient labeling system, differences in prescribed safety values between managing departments, and differences in response measures for damage relief can all lead to public distrust of a government’s systematic chemical substance safety management.
Another important issue is that risk assessment systems are operated separately by different governmental departments, resulting in poor risk assessment development methods and integrated safety control systems. From the viewpoint of consumers who use various products and risk exposure to harmful substances, this leads to the fear that inefficiency may arise in risk assessment and hazardous contaminants in products may go unidentified. This further shows the necessity for integrated risk assessments.
The number of different chemical substances used commercially worldwide is estimated to be between 70,000 and 100,000, and approximately 1,000 new substances are developed and used every year. Given these numbers, it is not surprising that the issue of trace chemicals is a cause of anxiety for consumers who expect appropriate chemical substance control with no blind spots.
Perception of trace chemical safety
Managing the safety of trace hazardous substances in humans is difficult in cases where: 1) there are no apparent guidelines for safe levels of the substance or setting such guidelines is difficult; 2) toxicity data are insufficient or limited; 3) other standards (source movement routes or detection limits) are unclear; or 4) there are limitations to applying existing chemical substance management system.
The issue of safety regarding trace hazardous substances has been raised repeatedly by consumer organizations, environmental organizations, National Assembly, and the media, as public transparent analysis of chemical substance levels in consumer products have increased over time. In July 2009, the safety issue on traces of formaldehyde and 1,4-dioxane detected in children’s bath products in South Korea was originally initiated by an American consumer organization, leading to governmental establishment of countermeasures. In March 2018, some of the cosmetic products were recalled following the detection of levels of antimony exceeding the government-defined standard 10 ppm (Joins Citation2018). In South Korea, the Cosmetics Act was fortunately revised in 2014 such that in cases where an ingredient for which use is prohibited is found in a cosmetic product, risk assessment need to be conducted to determine whether the ingredient is harmful and whether further actions, such as collection and destruction, need to be conducted (MFDS Citation2018).
Suggestions for industry
In cases where a product contains a hazardous substance, consumers are reluctant to use the product based upon only the fact that the substance exists at all, regardless of the amount or the manner in which it was detected. This may feel unfair to the product manufacturer. Completely eliminating products with unintentional hazardous contaminants is difficult due to technological, time, and financial limitations. Governments should be expected to maintain consumer confidence by creating product safety management systems without blind spots, integrating systems for the management of risk assessment, and maintaining effective labeling systems. This is why governmental organizations such as the U.S. CPSC, which manages product safety in an integrated manner, are important in maintaining consumer confidence.
Industry also needs to play a role in monitoring and risk assessment of trace chemical substances that are unintentionally introduced during production. Companies equipped with their own risk assessment capabilities need to make active efforts to share their safety information with the public. Government organizations can then use this information to monitor the influence of chemical substances that have been estimated to pose no apparent hazard and update any relevant standards.
Role of consumers
When communicating with consumers regarding t product hazards, more efforts to develop evidence-based communication skills and strategies are required. Although scientific fact-based communication cannot be built up in a day, one needs to establish such a culture over the long-term. In particular, the methods by which consumers obtain information have been rapidly changing in recent years and an era of information spread through images via new media and social networking has begun. By making expert scientific information easy to understand, it can be continuously communicated to the public in an easily comprehensible language, allowing consumers to have a better understanding and appreciation of safety. Such a step will also build a focus on consumer safety issues.
Consumers need to take more logical and rational approaches rather than emotional approaches, attaining a realistic scientific understanding before raising issues regarding chemical substance safety. Educating consumers on the topics of dosage and toxicology may be helpful to produce a better understanding of chemophobia and enable them to make rational decisions with respect to product safety.
Perspectives from industry
Manufacturers of consumer products are responsible for ensuring the safety of ingredients and products prior to their introduction into the marketplace. An exposure-based risk assessment approach is applied to the safety evaluation of ingredients and product formulations by considering both toxicity of the chemical of interest and exposure conditions typical of intended and reasonably foreseeable consumer uses of the product (Kwon, Holland, and Kern Citation2009; NAS Citation1983; SCCS Citation2018).
Consumers are increasingly concerned about the quality and safety of the products they use. With advances in analytical sciences, it is inevitable that trace amounts of chemical contaminants will be detected in consumer products. Certain trace chemicals have been identified as hazardous based upon current classification criteria, which do not take into consideration of consumer exposure and risk assessment due to the chemical use (GHS Citation2017). Thus, recent reports on the detection of certain trace chemicals classified as hazardous in consumer products have triggered misinformed public anxieties over the perceived safety issues of these chemicals.
In many of these recent reports, analytical measurements of trace chemicals were carried out in extreme conditions such as strong organic solvents, extreme pH values, prolonged contact time or total digestion that were not relevant to actual consumer product use scenarios. This is especially true for articles such as diapers. It is critical that one uses appropriate analytical methods employing physiologically relevant conditions (Dey et al. Citation2016). Further, it is imperative that one avoids misinterpreting analytical data on trace chemicals measured in extreme conditions, as these detections are not physiologically relevant. One should also not focus merely on the existence of a chemical of concern in a product. Safety assessments for trace chemicals in consumer products need to be based upon analytical data obtained in physiologically relevant conditions in conjunction with an understanding of the consumer exposure to a chemical of concern under intended product use conditions (ICCR Citation2011).
Conclusions
In conclusion, it is crucial that one makes science-based judgments on the safety of trace chemicals, thereby appropriately managing misinformed public concerns (sometimes exacerbated by social media) about the perceived safety of chemicals. A collaborative effort for effective risk communication between key stakeholders might help consumers make informed decisions on the safety of trace chemicals in consumer products.
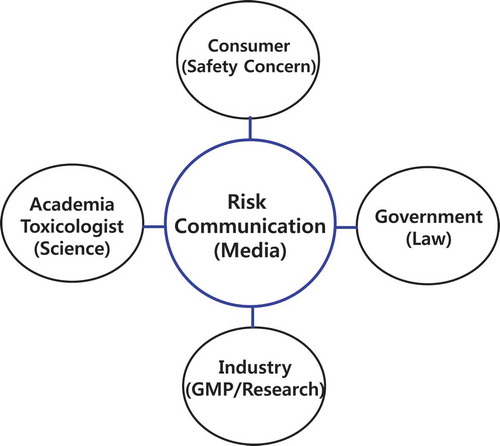
Discussing and analyzing the viewpoints of various stakeholder circles regarding the dangers of trace chemicals is meaningful and a necessary process in management of the risk of trace chemicals for consumer safety. However, providing opportunities for scientists, government organizations, consumer groups, and industry to get together and share their opinions is not easy, and the participants may be reluctant (). Before communication even begins, there may be anxiety or hostility toward other parties. In addition, there will naturally be vast differences in opinion between parties regarding safety of and plans for the management of trace chemicals. However, despite differences between understanding of and views on trace chemical safety, all participants were of the opinion that safe production and governmental management are fundamentally required not only for consumer safety but also for manufacturer profits. Consumers demanded greater effort from industries and governments, as well as greater scientific research for consumer safety. However, there are still great differences in the perceived importance of trace chemical safety, with many manufacturers shortsightedly pursuing short-term profits rather than investing in or putting effort into the safe manufacture of products. In addition, national regulatory agencies do not always efficiently manage the safety of trace chemicals primarily because of the absence of experts in safety management, disharmony of management systems, and inadequacy of legal systems. Although academics may be interested in studying safety issues and risk management, it is difficult to conduct independent research without financial support from industries or governmental agencies. Therefore, although the management of trace chemical safety issues needs to be in part the responsibility of consumers and consumer organizations, manufacturers and governmental agencies need to take these issues seriously and take the lead in research investments. In the future, the stakeholder circles examined here need to engage in mutual, trustworthy dialogues, such that ongoing science-based risk communication might lead to better solution of issues regarding chemical safety.
Disclosure statement
The authors have no conflict of interest to declare
Additional information
Funding
References
- Anzai, K., N. Ban, T. Ozawa, and S. Tokonami. 2012. Fukushima daiichi nuclear power plant accident: Facts, environmental contamination, possible biological effects, and countermeasures. J. Clin. Biochem. Nutr. 50:2–8. doi:10.3164/jcbn.D-11-00021.
- Asia Times. 2018. South Korean cosmetics recalled after inspection. Accessed September 21, 2018. http://www.atimes.com/article/south-korean-cosmetics-recalled-inspection/.
- ATSDR (Agency for Toxicological Substances and Disease Registry). 2012. Toxicological profile for 1,4-dioxane. Atlanta, GA: U.S. Department of Health and Human Services. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=955&tid=199.
- Bocca, B., A. Pino, A. Alimonti, and G. Forte. 2014. Toxic metals contained in cosmetics: A status report. Reg. Toxicol. Pharmacol. 68:447–67. doi:10.1016/j.yrtph.2014.02.003.
- Borowska, S., and M. M. Brzóska. 2015. Metals in cosmetics: Implications for human health. J. Appl. Toxicol. 35:551–72. doi:10.1002/jat.3129.
- CFR (Code of Federal Register). 2015. 40 CFR part 59, subpart C-national volatile organic compound emission standards for consumer products. https://www.law.cornell.edu/cfr/text/40/part-59/subpart-C
- CFR (Code of Federal Register). 2018a. 21 CFR 700.13. Use of mercury compounds in cosmetics including use as skin bleaching agents in cosmetic preparations also regarded as drugs. https://www.law.cornell.edu/cfr/text/21/700.13
- CFR (Code of Federal Register). 2018b. 21 CFR part 74-listing of color additives subject to certification, subpart C-cosmetics. https://www.ecfr.gov/cgi-bin/text-idx?c=ecfr&SID=d1e893bdb7e.
- Choi, S. M., T. H. Roh, D. S. Lim, S. Kacew, H. S. Kim, and B. M. Lee. 2018. Risk assessment of benzalkonium chloride in cosmetic products. J. Toxicol. Environ. Health B 21:8–23. doi:10.1080/10937404.2017.1408552.
- Choi, W., S. Kim, Y.-W. Baek, K. Choi, K. Lee, S. Kim, S. D. Yu, and K. Choi. 2017. Exposure to environmental chemicals among Korean adults-updated from the second Korean national environmental health survey (2012-2014). Int. J. Hyg. Environ. Health 220:29–35. doi:10.1016/j.ijheh.2016.10.002.
- CIR (Cosmetic Ingredient Review). 2005. Amended final report on the safety assessment of polyacrylamide and acrylamide residues in cosmetics. Int. J. Toxicol 24(Suppl 2):21–51. doi:10.1080/10915810590953842.
- Clewell, H. J., J. W. Yager, T. B. Greene, and P. R. Gentry. 2018. Application of the adverse outcome pathway (AOP) approach to inform mode of action (MOA): A case study with inorganic arsenic. J. Toxicol. Environ. Health A 81:893–912. doi:10.1080/15287394.2018.1500326.
- CPSC (Consumer Product Safety Commission). 2018. Regulations, laws & standards. https://www.cpsc.gov/Regulations-Laws–Standards.
- DeVito, M. J., and A. Schecter. 2002. Exposure assessment to dioxins from the use of tampons and diapers. Environ. Health Perspect. 110:23–28. doi:10.1289/ehp.0211023.
- Dey, S., M. Purdon, T. Kirsch, H. Helbich, K. Kerr, L. Li, and S. Zhou. 2016. Exposure Factor considerations for safety evaluation of modern disposable diapers. Reg. Toxicol. Pharmacol. 81:183–93. doi:10.1016/j.yrtph.2016.08.017.
- DMOE (Danish Ministry of Environment). 2013. Survey of methanol (Cas no. 67-56-1). https://www2.mst.dk/Udgiv/publications/2013/04/978-87-93026-01-8.pdf.
- Entine, J. 2011. Scared to death: How chemophobia threatens public health. New York, NY: American Council on Science and Health. https://www.acsh.org/sites/default/files/Scared-to-Death-How-Chemophobia-Threatens-Public-Health.pdf.
- EU (European Union). 2018. Regulation (EC) no 1223/2009 of the European parliament and of the council of 30 November 2009 on cosmetic products (recast) (Text with EEA relevance). http://ec.europa.eu/growth/sectors/cosmetics/legislation/.
- Francl, M. 2013. How to counteract chemophobia. Nat. Chem. 5:439–40. doi:10.1038/nchem.1661.
- Fukushima, S., M. Gi, M. Fujioka, A. Kakehashi, H. Wanibuchi, and M. Matsumoto. 2018. Quantitative approaches to assess key carcinogenic events of genotoxic carcinogens. Toxicol. Res. 34:291–96. doi:10.5487/TR.2018.34.4.291.
- GHS (globally harmonized system). 2017. The globally harmonized system of classification and labelling of chemicals (GHS). United Nations. http://www.unece.org/trans/danger/publi/ghs/ghs_rev00/00files_e.html.
- Haji-Saeid, M., M. H. O. Sampaa, and A. G. Chmielewski. 2007. Radiation treatment for sterilization of packaging materials. Radiat. Phys. Chem. 76:1535–41. doi:10.1016/j.radphyschem.2007.02.068.
- Harmon, M. E., J. Lewis, C. Miller, J. Hoover, A. S. Ali, C. Shuey, M. Cajero, S. Lucas, B. Pacheco, E. Erdei, et al. 2018. Arsenic association with circulating oxidized low-density lipoprotein in a Native American community. J. Toxicol. Environ. Health A. 81:535–48. doi:10.1080/15287394.2018.1443860.
- Hartings, M. R., and D. Fahy. 2011. Communicating chemistry for public engagement. Nat. Chem. 3:674–77. doi:10.1038/nchem.1094.
- Health Canada. 2018. Cosmetic ingredient hotlist. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/hotlist.html#tbl2.
- IARC (International Agency for Research on Cancer). 2018. Agents classified by the IARC monographs. vols. 1–123. IARC. https://monographs.iarc.fr/agents-classified-by-the-iarc/.
- ICCR (International Cooperation on Cosmetic Regulation). 2011. Principles for the handling of traces of impurities and/or contaminants in cosmetic products. http://ec.europa.eu/consumers/sectors/cosmetics/files/pdf/iccr5_contaminants_en.pdf.
- Inomata, S. 2014. Safety assurance of cosmetics in Japan: Current situation and future prospects. J. Oleo Sci. 63 (1):1–6.
- Joins. 2018. Cosmetics contaminated with heavy metals. https://news.joins.com/article/22459668
- Kaličanin, B., and D. Velimirović. 2016. A study of the possible harmful effects of cosmetic beauty products on human health. Biol. Trace Elem. Res. 170:476–84. doi:10.1007/s12011-015-0477-2.
- Kim, M. K., K. B. Kim, K. Yoon, S. Kacew, H. S. Kim, and B. M. Lee. 2018. IL-1a and IL-1b as alternative biomarkers for risk assessment and the prediction of skin sensitization potency. J. Toxicol. Environ. Health A 81:830–43. doi:10.1080/15287394.2018.1494474.
- Koo, H. J., and B. M. Lee. 2004. Estimated exposure to phthalates in cosmetics and risk assessment. J. Toxicol. Environ. Health A 67:1901–14. doi:10.1080/15287390490513300.
- Korea Herald. 2017. Fears mount over “toxic” sanitary pads. http://www.koreaherald.com/view.php?ud=20170824000747.
- Korea Pharma Today. 2017. No sanitary pad in the market is harmful to the human body. Accessed September 21, 2018. http://english.yakup.com/article/read/21699.
- Kroes, R., J. Kleiner, and A. Renwick. 2005. The threshold of toxicological concern concept in risk assessment. Toxicol. Sci. 86:226–30. doi:10.1093/toxsci/kfi169.
- Kwon, S., D. Holland, and P. Kern. 2009. Skin safety evaluation of laundry detergent products. J. Toxicol. Environ. Health A 72:1369–79. doi:10.1080/15287390903212675.
- Lee, J. H., Y. H. Kim, and J. H. Kwon. 2012. Fatal misuse of humidifier disinfectants in Korea: Importance of screening risk assessment and implications for management of chemicals in consumer products. Environ. Sci. Technol. 46:2498–500. doi:10.1021/es300567j.
- Lim, D. S., S. K. Lim, M. K. Kim, Y. C. Kwon, T. H. Roh, S. M. Choi, S. Yoon, H. S. Kim, and B. M. Lee. 2018c. Formation and inhibition of N-nitrosodiethanolamine in cosmetics under pH, temperature, and fluorescent, ultraviolet, and visual light. J. Toxicol. Environ. Health A. 81:241–53. doi:10.1080/15287394.2018.1440172.
- Lim, D. S., T. Roh, M. K. Kim, Y. C. Kwon, S. M. Choi, S. J. Kwack, K. B. Kim, S. Yoon, H. S. Kim, and B. M. Lee. 2018a. Non-cancer, cancer, and dermal sensitization risk assessment of heavy metals in cosmetics. J. Toxicol. Environ. Health A 81:432–52. doi:10.1080/15287394.2018.1451191.
- Lim, D. S., T. Roh, M. K. Kim, Y. C. Kwon, S. M. Choi, S. J. Kwack, K. B. Kim, S. Yoon, H. S. Kim, and B. M. Lee. 2018b. Risk assessment of N-nitrosodiethylamine (NDEA) and N-nitrosodiethanolamine (NDELA) in cosmetics. J. Toxicol. Environ. Health A 81:465–80. doi:10.1080/15287394.2018.1460782.
- The Local. 2017. ‘Toxic substances’ found in most nappies sold in France. https://www.thelocal.fr/20170124/weedkiller-found-in-nappies-in-france.
- MFDS (Ministry of Food and Drug Safety). 2017. Regulation on safety standards for cosmetics (in Korean). http://www.mfds.go.kr/brd/m_211/view.do?seq=13674&srchFr=&srchTo=&srchWord=%ED%99%94%EC%9E%A5%ED%92%88&srchTp=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1
- MFDS (Ministry of Food and Drug Safety). 2018. The cosmetics act. http://www.mfds.go.kr/.
- MHLW (Ministry of Health, Labour and Welfare). 2018a. Household product safety measures. https://www.mhlw.go.jp/english/wp/wp-hw5/dl/23010306e.pdf.
- MHLW (Ministry of Health, Labour and Welfare). 2018b. Standards for cosmetics in Japan. https://www.mhlw.go.jp/file/06-Seisakujouhou-11120000-Iyakushokuhinkyoku/0000032704.pdf.
- NAS (National Academy of Science). 1983. Risk assessment in the Federal government. Washington, DC: National Academy Press. National Research Council.
- Norberg, M., and G. F. Norberg. 2016. Trace element research-historical and future aspects. J. Trace Elem. Med. Biol. 38:46–52. doi:10.1016/j.jtemb.2016.04.006.
- PCC (Personal Care Council). 2018. Guidance – personal care products council. Raw material information form guidance. http://www.personalcarecouncil.org/sites/default/files/RMIFGuidance100806.doc.
- Pleil, J. D., M. A. G. Wallace, M. A. Stiegel, and W. E. Funk. 2018. Human biomarker interpretation: The importance of intra-class correlation coefficients (ICC) and their calculations based on mixed models, ANOVA, and variance estimates. J. Toxicol. Environ. Health B 21:161–80. doi:10.1080/10937404.2018.1490128.
- Roy, J. S., D. Chatterjee, N. Das, and A. K. Giri. 2018. Substantial evidences indicate that inorganic arsenic is a genotoxic carcinogen: A review. Toxicol. Res. 34:311–24. doi:10.5487/TR.2018.34.4.311.
- SCCS (Scientific Committee on Consumer Safety). 1999. Opinion of the scientific committee on cosmetic products and non-food products intended for consumers concerning acrylamide residues in cosmetics adopted by the plenary session of the SCCNFP of 30 September 1999. http://ec.europa.eu/health/scientific_committees/consumer_safety/opinions/sccnfp_opinions_97_04/sccp_out95_en.htm.
- SCCS (Scientific Committee on Consumer Safety). 2012. Opinion on NDELA in cosmetic products and nitrosamines in balloons. http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_100.pdf.
- SCCS (Scientific Committee on Consumer Safety). 2015. The report of the ICCR working group: Considerations on acceptable trace level of 1,4-dioxane in cosmetic products. https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_194.pdf.
- SCCS (Scientific Committee on Consumer Safety). 2018. The SCCS notes of guidance for the testing of cosmetic ingredients and their safety evaluation. 10th revision. SCCS/1602/18. https://ec.europa.eu/health/sites/health/files/scientific_committees/consumer_safety/docs/sccs_o_224.pdf.
- Serrazina, D. C., V. L. De Andrade, M. Cota, M. L. Mateus, M. Aschner, and A. P. Marreilha. 2018. Biomarkers of exposure and effect in a working population exposure to lead, manganese and arsenic. J. Toxicol. Environ. Health A 81:161–80. doi:10.1080/15287394.2018.1509408.
- SFDA (State Food and Drug Agency). 2015. Safety and technical standards for cosmetics 2015. http://www.nmpa.gov.cn/WS04/CL2193/300091.html.
- Shin, J. W., Y. G. Ahn, and J. J. Seo. 2005. Determination of PCDD/Fs in disposable diaper for infant. Korean Soc. Cloth. Text. 29:814–24.
- Siedel, A., ed. 2013. Kirk-Othmer chemical technology of cosmetics. Hoboken, NJ: John Wiley & Sons, Inc.
- Sivri, N. N., A. Y. Özer, M. Özalp, N. Atakan, and M. Polat. 2006. Decontamination of cosmetic products and raw materials by Gamma irradiation. FABAD J. Pharm. Sci. 31:198–209.
- Tohon, H., A. Nong, M. Moreau, M. Valcke, and S. Haddad. 2018. Reverse dosimetry modeling of toluene exposure concentrations based on biomonitoring levels from the Canadian health measures survey. J. Toxicol. Environ. Health A 81:1066–82. doi:10.1080/15287394.2018.1534174.
- Tsai, S. S., Y. H. Weng, Y. W. Chiu, and C. Y. Yang. 2018. Farming and mortality rates attributed to non-Hodgkin’s lymphoma in Taiwan. J. Toxicol. Environ. Health A. 81:31–36. doi:10.1080/15287394.2017.1408362.
- U.S. FDA (United States Food and Drug Administration). 2015. Pesticide residue monitoring program fiscal year 2015 pesticide report. https://www.fda.gov/downloads/Food/FoodborneIllnessContaminants/Pesticides/UCM582721.pdf.
- U.S. FDA (United States Food and Drug Administration). 2018a. FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. https://www.fda.gov/cosmetics/guidanceregulation/lawsregulations/ucm074162.htm.
- U.S. FDA (United States Food and Drug Administration). 2018b. FDA”s testing of cosmetics for arsenic, cadmium, chromium, cobalt, lead, mercury, and nickel content. https://www.fda.gov/Cosmetics/ProductsIngredients/PotentialContaminants/ucm452836. htm.
- Wallace, M. A., T. M. Kormos, and J. D. Pleil. 2016. Blood-borne biomarkers and bioindicators for linking exposure to health effects in environmental health science. J. Toxicol. Environ. Health B 19:380–409. doi:10.1080/10937404.2016.1215772.
- World Health Organization (WHO). 1973. Trace-elements in human nutrition. Report of a WHO expert committee. Geneva, Switzerland: World Health Organization;(WHO Technical Report Series, No. 532).
- World Health Organization (WHO). 2011. Summary of principles for evaluating health risks in children associated with exposure to chemicals. Geneva, Switzerland: World Health Organization.
- Yoo, M. S. 2018. A survey on public perception of chemical hazard in Korea. https://www.yna.co.kr/view/AKR20180418064400004.
- Zenobio, J. E., B. C. Sanchez, J. K. Leet, L. C. Archuleta, and M. S. Sepúlveda. 2015. Presence and effects of pharmaceutical and personal care products on the Baca National Wildlife Refuge, Colorado. Chemosphere 120:750–55. doi:10.1016/j.chemosphere.2014.10.050.