 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Bisphenol A (BPA) and phthalate diesters are ubiquitous environmental contaminants. While these compounds have been reported as reproductive toxicants, their effects may partially be attributed to metabolites. The aim of this study was to examine reproductive organ development in chicken embryos exposed to the BPA metabolite, 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene (MBP; 100 µg/g egg) or a human-relevant mixture of 4 phthalate monoesters (85 µg/g egg). The mixture was designed within the EU project EDC-MixRisk based upon a negative association with anogenital distance in boys at 21 months of age in a Swedish pregnancy cohort. Chicken embryos were exposed in ovo from an initial stage of gonad differentiation (embryonic day 4) and dissected two days prior to anticipated hatching (embryonic day 19). No discernible effects were noted on reproductive organs in embryos exposed to the mixture. MBP-treated males exhibited retention of Müllerian ducts and feminization of the left testicle, while MBP-administered females displayed a diminished the left ovary. In the left testicle of MBP-treated males, mRNA expression of female-associated genes was upregulated while the testicular marker gene SOX9 was downregulated, corroborating a feminizing effect by MBP. Our results demonstrate that MBP, but not the phthalate monoester mixture, disrupts both male and female reproductive organ development in an avian embryo model.
Introduction
Humans and wildlife are exposed to complex mixtures of various environmental contaminants. Some of these chemicals may produce long-lasting or even permanent effects, in particular, if exposure occurs during sensitive windows of development (Yoon. et al. Citation2014). Xenobiotics may perturb the development of reproductive organs during early life by interfering with the endocrine systems or by disrupting other pathways critical to reproductive organ development (Dorman et al. Citation2018). Such developmental effects may lead to impaired reproductive capacity in adulthood (WHO Citation2012). It is therefore essential to determine whether mixtures that are relevant for humans and wildlife affect early development of reproductive organs.
Bisphenol A (BPA) is one of the most well-known endocrine-disrupting chemicals (EDCs) and reproductive toxicants. Bisphenol A (BPA) is used as a precursor in the production of polycarbonate plastics and epoxy resins, and found in a variety of consumer products (Michałowicz Citation2014). BPA was present in the majority of tissue, plasma, and urine samples taken from humans (Vandenberg et al. Citation2007), and found in surface waters, sediments, and wildlife (Corrales et al. Citation2015; Nehring, Staniszewska, and Falkowska Citation2017). Exposure to BPA has been associated with a wide range of adverse effects, including reproductive and developmental effects, in experimental and epidemiological studies (Michałowicz Citation2014; Rochester Citation2013; Yoon. et al. Citation2014). Experimental studies in fish, amphibians, rodents, and birds reported that BPA affected testicular development (Berg, Halldin, and Brunström Citation2001a; De Campos et al. Citation2019; Tamschick et al. Citation2016; Williams et al. Citation2014) and perinatal exposure to BPA was implicated in chronic adverse effects on female reproductive organs (Newbold, Jefferson, and Padilla-Banks Citation2007; Suvorov and Waxman Citation2015). Despite the large number of published studies on BPA, there is a continuing controversy over the risk that BPA exposure may pose to human health (Camacho et al. Citation2019; Prins et al. Citation2019). There are low-dose studies that show adverse reproductive and developmental effects (Christiansen et al. Citation2014) whereas other investigators reported lack of such effects (Howdeshell et al. Citation2008; Ryan et al. Citation2010). Some of the effects attributed to BPA may be associated with activation of the estrogen receptors (ERs), but there is also evidence for interaction with other receptors and induction of epigenetic changes (Acconcia, Pallottini, and Marino Citation2015; Santangeli et al. Citation2017).
Previously, Yoshihara et al. (Citation2001) demonstrated that the estrogenic activity of BPA was increased when it was incubated with S9 fraction from rat liver. Further investigation suggested that the enhanced estrogenic activity was induced by 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene (MBP) (Yoshihara et al. Citation2004). The estrogenic activity of MBP was noted in vivo in fish (Brown et al. Citation2019; Ishibashi et al. Citation2005; Moreman et al. Citation2018) and ovariectomized rats (Okuda, Takiguchi, and Yoshihara Citation2010) where it was 250-1000-fold more potent than BPA. Exposure to MBP induced cell proliferation and reduced protein levels of ERα in a human breast cancer cell line (Hirao-Suzuki et al. Citation2019). Further, in silico-docking simulations indicated that MBP activated the human ERs (Baker and Chandsawangbhuwana Citation2012) and interacted with human androgen and progesterone receptors (Rehan et al. Citation2015). To the best of our knowledge, MBP has not previously been studied in an avian model.
Phthalates, or phthalic acid esters, are utilized as plasticizers and solvents. Similar to BPA, the use of phthalates in numerous products results in widespread human exposure (Wormuth et al. Citation2006), and various phthalates are ubiquitously present in the environment (Gani, Tyagi, and Kazmi Citation2017). Phthalates have been suggested to contribute to male reproductive disorders, including testicular dysgenesis syndrome which may have fetal origin (Bornehag et al. Citation2015; Dorman et al. Citation2018; Habert et al. Citation2009; Radke et al. Citation2018; Skakkebæk, Rajpert-De Meyts, and Main Citation2001; Swan et al. Citation2015). The epidemiological evidence is not entirely consistent; associations between phthalate exposure and testosterone levels range from a negative association (Meeker and Ferguson Citation2014) to no association (Mieritz et al. Citation2012). Recently, Radke et al. (Citation2018) examined the epidemiological evidence and found that it supports the hypothesis that phthalate exposure may result in adverse male reproductive effects, albeit the authors recognized inconsistencies between studies and the difficulty to draw conclusions regarding all phthalates as a group. Experimental evidence generally supports the idea that early exposure to phthalates has implications for reproductive health (De Campos et al. Citation2019; Gray et al. Citation2000; Mylchreest, Cattley, and Foster Citation1998; Mylchreest et al. Citation2000; Parks et al. Citation2000; Shono et al. Citation2000) but there are species differences in both effects and sensitivity (Gray et al. Citation1982; Johnson, Heger, and Boekelheide Citation2012) and non-monotonic dose-response have been observed (Lin et al. Citation2008).
The Swedish environmental longitudinal mother and child, asthma and allergy (SELMA) study is a pregnancy cohort study, designed to investigate early life exposure to environmental chemicals and health outcomes in children, including altered reproductive development (Bornehag et al. Citation2012). Between 2007 and 2010, more than 2,300 pregnant women from the county of Värmland, Sweden, were recruited to the study (Bornehag et al. Citation2012). At gestational week 10, urine and serum were collected from the women and analyzed for 20 chemicals, including phthalates, phenols, and perfluorinated compounds. Using weighted quantile sum (WQS) regression, levels of 4 phthalate monoesters in the urine of 198 mothers were associated with shorter anogenital distance (AGD) in their baby boys at 21 months of age (Bornehag et al. Citation2019). A mixture (Mixture S) was established for these four phthalates, i.e. monobutyl phthalate, monoisononyl phthalate, monoethylhexyl phthalate, and monobenzyl phthalate, with mixing proportions based on geometric means of estimated serum concentrations in the SELMA mothers (Bornehag et al. Citation2019). Gestational exposure to Mixture S in mice led to reduced AGD and gonadal weight, histological changes in the gonads, and altered mRNA expression of regulators of steroidogenesis in the offspring (Repouskou et al. Citation2019). Elevated levels of testosterone, estradiol, and luteinizing hormone have been found in male offspring (Repouskou et al. Citation2019). The indication that Mixture S may disrupt reproductive organ development in humans and the effects found in mice warranted us to further explore the potential influence on reproductive organ development in an avian model.
The avian embryo is an established model for examining the effects of endocrine-disrupting compounds on the developing female and male reproductive systems (Berg et al. Citation1998; Brunström et al. Citation2009). Birds and mammals share many cellular and molecular mechanisms involved in sex differentiation of the reproductive organs but there are also some important differences (DeFalco and Capel Citation2009). In contrast to mammals, the development of the female reproductive organs is asymmetric; the left gonad develops into an ovary and the left Müllerian duct differentiates into an oviduct, while the gonad and Müllerian duct on the right side regress (Romanoff Citation1960). In males, the early gonads differentiate into testes of similar size and both Müllerian ducts regress (Romanoff Citation1960). In the presence of xenoestrogens, the left gonad in male embryos develops into an ovotestis, and retention and malformations of Müllerian ducts occur in both sexes (Berg et al. Citation1999; Mattsson, Olsson, and Brunström Citation2011). The hormone-dependent differentiation of reproductive organs makes the avian embryo a suitable model in studies of EDCs (Jessl, Scheider, and Oehlmann Citation2018a).
The aim of this study was to investigate whether the suggested BPA metabolite MBP and a human-relevant mixture of phthalate monoesters (metabolites of phthalate diesters) interfere with reproductive organ development in the chicken embryo model.
Methods and materials
Chemicals
4-Methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene (MBP; CAS: 13464-24-9) was purchased from Sigma Aldrich. Mixture S was composed within the project EDC MixRisk (http://edcmixrisk.ki.se/) as described in (Bornehag et al. Citation2019). The mixture consisted of monobutyl phthalate, monoisononyl phthalate, monoethylhexyl phthalate, and monobenzyl phthalate (). Dimethyl sulfoxide (DMSO; CAS: 67-68-5; Sigma Aldrich, Saint Louis, MO, USA) was used as a solvent.
Table 1. Composition of mixture S and the doses injected.
Exposure and sampling
Chicken eggs (Gallus gallus domesticus) were purchased from OVA Production AB, Vittinge, Sweden. The experimental procedures were approved by the Uppsala Ethical Committee for Research on Animals (Permit no: C 90/15).
In a first experiment, chicken embryos were exposed to MBP. Thirty eggs were randomly sampled and weighed to estimate the mean weight of the batch of eggs. The study was divided into five replicate experiments that were initiated at consecutive days. Eggs randomly allocated to replicate experiments were incubated horizontally at 37.7 ± 0.6°C with 65 ± 4% relative humidity (mean ± sd) and with automatized turning every 6 hr. The exposure started on embryonic day 4 (E4), an initial stage of gonadal differentiation. On that day, unfertilized eggs were removed (fertilization degree: 94%) and embryos exposed via air sac-injection to DMSO (vehicle control, n = 37) or 100 µg MBP/g egg (n = 38) as follows. The eggs were candled to locate the air sac and the area was wiped with ethanol (70%) to sterilize it. A hole was drilled in the shell above the center of the air sac through which the substances were placed on the inner shell membrane with a Hamilton syringe (injection volume: 20 µl). After injection, the hole in the shell was sealed with paraffin wax. The eggs were candled at E9-12 and any eggs with dead embryos were removed and recorded.
A study with Mixture S was conducted in a similar manner as that with MBP, but with two replicate experiments that were initiated at consecutive days. The temperature in the incubator was 37.3 ± 0.2°C with 65 ± 6% relative humidity (mean ± sd). The fertilization degree was 86%. The embryos were exposed via air sac-injection to DMSO (vehicle control; n = 22) or Mixture S dissolved in DMSO (in total 85 µg/g egg; n = 22). The whole-egg concentration of the mixture corresponds to approximately 4700x the geometrical mean of the estimated serum levels in the SELMA-women (Bornehag et al. Citation2019). An additional control group with unexposed embryos (eggs neither drilled nor injected) was included in the study (n = 16).
Embryo dissections and tissue sampling were performed at E19, i.e. two days before anticipated hatching. At this developmental stage, the sexes are easily distinguishable in control animals by the morphology of the gonads and presence/absence of Müllerian ducts. The embryos were euthanized by decapitation. Body weight and liver weight were recorded. The hepatosomatic index (HSI) was calculated as 100 × liver weight/body weight. The reproductive organs were visually inspected under a stereo microscope. Gonads were photographed through a stereo microscope and placed in RNA-later for subsequent qPCR analysis. Length of the right Müllerian duct was measured, if present, using a slide caliper. Due to the possibility of sexual differentiation being altered by the exposure, a piece of the liver was sampled for determination of genetic sex. All samplings and measurements apart from body weight and liver weight were recorded without knowledge of the exposure group.
Gonad size
Gonad size was measured by image analysis using the open-source software ImageJ (Schneider, Rasband, and Eliceiri Citation2012). The gonad size was defined as the gonad surface area visible in the images. The analysis was performed without knowledge of the exposure group or genetic sex.
Genetic sex determination
Female birds are heterogametic, having one copy each of the sex chromosomes Z and W, whereas males are homogametic and have two Z chromosomes. Genetic sex was determined from the liver samples, using a method based on the allele-specific products formed by the CDH1 gene (Fridolfsson and Ellegren Citation1999). DNA was extracted using TRIzol® Reagent (Invitrogen™, Thermo Fisher Scientific, Waltham, MA, USA), precipitated with absolute ethanol, washed in 0.1 M trisodium citrate buffer containing 10% ethanol, and dissolved in 8 mM NaOH. The PCR reaction mixtures (20 μl) consisted of iQ SYBR Green Supermix (Bio-Rad), 5 pmoles of each of forward and reverse primers and 1 µl of the extracted DNA. Amplification with CDH1 primers () was performed using a Rotor Gene 6000 real-time PCR machine (software version 1.7; Qiagen, Hilden, Germany) with the following program: hold at 94°C for 2 min followed by 40 cycles of 94°C for 30 sec, annealing for 30 sec, and 72°C for 35 sec. To increase product specificity, a touch-down protocol was used for the first cycles; i.e. the annealing temperature was lowered with 1 degree per cycle from 60°C down to 50°C and then was maintained at 50°C. The Z- and W-linked products were identified through melt curve analysis (55–95°C, 1°C per min) (Supplementary Figure 1).
Table 2. Primer information.
Quantitative real-time RT-PCR
Relative mRNA levels of genes involved in steroidogenesis, meiosis, and gonad differentiation were analyzed in the gonads. In the experiment on mixture S, the left ovary and both the right and left testis were analyzed (the unexposed controls were not included in this analysis). In the experiment on MBP, the left gonad of both sexes was analyzed. Isolation of RNA, cDNA synthesis, and real-time qPCR were performed using commercial kits according to the manufacturers’ instructions. In short, RNA isolation was performed using Aurum™ total RNA fatty and fibrous tissue kit (Bio-Rad, Hercules, CA, USA). Integrity of RNA was verified with agarose gel electrophoresis. The quantity and purity of RNA was determined spectrophotometrically (NanoDrop ND-1000; NanoDrop Technologies, Wilmington, DE, USA). For each sample, 450 ng (MBP experiment) or 1 µg (mixture S experiment) of total RNA was used to synthesize cDNA using the iScript cDNA Synthesis kit (Bio-Rad). Gene-specific primers were synthesized by Sigma-Aldrich (). The qPCR analysis was conducted using a Rotor Gene 6000 real-time PCR machine (Qiagen, Hilden, Germany). The reaction mixture consisted of iQ SYBR Green Supermix (Bio-Rad), forward and reverse primers at concentrations of 0.25 µM, and cDNA derived from 17 ng (mixture S experiment) or 5 ng (MBP experiment) of total RNA. The total reaction volume was 20 µl. All samples were analyzed in duplicate with the following PCR protocol: 95°C for 4 min, followed by 40 cycles of 95°C for 15 sec and 62°C for 45 sec. Melt curve analysis was performed at 55–95°C and the curve was studied to ensure that only one product was formed. β-Actin (ACTB) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were unaffected by the treatments and thus used as reference genes. The mean efficiency (E) of each primer pair was determined by the LinRegPCR program (Ruijter et al. Citation2009). The relative mRNA level of each gene of interest was calculated using Equation 1:
Data, regardless of sex, was normalized to the mean expression level of the left ovary in the DMSO control group.
Statistical analysis
Statistical analysis was performed using Prism 5 by GraphPad Software Inc. (San Diego, CA, USA). D’Agostino & Pearson omnibus normality test was used for assessing normality. If the data passed the normality test, a one-way analysis of variance (ANOVA) was performed followed by Dunnett’s test. When only two groups were compared a student’s t-test was used, with Welch’s correction if the variance differed between groups. If the normality test was not passed, even after transformation, Kruskal Wallis H test was performed followed by Dunn’s multiple comparisons test or Mann Whitney U test. Fisher’s exact test was applied on quantal response data. The treatment groups were compared with their respective control group. Pearson correlation was performed to assess the correlation between SOX9 expression and left testis size.
Results
General toxicity
Mortality was not significantly affected compared with the DMSO control in any of the exposed groups (), and was within the range of control animals in previous studies (Berg et al. Citation2004; Berg, Halldin, and Brunström Citation2001a; Biau et al. Citation2006; Brunström and Andersson Citation1988; Jönsson et al. Citation2016; Mattsson et al. Citation2015, Citation2019). The treatments did not induce any visible malformations but two control and two MBP-exposed animals exhibited necrotic tissue at the tip of the left lobe of the liver and one Mixture S-exposed animal displayed white dots on the kidneys. Body weight, liver weight, and hepatosomatic index (HSI) were not markedly affected by the exposures (), and did not differ between sexes.
Table 3. Cumulative mortality, body weight, liver weight, and hepatosomatic index (HSI) of 19-day-old unexposed chicken embryos and embryos exposed to MBP, the mixture S, or DMSO from E4a.
Gonad morphology and genetic sex
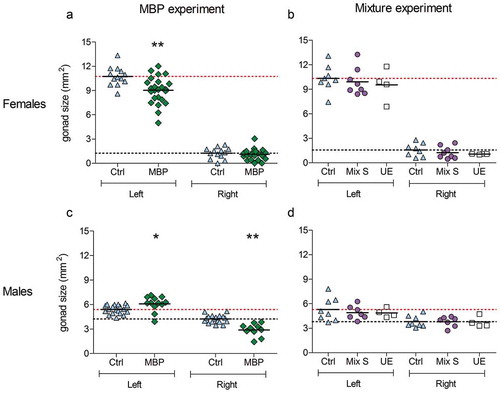
Representative images of control and MBP-exposed embryos of both sexes are illustrated in and the left and right gonad sizes of all groups are shown in . Control females exhibited a large left and a small, largely regressed, right ovary. Control males showed two testes of similar size and shape. The mean left ovary area was significantly smaller in MBP-exposed females than in control females ()) and in several MBP-exposed individuals the ovary appeared thinner than controls. On average, the right testis was significantly smaller and the left testis significantly larger in MBP-exposed males than in control males ()). All males exposed to MBP developed a left ovotestis, as assessed by the shape and structure of the left gonad. Four out of 12 MBP-exposed males were feminized to the degree that they were mistaken for females at the visual inspection during the dissection. These were determined to be males by genetic sex determination. In mixture-exposed embryos, the gonads appeared unaffected and the mean gonad area did not differ markedly from that of the control group in either of the sexes.
Figure 1. Representative images of male (♂) and female (♀) chicken gonads on embryonic day 19 after exposure to DMSO (control) or MBP (100 µg/g). The control embryos are shown in the left column and the MBP-exposed ones in the right. The right testicle (gray arrow) was smaller and the left testicle (white arrow) was larger in MBP-exposed males compared with those of control males. The left testicle in all MBP-exposed males was ovary-like in shape. The left ovary of the MBP-exposed females (black arrow) was smaller than in the control animals and appeared thin in some individuals, like the one in the image. The gonads of Mixture S-exposed females and males did not differ in appearance from those of control animals (not shown).
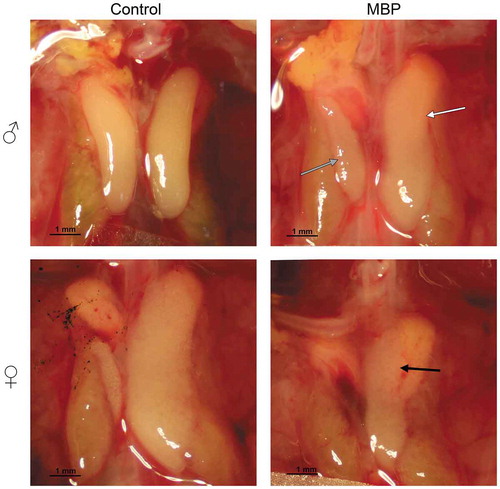
Figure 2. Effect of MBP and Mixture S on the size of the left and right gonad of 19-day-old female (a,b) and male (c,d) chicken embryos. In the MBP experiment, the embryos were exposed to DMSO (n = 13 females, 19 males) or MBP (n = 24 females, 12 males) from embryonic day 4. In the mixture experiment, the embryos were unexposed (unexposed control; UE; n = 4 of each sex) or exposed to DMSO (vehicle control; n = 8 of each sex) or the mixture S (n = 8 females, 7 males) from embryonic day 4. The solid lines represent the mean of each group. The upper (red) dotted lines represent the control mean of the left gonad whereas the lower (black) dotted lines show the control mean of the right gonad. The mean sizes of the left and right gonad within each group were compared with those in the vehicle control group. Data from the MBP experiment were analyzed with unpaired t-tests (with Welch’s correction when applicable). If needed to achieve normality, the data were transformed, either squared (male left) or log transformed (female right). One-way ANOVA followed by Dunnett’s test was used in the mixture experiment. Significance levels are indicated as * (p ≤ 0.05) or ** (p ≤ 0.01).
Müllerian ducts
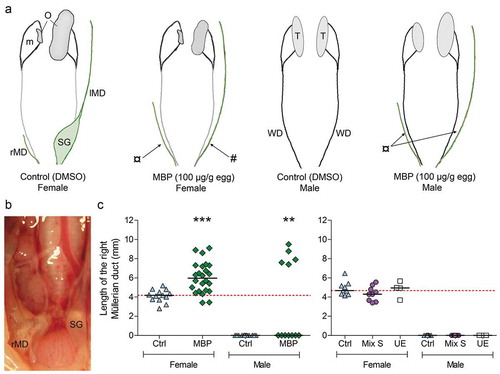
The Müllerian ducts on both sides appeared normal in all control females, and a shell gland had started to form at the caudal end of the left duct (). The major part of the right Müllerian duct was regressed and approximately 4 mm remained in female controls ()). The Müllerian ducts on both sides were completely regressed in control males ()). In MBP-exposed females, the right Müllerian duct was significantly longer than in controls, suggesting that MBP inhibited normal Müllerian duct regression ()). Seven out of 24 MBP-exposed females did not display a visible primordial shell gland ()) and one exhibited a noticeably small gland. One MBP-exposed female had small fluid-filled vesicles on the left Müllerian duct. Five out of 12 MBP-exposed males still possessed Müllerian ducts (,c)). The Müllerian ducts in mixture-exposed embryos did not differ markedly from those in the control in length or appearance.
Figure 3. (a) Schematic representations of gonads and accessory ducts in female and male chicken embryos at E19 following exposure to DMSO (vehicle control) or MBP from E4. The control embryos developed normally: at this stage, the shell gland (SG) has started to form in the caudal part of the left Müllerian duct (lMD) while the right Müllerian duct (rMD) is almost completely regressed in females, and both Müllerian ducts are completely regressed in males. The Wolffian ducts (WD) are present in both sexes but they have started to degenerate in females. The gonads (O: ovary, T: testis) lie ventrally to the mesonephros (m). Effects on Müllerian ducts are indicated by arrows. Seven out of 24 MBP-exposed females lacked the primordial shell gland (#) of the left Müllerian duct. Müllerian duct retention (¤) was observed in both females (right side) and males (both sides). (b) Müllerian ducts in the abdominal cavity of a control female at E19. The primordial shell gland can be seen on the left duct. (c) Mean length of the right Müllerian duct in unexposed embryos (unexposed control; UE) and in embryos exposed to either DMSO (vehicle control; Ctrl), MBP (100 µg/g egg), or Mixture S (in total 85 µg/g egg). Each exposure group was compared with the respective vehicle control group using either Kruskal Wallis H test followed by Dunn’s multiple comparison test or an unpaired t-test. Males which still had Müllerian ducts were only present in the MBP group. Fisher’s exact test was used to analyze the difference in frequency of embryos that still had a right Müllerian duct in control versus MBP-exposed males. Significance levels are indicated as ** (p < 0.01) and *** (p ≤ 0.001).
Gene expression
The mRNA expression of three analyzed genes implicated in ovarian differentiation, i.e. DNA meiotic recombinase 1 (DMC1), forkhead box L2 (FOXL2), and cytochrome P450 family 19 subfamily A member 1 (CYP19A1; aromatase), was upregulated in the left testis in MBP-treated males. Sex determining region Y-box 9 (SOX9), which is involved in testis differentiation, was downregulated in the same group. The analyzed genes were unaffected by MBP exposure in females. The mRNA expression results from the MBP experiment are presented in . The expression of SOX9 in MBP-treated males was significantly negatively correlated with size of the left gonad (). Exposure to Mixture S did not markedly affect gonadal mRNA expression of the analyzed genes in either sex (Supplementary Figure 2).
Figure 4. Levels of mRNA expression in the left gonad of female and male chicken embryos at embryonic day 19 following exposure to MBP (100 µg/g egg) or DMSO (vehicle control) from embryonic day 4. The values have been normalized to the geometric mean expression of two reference genes: ACTB and GAPDH. Each value is expressed relative to the mean of the female control group. The lines represent the group means. The mean levels in the MBP group were compared with those of the control group for the respective sex and gene using student’s t-test (DMRT1, SOX9 males, AMH, DMC1, CYP19A1, CYP17A1). Welch’s correction (DMC1 males, CYP19A1 females) or log transformation of values (AMH) were performed when required. Mann–Whitney U-test was used when the normality test was not passed (FOXL2 females). Samples with expression levels below the level of reliable quantification (LOQ, defined as a CT-value 3.4 times lower than the reverse transcriptase control) are denoted as x. The value below LOQ in FOXL2 (females; MBP) was excluded from the statistical analysis. For SOX9 (females), FOXL2 (males), and CYP19A1 (males), Fisher’s exact test was applied on the number of individuals that had expression values below LOQ versus those that did not. Significance levels are indicated as * (p ≤ 0.05) *** (p ≤ 0.001).
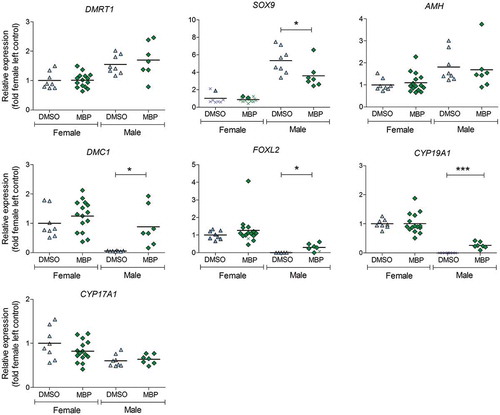
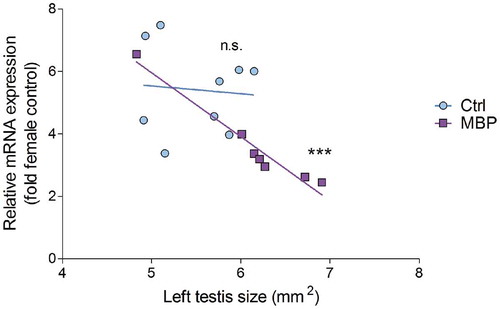
Figure 5. The relationship between relative mRNA expression of SOX9 in the left testicle and the size of this organ in control (light blue circles) and MBP-treated (dark purple squares) chicken embryos. Pearson correlation analysis was performed to assess whether the SOX9 expression correlates with the size of the left testicle in the control group (correlation coefficient: −0.086; R2: 0.007; p-value: 0.83) and in the MBP group (correlation coefficient: −0.98; R2: 0.95; p-value: 0.0002).
Some gene expressions fell below the level of a reliable quantification (LOQ), defined as a CT-value 3.4-fold lower than the reverse transcriptase control. The samples that were below LOQ are included in the graphs for visualization and transparency but it should be noted that there are uncertainties regarding their actual expression levels. The expression of the female-biased genes FOXL2 and CYP19A1 fell below LOQ in all control males, while the male-specific gene SOX9 was below LOQ in almost all females. For this reason, the differences in expression of these genes between treatment groups and their respective DMSO control group were analyzed by comparing the frequencies of embryos that displayed expression values below versus above LOQ.
Discussion
In this study, estrogenic effects of the suggested BPA metabolite MBP was demonstrated for the first time in an avian model. No marked effect was observed with a mixture of phthalate monoesters (Mixture S) that has been negatively associated with AGD in 21-month-old boys in a Swedish pregnancy cohort (Bornehag et al. Citation2019).
Chicken and quail embryos have been widely used for studying effects of xenoestrogens on sex differentiation (Berg et al. Citation1998, Citation1999; Brunström et al. Citation2009; Jessl, Scheider, and Oehlmann Citation2018a). Development of the reproductive tract of females in most bird species is asymmetric, so that the ovary and Müllerian duct on the left side differentiate and grow while those on the right side regress. In males, the early gonads differentiate into two similar testes and Müllerian ducts regress on both sides. In the present study, treatment of chicken embryos with MBP affected gonad size in a sex-dependent manner. Among the MBP-exposed embryos, females exhibited a smaller left ovary, and in males a left ovotestis and smaller right testis compared with controls. Developmental exposure to potent synthetic estrogens produces feminization of male gonads and retention as well as malformation of Müllerian ducts in both chicken and quail (Berg, Halldin, and Brunström Citation2001a; Berg et al. Citation1999; Mattsson, Olsson, and Brunström Citation2011; Rissman et al. Citation1984). Thus, the finding that MBP induced ovotestis in males harmonizes with the interpretation that MBP exerts an estrogenic mode of action. On the other hand, the smaller left ovary in MBP-exposed females is consistent with exposure to antiestrogens (Jessl et al. Citation2018b; Scheib Citation1983). This may be explained if MBP acts as a partial agonist, inferring it activates ER but does not reach the efficacy of endogenous estrogen. Such partial ER agonism might manifest differently in the sexes. In the female, where high levels of endogenous estrogens are present (Woods and Erton Citation1978), partial activation of ER by MBP may interfere with the normal estrogen-induced activation of ERα and hence result in a smaller ovary. In the male, however, where levels of endogenous estrogens are low, the presence of a xenoestrogenic compound would produce a net increase in estrogenic effect.
The males in the MBP group developed a left ovotestis. Similar results have been obtained with BPA (Berg, Halldin, and Brunström Citation2001a; Jessl, Scheider, and Oehlmann Citation2018a). Berg, Halldin, and Brunström (Citation2001a) found that BPA induced ovotestis formation in male chicken embryos as determined histologically at 200 µg/g egg but not at 67 µg/g egg. Jessl, Scheider, and Oehlmann (Citation2018a) noted an increase in cortex thickness in the left testis and reduction in cortex thickness in the left ovary after exposure to 75, 100, or 300 µg BPA/g egg. All 12 males exposed to 100 µg MBP/g egg in our study developed a left ovotestis, while approximately half of the males developed ovotestis at 200 µg BPA/g egg in the study by Berg, Halldin, and Brunström (Citation2001a). Jessl, Scheider, and Oehlmann (Citation2018a) observed an effect of BPA on cortex thickness already at 75 µg/g egg in both sexes, but there no marked effect on gonad size in males at this dose. These results suggest that MBP is at least as potent as BPA in terms of inducing ovotestis in chicken embryos.
Five males exposed to MBP still possessed Müllerian ducts at E19, a time when they normally have regressed completely. In females, the right Müllerian duct was not regressed to the same extent in the MBP group as in controls. Further, left Müllerian ducts lacking a shell gland were observed in approximately one-third of the MBP-exposed females. Berg, Halldin, and Brunström (Citation2001a) reported a significantly elevated frequency of Müllerian duct deformations in female quail exposed to BPA at 200 µg/g egg but not in those exposed to 67 µg/g egg, while no significant effect on Müllerian ducts was noted in chickens at either dose. Embryonic exposure to estrogens has also been reported to induce long-term effects on the oviducts. In some studies, estrogen exposure during development resulted in hens laying fewer eggs or eggs devoid of shell and/or membranes, consequences ascribed to morphological abnormalities in the oviducts (Greenwood and Blyth Citation1938; Rissman et al. Citation1984). Injection of Japanese quail eggs with the synthetic estrogen ethinylestradiol (EE2) resulted in morphological and histological malformations in the oviducts of the adult hens (Berg et al. Citation2001b). Similar treatment of chicken embryos altered the distribution and expression of carbonic anhydrase in the shell gland of the adult bird and initiated egg shell thinning (Berg et al. Citation2004). Thus there is strong evidence that the presence of xenoestrogenic compounds during embryonic development affects the shell gland in birds, even though the particular effect found in our study (lack of shell gland) has not previously been reported to our knowledge.
The analyzed genes were selected because of their implications in sex differentiation of the reproductive organs. All of the genes except AMH and CYP17A1 demonstrated clear sex-dependent expression in control gonads at E19. In male chicken embryos exposed to MBP, the female-associated gonadal genes CYP19A1, FOXL2, and DMC1 were induced and the expression of the testicular marker gene SOX9 was reduced. This feminized expression pattern corroborates the observed feminizing effect of MBP on testis morphology. Our results are consistent with effects from BPA exposure found previously. In a study by Yu et al. (Citation2018), induced mRNA expression of the meiosis-specific gene DMC1 was detected in 12- to 18-day-old chicken embryos exposed to 0.05 and 0.5 mg BPA/egg from E10.5. Expression of CYP19A1 mRNA was enhanced in the testes of 5–15-week-old White Leghorn male chicks following oral exposure to BPA from 2 weeks of age (Furuya et al. Citation2006) and expression of SOX9 was suppressed by BPA in mouse embryonic stem cells (Aoki and Takada Citation2012). In the present study, SOX9 expression, which is involved in testicular development, was markedly negatively correlated with the left testis area in MBP-treated males. As the testis becomes feminized it grows larger, and thus our results demonstrated that the more feminized testis (using size as an indicator), the lower expression of SOX9. In summary, the changes in mRNA expression in the left testis of MBP-treated males indicated feminization and are consistent with previously published effects attributed to BPA.
Exposure of humans and wildlife to this substance is unknown. A hypothesis on the potential mechanism for formation of MBP from BPA has been presented (Yoshihara et al. Citation2004) but to our knowledge no studies has been performed to verify this in vivo. It is therefore difficult to assess the environmental relevance of this substance. The main metabolite of BPA in mammals is a monoglucuronide (Kurebayashi et al. Citation2002; Snyder et al. Citation2000; Völkel et al. Citation2002). However, glucuronidation capacity is limited during early development (Bjerregaard et al. Citation2008; Coughtrie et al. Citation1988; Domoradzki et al. Citation2004; Matsumoto, Yokota, and Yuasa Citation2002; Strassburg et al. Citation2002) and it was therefore suggested that MBP is more likely to be formed in developing organisms than in adults (Yoshihara et al. Citation2004). The dose of MBP in the current study was selected such that it would be in the same range as doses in previous studies examining BPA effects in chicken (Berg, Halldin, and Brunström Citation2001a; Jessl, Scheider, and Oehlmann Citation2018a) rather than of environmental relevance. A future study on the dose–response relationship of MBP would provide information on potential effects at doses that are environmentally relevant for BPA. This is especially important considering the reported non-monotonic response to increasing BPA doses in experimental studies (Vandenberg Citation2013).
There were no significant effects on morphology of either gonads or Müllerian ducts induced by the phthalate monoester mixture. No marked effect on mRNA expression of any of the genes was detected. The dose of phthalates administered to the eggs (85 µg/g egg = 331 nmol/mL, assuming 1 g egg = 1 ml in volume) was 4700-fold higher than the geometrical mean of the estimated plasma levels (0.07 nmol/ml) in the SELMA women (Bornehag et al. Citation2019). In an unpublished study, a mixture of several common environmental pollutants (including the two mixture S components monoethylhexyl phthalate and monobenzyl phthalate) was administered to chicken embryos in the same manner as in this study and the plasma level of each of the substances was analyzed at E11 and E16. Both mixture S components were present in the plasma at these times at levels that corresponded to 0.2–3.6% of the administered dose. In the present study, this would represent a 9-169-fold higher concentration in the plasma of embryos than the geometrical mean of the serum concentration in the SELMA women, assuming similar kinetics in the chicken eggs as in the previous study. Based on this, it was assumed that the phthalates were absorbed and present in the plasma during gonadal differentiation.
Phthalate monoesters are metabolites of phthalate diesters that are extensively used as plasticizers. In mammals, phthalate exposure in utero results in shortened AGD and other symptoms that indicate an antiandrogenic mode of action (Dorman et al. Citation2018; Lee and Koo Citation2007; Mylchreest, Cattley, and Foster Citation1998; Parks et al. Citation2000). It is possible that potential antiandrogenic effects of the tested substances might remain undetected in the present study. The role of androgens and effects of antiandrogens on reproductive organ development in the chicken are not completely established but there are studies suggesting that disruption of androgen signaling may initiate changes in ovarian histology. For instance, exposure to androgens prevents regression of ovarian medulla and at high doses even stimulates development of testicular-like cords in the ovary (Willier, Rawles, and Koch Citation1938). Exposure to the antiandrogenic substance flutamide produced disorganization of the sex cords in the cortex of the ovary of developing chicken embryos, an effect counteracted by testosterone (Katoh, Ogino, and Yamada Citation2006). One cannot exclude the possibility that similar effects occurred in our study as a histological analysis of the gonads was not performed. On the other hand, the unaffected gene expression profile in gonads after exposure to mixture S suggests no major effects on ovarian (or testicular) differentiation in the chicken embryos.
Conclusions
4-Methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene (MBP) induced abnormal Müllerian duct retention and altered gonadal morphology in both male and female chicken embryos, suggesting estrogenic and possibly also antiestrogenic properties. The altered mRNA expression patterns in the left testis of MBP-treated males further strengthen the evidence of MBP-induced feminization of the embryos. Further, some MBP-exposed females lacked a shell gland, a feature that to our knowledge has not previously been reported following exposure to BPA or other estrogenic compounds. This finding calls for further studies to elucidate potential additional targets of MBP. The mixture of phthalate monoesters did not produce any apparent visible effects on morphology of gonads or Müllerian ducts and did not alter gonadal mRNA expression of genes involved in sex differentiation. We conclude that MBP, but not the mixture of phthalate monoesters, disrupts sexual development in the avian model at the studied doses.
Declaration of interest
The authors declare no conflict of interest.
Supplemental Material
Download MS Word (524 KB)Acknowledgments
This work was supported by the European Union’s Horizon 2020 research and innovation programme under grant agreement No 634880. The study was performed within the EU project EDC-MixRisk. The phthalate mixture was prepared by Dr Christian Lindh, Division of Occupational and Environmental Medicine, Lund University. We would like to thank Professor Björn Brunström, Department of Environmental Toxicology, Uppsala University, for valuable scientific discussions.
Supplementary Data
Supplementary material for this article can be accessed here.
Additional information
Funding
References
- Acconcia, F., V. Pallottini, and M. Marino. 2015. Molecular mechanisms of action of BPA. Dose-Response 13:1559325815610582. doi:10.1177/1559325815610582.
- Aoki, T., and T. Takada. 2012. Bisphenol A modulates germ cell differentiation and retinoic acid signaling in mouse ES cells. Reprod. Toxicol. 34:463–70. doi:10.1016/j.reprotox.2012.06.001.
- Baker, M. E., and C. Chandsawangbhuwana. 2012. 3D models of MBP, a biologically active metabolite of Bisphenol A, in human estrogen receptor α and estrogen receptor β. PLoS ONE 7:e46078. doi:10.1371/journal.pone.0046078.
- Berg, C., A. Blomqvist, L. Holm, I. Brandt, B. Brunström, and Y. Ridderstrale. 2004. Embryonic exposure to oestrogen causes eggshell thinning and altered shell gland carbonic anhydrase expression in the domestic hen. Reproduction 128:455–61. doi:10.1530/rep.1.00211.
- Berg, C., K. Halldin, and B. Brunström. 2001a. Effects of bisphenol A and tetrabromobisphenol A on sex organ development in quail and chicken embryos. Environ. Toxicol. Chem. 20:2836–40. doi:10.1002/etc.v20:12.
- Berg, C., K. Halldin, A. K. Fridolfsson, I. Brandt, and B. Brunström. 1999. The avian egg as a test system for endocrine disrupters: Effects of diethylstilbestrol and ethynylestradiol on sex organ development. Sci. Total Environ. 233:57–66. doi:10.1016/S0048-9697(99)00179-5.
- Berg, C., L. Holm, I. Brandt, and B. Brunström. 2001b. Anatomical and histological changes in the oviducts of Japanese quail, Coturnix japonica, after embryonic exposure to ethynyloestradiol. Reproduction 121:155–65. doi:10.1530/rep.0.1210155.
- Berg, C., K. Halldin, B. Brunström, and I. Brandt. 1998. Methods for studying xenoestrogenic effects in birds. Toxicol. Lett. 102-103:671–76. doi:10.1016/S0378-4274(98)00285-9.
- Biau, S., S. Bayle, P. de Santa Barbara, and B. Roig. 2006. The chick embryo: An animal model for detection of the effects of hormonal compounds. Anal. Bioanal. Chem. 38:1397.
- Bjerregaard, L. B., C. Lindholst, B. Korsgaard, and P. Bjerregaard. 2008. Sex hormone concentrations and gonad histology in brown trout (Salmo trutta) exposed to 17β-estradiol and bisphenol A. Ecotoxicology 17:252–63. doi:10.1007/s10646-008-0192-2.
- Bornehag, C.-G., F. Carlstedt, B. A. G. Jönsson, C. H. Lindh, T. K. Jensen, A. Bodin, C. Jonsson, S. Janson, and S. H. Swan. 2015. Prenatal phthalate exposures and anogenital distance in Swedish boys. Environ. Health Perspect. 123:101–07. doi:10.1289/ehp.1408163.
- Bornehag, C.-G., E. Kitraki, A. Stamatakis, E. Panagiotidou, C. Rudén, H. Shu, C. Lindh, J. Ruegg, and C. Gennings. 2019. A novel approach to chemical mixture risk assessment – Linking data from population based epidemiology and experimental animal tests. Risk Anal. doi:10.1111/risa.13323.
- Bornehag, C. G., S. Moniruzzaman, M. Larsson, C. B. Lindstrom, M. Hasselgren, A. Bodin, L. B. von Kobyletzkic, F. Carlstedt, F. Lundin, E. Nanberg, et al. 2012. The SELMA study: A birth cohort study in Sweden following more than 2000 mother-child pairs. Paediatr. Perinat. Epidemiol. 26:456–67. doi:10.1111/j.1365-3016.2012.01314.x.
- Brown, A. R., J. M. Green, J. Moreman, L. M. Gunnarsson, S. Mourabit, J. Ball, M. J. Winter, M. Trznadel, A. Correia, C. Hacker, et al. 2019. Cardiovascular effects and molecular mechanisms of Bisphenol A and its metabolite MBP in Zebrafish. Environ. Sci. Technol. 53:463–74. doi:10.1021/acs.est.8b04281.
- Brunström, B., and L. Andersson. 1988. Toxicity and 7-ethoxyresorufin O-deethylase-inducing potency of coplanar polychlorinated biphenyls (PCBs) in chick embryos. Arch. Toxicol. 62:263–66. doi:10.1007/BF00332485.
- Brunström, B., J. Axelsson, A. Mattsson, and K. Halldin. 2009. Effects of estrogens on sex differentiation in Japanese quail and chicken. Gen. Comp. Endocrinol. 163:97–103. doi:10.1016/j.ygcen.2009.01.006.
- Camacho, L., S. M. Lewis, M. M. Vanlandingham, G. R. Olson, K. J. Davis, R. E. Patton, N. C. Twaddle, D. R. Doerge, M. I. Churchwell, M. S. Bryant, et al. 2019. A two-year toxicology study of bisphenol A (BPA) in Sprague-Dawley rats: CLARITY-BPA core study results. Food Chem. Toxicol. 132:110728. doi:10.1016/j.fct.2019.110728.
- Christiansen, S., M. Axelstad, J. Boberg, A. M. Vinggaard, G. A. Pedersen, and U. Hass. 2014. Low-dose effects of bisphenol A on early sexual development in male and female rats. Reproduction 147:477–87. doi:10.1530/REP-13-0377.
- Corrales, J., L. A. Kristofco, W. B. Steele, B. S. Yates, C. S. Breed, E. S. Williams, and B. W. Brooks. 2015. Global assessment of Bisphenol A in the environment: Review and analysis of its occurrence and bioaccumulation. Dose-Response 13:1559325815598308. doi:10.1177/1559325815598308.
- Coughtrie, M. W., B. Burchell, J. E. Leakey, and R. Hume. 1988. The inadequacy of perinatal glucuronidation: Immunoblot analysis of the developmental expression of individual UDP-glucuronosyltransferase isoenzymes in rat and human liver microsomes. Mol. Pharmacol. 34:729–35.
- De Campos, P., I. M. Oliveira, J. S. deSouza, R. R. da Concelicao, G. Giannocco, M. I. Chiamolera, M. R. D. Silva, M. A. Romano, and M. Romano. 2019. Maternal bisphenol A exposure disrupts permatogenesis in adult rat offspring. J. Toxicol. Environ. Health Part A 82:163–75. doi:10.1080/15287394.2019.1572557.
- DeFalco, T., and B. Capel. 2009. Gonad morphogenesis in vertebrates: Divergent means to a convergent end. Annu. Rev. Cell Dev. Biol. 25:457–82. doi:10.1146/annurev.cellbio.042308.13350.
- Domoradzki, J. Y., C. M. Thornton, L. H. Pottenger, S. C. Hansen, T. L. Card, D. A. Markham, M. D. Dryzga, R. N. Shiotsuka, and J. M. Waechter Jr. 2004. Age and dose dependency of the pharmacokinetics and metabolism of bisphenol A in neonatal sprague-dawley rats following oral administration. Toxicol. Sci. 77:230–42. doi:10.1093/toxsci/kfh054.
- Dorman, D. C., W. Chiu, B. F. Hales, R. Hauser, K. J. Johnson, E. Mantus, S. Martel, K. A. Robinson, A. A. Rooney, R. Rudel, et al. 2018. Systematic reviews and meta-analyses of human and animal evidence of prenatal diethylhexyl phthalate exposure and changes in male anogenital distance. J. Toxicol. Environ. Health B 21:207–26. doi:10.1080/10937404.2018.1505354.
- Fridolfsson, A.-K., and H. Ellegren. 1999. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 30:116–21. doi:10.2307/3677252.
- Furuya, M., K. Adachi, S. Kuwahara, K. Ogawa, and Y. Tsukamoto. 2006. Inhibition of male chick phenotypes and spermatogenesis by Bisphenol-A. Life Sci. 78:1767–76. doi:10.1016/j.lfs.2005.08.016.
- Gani, K. M., V. K. Tyagi, and A. A. Kazmi. 2017. Occurrence of phthalates in aquatic environment and their removal during wastewater treatment processes: A review. Environ. Sci. Pollut. Res. 24:17267–84. doi:10.1007/s11356-017-9182-3.
- Gray, L. E., Jr., J. Ostby, J. Furr, M. Price, D. N. Veeramachaneni, and L. Parks. 2000. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol. Sci. 58:350–65. doi:10.1093/toxsci/58.2.350.
- Gray, T. J. B., I. R. Rowland, P. M. D. Foster, and S. D. Gangolli. 1982. Species differences in the testicular toxicity of phthalate esters. Toxicol. Lett. 11:141–47. doi:10.1016/0378-4274(82)90119-9.
- Greenwood, A. W., and J. S. S. Blyth. 1938. Experimental modification of the accessory sexual apparatus in the hen. Quart. J. Exp. Physiol. Cogn. Med. Sci. 28:61–69.
- Habert, R., V. Muczynski, A. Lehraiki, R. Lambrot, C. Lécureuil, C. Levacher, H. Coffigny, C. Pairault, D. Moison, R. Frydman, et al. 2009. Adverse effects of endocrine disruptors on the foetal testis development: Focus on the phthalates.Folia Histochem Cytobiol.47:S67–S74. doi:10.2478/v10042-009-0056-5.
- Hirao-Suzuki, M., S. Takeda, K. Okuda, M. Takiguchi, and S. Yoshihara. 2019. Repeated exposure to 4-Methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene (MBP), an active metabolite of Bisphenol A, aggressively stimulates breast cancer cell growth in an estrogen receptor β (ERβ)-dependent manner. Mol. Pharmacol. 95:260–68. doi:10.1124/mol.118.114124.
- Howdeshell, K. L., J. Furr, C. R. Lambright, V. S. Wilson, B. C. Ryan, and L. E. Gray Jr. 2008. Gestational and lactational exposure to ethinyl estradiol, but not bisphenol A, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male long evans hooded rat. Toxicol. Sci. 102:371–82. doi:10.1093/toxsci/kfm306.
- Ishibashi, H., N. Watanabe, N. Matsumura, M. Hirano, Y. Nagao, H. Shiratsuchi, S. Kohra, S.-I. Yoshihara, and K. Arizono. 2005. Toxicity to early life stages and an estrogenic effect of a bisphenol A metabolite, 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene on the medaka (Oryzias latipes). Life Sci. 77:2643–55. doi:10.1016/j.lfs.2005.03.025.
- Jessl, L., R. Lenz, F. G. Massing, J. Scheider, and J. Oehlmann. 2018b. Effects of estrogens and antiestrogens on gonadal sex differentiation and embryonic development in the domestic fowl (Gallus gallus domesticus). PeerJ 6:e5094. doi:10.7717/peerj.5094.
- Jessl, L., J. Scheider, and J. Oehlmann. 2018a. The domestic fowl (Gallus gallus domesticus) embryo as an alternative for mammalian experiments – Validation of a test method for the detection of endocrine disrupting chemicals. Chemosphere 196:502–13. doi:10.1016/j.chemosphere.2017.12.131.
- Johnson, K. J., N. E. Heger, and K. Boekelheide. 2012. Of mice and men (and rats): Phthalate-induced fetal testis endocrine disruption is species-dependent. Toxicol. Sci. 129:235–48. doi:10.1093/toxsci/kfs206.
- Jönsson, M. E., A. Mattsson, S. Shaik, and B. Brunström. 2016. Toxicity and cytochrome P450 1A mRNA induction by 6-formylindolo[3,2-b]carbazole (FICZ) in chicken and Japanese quail embryos. Comp. Biochem. Physiol. Toxicol. Pharmacol. 179:125–36. doi:10.1016/j.cbpc.2015.09.014.
- Katoh, H., Y. Ogino, and G. Yamada. 2006. Cloning and expression analysis of androgen receptor gene in chicken embryogenesis. FEBS Lett. 580:1607–15. doi:10.1016/j.febslet.2006.01.093.
- Kurebayashi, H., R. Harada, R. K. Stewart, H. Numata, and Y. Ohno. 2002. Disposition of a low dose of Bisphenol A in male and female Cynomolgus monkeys. Toxicol. Sci. 68:32–42. doi:10.1093/toxsci/68.1.32.
- Lee, B. M., and H. J. Koo. 2007. Hershberger assay for antiandrogenic effects of phthalates. J. Toxicol. Environ. Health Part A 70:1365–70. doi:10.1080/15287390701432285.
- Lin, H., R.-S. Ge, G.-R. Chen, G.-X. Hu, L. Dong, -Q.-Q. Lian, D. O. Hardy, C. M. Sottas, X.-K. Li, and M. P. Hardy. 2008. Involvement of testicular growth factors in fetal Leydig cell aggregation after exposure to phthalate in utero. Proc. Natl. Acad. Sci. 105:7218–22. doi:10.1073/pnas.0709260105.
- Matsumoto, J., H. Yokota, and A. Yuasa. 2002. Developmental increases in rat hepatic microsomal UDP-glucuronosyltransferase activities toward xenoestrogens and decreases during pregnancy. Environ. Health Perspect. 110:193–96. doi:10.1289/ehp.02110193.
- Mattsson, A., A. Kärrman, R. Pinto, and B. Brunström. 2015. Metabolic profiling of chicken embryos exposed to perfluorooctanoic acid (PFOA) and agonists to peroxisome proliferator-activated receptors. PLoS ONE 10:e0143780–e0143780. doi:10.1371/journal.pone.0143780.
- Mattsson, A., J. A. Olsson, and B. Brunström. 2011. Activation of estrogen receptor alpha disrupts differentiation of the reproductive organs in chicken embryos. Gen. Comp. Endocrinol. 172:251–59. doi:10.1016/j.ygcen.2011.03.010.
- Mattsson, A., S. Sjöberg, A. Kärrman, and B. Brunström. 2019. Developmental exposure to a mixture of perfluoroalkyl acids (PFAAs) affects the thyroid hormone system and the bursa of Fabricius in the chicken. Sci. Rep. 9:19808. doi:10.1038/s41598-019-56200-9.
- Meeker, J., and K. K. Ferguson. 2014. Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011–2012. J. Clin. Endocrinol. Metab. 99:4346–52. doi:10.1210/jc.2014-2555.
- Michałowicz, J. 2014. Bisphenol A – Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 37:738–58. doi:10.1016/j.etap.2014.02.003.
- Mieritz, M., G., . H. Frederiksen, K. Sørensen, L. Aksglaede, A. Mouritsen, C. P. Hagen, N. E. Skakkebaek, A.-M. Andersson, and A. Juul. 2012. Urinary phthalate excretion in 555 healthy Danish boys with and without pubertal gynaecomastia. Int. J. Androl. 35:227–35. doi:10.1111/j.1365-2605.2012.01279.x.
- Moreman, J., A. Takesono, M. Trznadel, M. J. Winter, A. Perry, M. E. Wood, N. J. Rogers, T. Kudoh, and C. R. Tyler. 2018. Estrogenic mechanisms and cardiac responses following early life exposure to Bisphenol A (BPA) and its metabolite 4-Methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene (MBP) in zebrafish. Environ. Sci. Technol. 52:6656–65. doi:10.1021/acs.est.8b01095.
- Mylchreest, E., R. C. Cattley, and P. M. D. Foster. 1998. Male reproductive tract malformations in rats following gestational and lactational exposure to di(n-butyl) phthalate: An antiandrogenic mechanism? Toxicol. Sci. 43:47–60. doi:10.1093/toxsci/43.1.47.
- Mylchreest, E., D. G. Wallace, R. C. Cattley, and P. M. D. Foster. 2000. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to di(n-butyl) phthalate during late gestation. Toxicol. Sci. 55:143–51. doi:10.1093/toxsci/55.1.143.
- Nehring, I., M. Staniszewska, and L. Falkowska. 2017. Human hair, Baltic grey seal (Halichoerus grypus) fur and herring gull (Larus argentatus) feathers as accumulators of Bisphenol A and alkylphenols. Arch. Environ. Contam. Toxicol. 72:552–61. doi:10.1007/s00244-017-0402-0.
- Newbold, R. R., W. N. Jefferson, and E. Padilla-Banks. 2007. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod. Toxicol. 24:253–58. doi:10.1016/j.reprotox.2007.07.006.
- Okuda, K., M. Takiguchi, and S. Yoshihara. 2010. In vivo estrogenic potential of 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene, an active metabolite of bisphenol A, in uterus of ovariectomized rat. Toxicol. Lett. 197:7–11. doi:10.1016/j.toxlet.2010.04.017.
- Parks, L. G., J. S. Ostby, C. R. Lambright, B. D. Abbott, G. R. Klinefelter, N. J. Barlow, and L. E. Gray. 2000. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol. Sci. 58:339–49. doi:10.1093/toxsci/58.2.339.
- Prins, G. S., H. B. Patisaul, S. M. Belcher, and L. N. Vandenberg. 2019. CLARITY-BPA academic laboratory studies identify consistent low-dose Bisphenol A effects on multiple organ systems. Basic Clin. Pharmacol. Toxicol. 125 (S3):14–31. doi:10.1111/bcpt.13125.
- Radke, E. G., J. M. Braun, J. D. Meeker, and G. S. Cooper. 2018. Phthalate exposure and male reproductive outcomes: A systematic review of the human epidemiological evidence. Environ. Int. 121:764–93. doi:10.1016/j.envint.2018.07.029.
- Rehan, M., E. Ahmad, I. A. Sheikh, A. M. Abuzenadah, G. A. Damanhouri, O. S. Bajouh, S. F. AlBasri, M. M. Assiri, and M. A. Beg. 2015. Androgen and progesterone receptors are targets for bisphenol A (BPA), 4-Methyl-2,4-bis-(p-hydroxyphenyl)pent-1-ene – A potent metabolite of BPA, and 4-tert-octylphenol: A computational insight. PLoS ONE 10:e0138438. doi:10.1371/journal.pone.0138438.
- Repouskou, A., E. Panagiotidou, L. Panagopoulou, P. L. Bisting, A., . R. Tuck, M. O. D. Sjödin, J. Lindberg, E. Bozas, J. Rüegg, C. Gennings, et al. 2019. Gestational exposure to an epidemiologically defined mixture of phthalates leads to gonadal dysfunction in mouse offspring of both sexes. Sci. Rep. 9:6424. doi:10.1038/s41598-019-42377-6.
- Rissman, E. F., M. Ascenzi, P. Johnson, and E. Adkins-Regan. 1984. Effect of embryonic treatment with oestradiol benzoate on reproductive morphology, ovulation and oviposition and plasma LH concentrations in female quail (Coturnix coturnix japonica). J. Reprod. Fertil. 71:411–17. doi:10.1530/jrf.0.0710411.
- Rochester, J. R. 2013. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 42:132–55. doi:10.1016/j.reprotox.2013.08.008.
- Romanoff, A. L. 1960. The Avian Embryo; Structural and Functional Development. New York: Macmillan.
- Ruijter, J. M., C. Ramakers, W. M. H. Hoogaars, Y. Karlen, O. Bakker, M. J. B. van den Hoff, and A. F. M. Moorman. 2009. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucl. Acids Res. 37:e45–e45. doi:10.1093/nar/gkp045.
- Ryan, B. C., A. K. Hotchkiss, K. M. Crofton, and L. E. Gray Jr. 2010. In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicol. Sci. 114:133–48. doi:10.1093/toxsci/kfp266.
- Santangeli, S., F. Maradonna, I. Olivotto, C. C. Piccinetti, G. Gioacchini, and O. Carnevali. 2017. Effects of BPA on female reproductive function: The involvement of epigenetic mechanism. Gen. Comp. Endocrinol. 245:122–26. doi:10.1016/j.ygcen.2016.08.010.
- Scheib, D. 1983. Effects and role of estrogens in avian gonadal differentiation. Mechanisms of Gonadal Differentiation in Vertebrates: Contributions of an EMBO-Workshop held in Freiburg, Berlin, Heidelberg: Springer Berlin Heidelberg, November 5–8, 1982.
- Schneider, C. A., W. S. Rasband, and K. W. Eliceiri. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9:671. doi:10.1038/nmeth.2089.
- Shono, T., H. Kai, S. Suita, and H. Nawata. 2000. Time-specific effects of mono-n-butyl phthalate on the transabdominal descent of the testis in rat fetuses. Br. J. Urol. Int. 86:121–25. doi:10.1046/j.1464-410x.2000.00710.x.
- Skakkebæk, N. E., E. Rajpert-De Meyts, and K. M. Main. 2001. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environmental aspects: Opinion. Human Reprod. 16:972–78. doi:10.1093/humrep/16.5.972.
- Snyder, R. W., S. C. Maness, K. W. Gaido, F. Welsch, S. C. J. Sumner, and T. R. Fennell. 2000. Metabolism and disposition of Bisphenol A in female rats. Toxicol. Appl. Pharmacol. 168:225–34. doi:10.1006/taap.2000.9051.
- Strassburg, C. P., A. Strassburg, S. Kneip, A. Barut, R. H. Tukey, B. Rodeck, and M. P. Manns. 2002. Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut 50:259. doi:10.1136/gut.50.2.259.
- Suvorov, A., and D. J. Waxman. 2015. Early programing of uterine tissue by bisphenol A: Critical evaluation of evidence from animal exposure studies. Reprod. Toxicol. 57:59–72. doi:10.1016/j.reprotox.2015.05.008.
- Swan, S. H., S. Sathyanarayana, E. S. Barrett, S. Janssen, F. Liu, R. H. Nguyen, and J. B. Redmon. 2015. First trimester phthalate exposure and anogenital distance in newborns. Human Reprod. 30:963–72. doi:10.1093/humrep/deu363.
- Tamschick, S., B. Rozenblut-Kościsty, M. Ogielska, D. Kekenj, F. Gajewski, A. Krüger, W. Kloas, and M. Stöck. 2016. The plasticizer bisphenol A affects somatic and sexual development, but differently in pipid, hylid and bufonid anurans. Pollut 216:282–91. doi:10.1016/j.envpol.2016.05.091.
- Vandenberg, L. N. 2013. Non-monotonic dose responses in studies of endocrine disrupting chemicals: Bisphenol a as a case study. Dose-response 12:259–76. doi:10.2203/dose-response.13-020.Vandenberg.
- Vandenberg, L. N., R. Hauser, M. Marcus, N. Olea, and W. V. Welshons. 2007. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 24:139–77. doi:10.1016/j.reprotox.2007.07.010.
- Völkel, W., T. Colnot, G. A. Csanády, J. G. Filser, and W. Dekant. 2002. Metabolism and kinetics of Bisphenol A in humans at low doses following oral administration. Chem. Res. Toxicol. 15:1281–87. doi:10.1021/tx025548t.
- WHO. 2012. State of the science of endocrine disrupting chemicals–2012. Geneva, Switzerland: World Health Organization.
- Williams, C., M. Bondesson, D. N. Krementsov, and C. Teuscher. 2014. Gestational bisphenol A exposure and testis development. Endocr. Disruptors (Austin) 2:e29088.
- Willier, B. H., M. E. Rawles, and F. C. Koch. 1938. Biological differences in the action of synthetic male hormones on the differentiation of sex in the chick embryo. Proc. Natl. Acad. Sci. USA 24:176. doi:10.1073/pnas.24.4.176.
- Woods, J. E., and L. H. Erton. 1978. The synthesis of estrogens in the gonads of the chick embryo. Gen. Comp. Endocrinol. 36:360–70. doi:10.1016/0016-6480(78)90117-X.
- Wormuth, M., M. Scheringer, M. Vollenweider, and K. Hungerbühler. 2006. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 26:803–24. doi:10.1111/j.1539-6924.2006.00770.x.
- Yoon., K., S. J. Kwack, H. S. Kim, and B.-M. Lee. 2014. Estrogenic endocrine-disrupting chemicals: Molecular mechanisms of action on putative human diseases. J. Toxicol. Environ. Health B 17:127–74. doi:10.1080/10937404.2014.882194.
- Yoshihara, S., M. Makishima, N. Suzuki, and S. Ohta. 2001. Metabolic activation of Bisphenol A by rat liver S9 fraction. Toxicol. Sci. 62:221–27. doi:10.1093/toxsci/62.2.221.
- Yoshihara, S., T. Mizutare, M. Makishima, N. Suzuki, N. Fujimoto, K. Igarashi, and S. Ohta. 2004. Potent estrogenic metabolites of Bisphenol A and Bisphenol B formed by rat liver S9 fraction: Their structures and estrogenic potency. Toxicol. Sci. 78:50–59. doi:10.1093/toxsci/kfh047.
- Yu, M., Y. Xu, M. Li, D. Li, Y. Lu, D. Yu, and W. Du. 2018. Bisphenol A accelerates meiotic progression in embryonic chickens via the estrogen receptor β signaling pathway. Gen. Comp. Endocrinol. 259:66–75. doi:10.1016/j.ygcen.2017.11.004.
