ABSTRACT
Sarcoidosis is a chronic granulomatous disease predominantly affecting the lungs and inducing significant morbidity and elevated mortality rate. The etiology of the disease is unknown but may involve exposure to an antigenic agent and subsequent inflammatory response resulting in granuloma formation. Various environmental and occupational risk factors have been suggested by previous observations, such as moldy environments, insecticides, and bird breeding. Our study investigated the association of air pollution with diagnosis of sarcoidosis using a case-control design. Penn State Health electronic medical records from 2005 to 2018 were examined for adult patients with (cases) and without (controls) an International Classification of Disease (ICD)-9 or −10 code for sarcoidosis. Patient addresses were geocoded and 24-hr residential-level air pollution concentrations were estimated using spatio-temporal models of particulate matter <2.5 μm (PM2.5), ozone, and PM2.5 elemental carbon (EC) and moving averages calculated. In total, 877 cases and 34,510 controls were identified. Logistic regression analysis did not identify significant associations between sarcoidosis incidence and air pollution exposure estimates. However, the odds ratio (OR) for EC for exposures occurring 7–10 years prior did approach statistical significance, and ORs exhibited an increasing trend for longer averaging periods. Data suggested a latency period of more than 6 years for PM2.5 and EC for reasons that are unclear. Overall, results for PM2.5 and EC suggest that long-term exposure to traffic-related air pollution may contribute to the development of sarcoidosis and emphasize the need for additional research and, if the present findings are substantiated, for public health interventions addressing air quality as well as increasing disease surveillance in areas with a large burden of PM2.5 and EC.
Introduction
Sarcoidosis is a chronic, multi-system disease characterized by granulomatous inflammation (Nunes et al. Citation2007; Sève et al. Citation2021). The granulomas may be located in various organs (Cooper and Suau Citation2023). The most common sites in the general population are lung, skin, and eyes (Brito-Zerón, Pérez-Álvarez, and Ramos-Casals Citation2022; Iannuzzi, Rybicki, and Teirstein Citation2007; Rossides et al. Citation2023). Almost all patients with sarcoidosis, even at sites other than the lung, develop pulmonary disease during the course of disease (Spagnolo et al. Citation2018; Wanat and Rosenbach Citation2015). Deposition of granulomas in the lungs may initiate respiratory symptoms including cough or dyspnea or may be detected incidentally on chest X-ray (Spagnolo et al. Citation2018). These granulomas may resolve spontaneously or produce permanent pulmonary scarring leading to significant morbidity and increased mortality rates (Grunewald et al. Citation2019).
The incidence of disease varies with age, gender, and race (Arkema and Cozier Citation2020; Duvall et al. Citation2023; Nunes et al. Citation2007; Rossides et al. Citation2023). In the US, evidence suggests race is a significant risk factor in development of sarcoidosis with Black patients being diagnosed with sarcoidosis at an approximately 3-fold higher rate than White patients (Baughman et al. Citation2016). The annual prevalence of sarcoidosis in Black patients is estimated at approximately 141.4 cases per 100,000 compared with 49.8 per 100,000 in White individuals residing in the US (Baughman et al. Citation2016; Hena Citation2020; Rybicki et al. Citation1997). Sarcoidosis is also more common in women than men (Baughman et al. Citation2001; Wanat and Rosenbach Citation2015).
Although the etiology of sarcoidosis is currently unknown, several investigators suggested exposure to an immunogenic antigen in a genetically susceptible individual is involved (Bennett et al. Citation2019; Llanos and Hamzeh Citation2019; Valeyre et al. Citation2014). Following exposure, antigen presenting cells interact with Th1 CD4+ helper T-cells resulting in hyperactivity of the cell-mediated immune system and formation of non-caseating, epithelioid granulomas (Bennett et al. Citation2019; Llanos and Hamzeh Citation2019). Research into the antigenic agent that initiates immunologic activation is ongoing and many possible mechanistic pathways are currently being explored. Some possible antigenic triggers for sarcoidosis include autoimmune reactions, infections, medications, and tattoos, as well as exposure to certain minerals such as silica, calcium phosphate, and calcium carbonate (Celada, Hawkins, and Drake Citation2015; Colboc et al. Citation2016, Citation2019; Cornejo et al. Citation2019; Kushima et al. Citation2020; Marcoval et al. Citation2001).
Previous studies also reported that multiple occupations such as bird breeders and those working in the automotive industry exhibit higher rates of sarcoidosis (Grunewald et al. Citation2019; Llanos and Hamzeh Citation2019; Newman et al. Citation2004). In addition, firefighters and first responders who responded to the World Trade Center disaster were also found to be at higher risk of sarcoidosis, possibly due to silica exposures (Crowley et al. Citation2011; Perlman et al. Citation2011). Specific air pollutants such as particulate silica and beryllium were also noted to enhance worker’s risk of developing sarcoidosis in miners and coal workers (Graff et al. Citation2020; Hayashi et al. Citation2020; Jonsson, Järvholm, and Andersson Citation2019; Seaton Citation2020). Consequently, Grunewald et al. (Citation2019) suggested that environmental exposures such as air pollution may serve as the antigenic trigger for disease development.
Air pollution poses a major threat to human health representing the fourth highest risk factor for global attributable deaths (Murray et al. Citation2020). The Global Burden of Disease report in 2019 indicated that outdoor air pollution is a leading cause of death contributing to approximately three million premature deaths per year (Murray et al. Citation2020). Exposure to air pollution was also been identified as a risk factor for many chronic conditions including ischemic heart disease, chronic obstructive pulmonary disease, lung cancer, and stroke (Bhatnagar Citation2022; Lei et al. Citation2023; Shkirkova et al. Citation2020; Sin et al. Citation2023; Thurston et al. Citation2017; Vargas et al. Citation2023).
Atmospheric particulate matter <2.5 μm in aerodynamic diameter (PM2.5) has been linked with occurrence of cardiovascular diseases, respiratory diseases, and poor neurological outcomes (Dockery et al. Citation1993; Ehsanifar, Yavari, and Rafati Citation2022; Shkirkova et al. Citation2020; Weuve et al. Citation2021). One component of PM2.5, elemental carbon (EC), is a product of incomplete combustion processes and a traffic-related air pollutant (TRAP). Of note, EC is enriched in diesel exhaust particulate. Previous investigators documented that exposure to PM2.5 and TRAPs might lead to oxidative stress and systemic and endothelial inflammation, which contribute to disease processes (Alexeeff et al. Citation2011; Brugge, Durant, and Rioux Citation2007; Cheng et al. Citation2022; Lane et al. Citation2023; Lanki et al. Citation2015; Puett et al. Citation2014, Citation2019). Exposure to ozone (O3) was also linked to pulmonary and systemic inflammation as well as other pulmonary outcomes (Mumby, Chung, and Adcock Citation2019).
To date, few research studies have examined whether concentrations of specific air pollutants are related to sarcoidosis. One study, Pirozzi et al. (Citation2018), examined whether symptom severity was related to short-term PM2.5 and O3 exposures and found that PM2.5 over 10 and 14 days was associated with increased symptom frequency, but O3 was not. However, no apparent investigations examined incident sarcoidosis regarding long-term air pollution exposures estimated using highly spatially- and temporally-resolved exposure estimates. The aim of this study was to investigate whether long-term exposure to air pollutants PM2.5, O3, and EC increased risk of incident sarcoidosis.
Methods
A retrospective case-control study was conducted using data from the Penn State Health electronic medical record (EMR); both cases and controls were adult patients who sought care at Penn State Health in Hershey, Pennsylvania, between 2005 and 2018 and had a non-missing address listed. Cases were identified as patients who had an International Classification of Disease (ICD)-9 or −10 code for sarcoidosis at least twice, separated by a minimum of one year (Chung et al. Citation2013). Patients were listed as controls if they never had an ICD-9 or −10 code for sarcoidosis and had an inpatient or ambulatory visit at Penn State Medical Center in Hershey, PA during the study period. Controls were not obtained from areas with lower levels of air pollution specifically, but rather represent a sample of the source population in the catchment area of Penn State Health in Hershey, PA. Inclusion and exclusion criteria are summarized in Supplemental Table S1.
In our analyses, the first encounter date at Penn State Health was used for both cases and controls (for cases this represented the date of diagnosis). All patients were grouped into 5-year age categories (25–29, 30–34,35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–60, 70–74, and 75–79). Due to small numbers of cases in 18–19-year-olds, patients of those ages were combined with 20–24-year-olds. Cases and controls were also grouped into race categories: 1) White or Caucasian, 2) Black or African American, 3) Asian and Pacific Islander, 4) Other Race, 5) Two or More races, and 6) Unavailable. Cases and controls were matched on age category, biological sex at birth, and race category as well as on the month and year of initial visit to Penn State Medical Center. All matching controls were included in the analysis (one-to-many matching). This study was reviewed and approved by the Institutional Review Board (IRB) at Pennsylvania State University Human Subjects Protection Office (STUDY00019450).
Patient addresses at the time of diagnosis (cases) or the time of first medical encounter (controls) were geocoded. Mapping of geocoded addresses was completed using ArcGIS 9.3 software (ESRI Inc., Redlands, CA). Data on median household income at the US Census tract level was obtained from the 2017 American Communities Survey and used as a measure of socioeconomic position(US Census Bureau American Communities Survey Citation2017).
For each of the three air pollutants examined, PM2.5, O3, and EC, a spatio-temporal generalized additive mixed model (GAMM) was used to estimate 24-hr concentrations (Yanosky et al. Citation2014, Citation2018). These three air pollutants are of each of interest and may be related to sarcoidosis risk, as discussed above. However, other are pollutants that are also of interest in this regard, including nitrogen dioxide and other TRAPs, but data on their concentrations at participant residences were unavailable. The exposure models included geospatial covariates such as urban land use, elevation, and smoothed population density and time-varying covariates representing: 1) daily gridded and interpolated output from a deterministic Eulerian air quality model (CMAQ); 2) daily smoothed meteorological parameters such as wind speed, total liquid precipitation and air temperature and 3) micro-scale traffic-related PM levels from a line-source Gaussian plume dispersion model (ADMS-Roads) to produce high spatial-resolution estimates of 24 hr concentrations for each patient outdoors at their geocoded address. For PM2.5 and O3, model-predicted concentrations were made daily from 1999–2019 based upon daily measurements. For EC, concentrations were predicted for one in every three days from 2004–2019 based upon one-in-three-day measurements.
Residential 24-hr levels of each air pollutant were then used to calculate moving averages 1, 3, 6, and 10 years prior to each patients’ encounter date, ending one day before this date. In addition, moving averages were calculated for 7–8, 9–10, and 7–10 years prior to the encounter date. Any moving averages with less than the corresponding data completeness criterion were removed. For PM2.5 and O3, the completeness criterion used was 70%. Since EC values were available for only one in every three days, 70% was divided by three to yield a completeness criterion of 23.3%. Interquartile ranges (IQRs) were calculated for each pollutant and moving average period and moving averages were then divided by the corresponding IQR to facilitate comparisons across pollutants and moving average periods. Associations between air pollutant exposures and incident sarcoidosis were calculated using conditional logistic regression, adjusting for socioeconomic position, with a separate stratum for each unique set of matching factors as follows: age, biological sex at birth, race, month, and year. SAS Version 9.4 (SAS Institute Inc., Cary, NC) was used for all statistical analyses. Odds ratios (ORs) and their 95% Confidence Intervals (CIs) were plotted using R (R Core Team 2020).
Results
Patient characteristics are summarized in . Our EMR query yielded a total of 877 cases. The age of cases showed a unimodal distribution with a peak age of diagnosis 50–55 years and a mean of 50.2 years across the study period. Of the cases, 345 (39.3%) patients were listed as female biological sex at birth. Most cases identified as White (77.5%) followed by Black or African American (17.8%), Other Race (2.6%), Two or More Races (1.3%), Unavailable (0.5%), and Asian and Pacific Islander (0.3%). A total of 34,510 matching controls were identified. Summary statistics on air pollution concentrations over 7–10 years prior to the encounter date are presented in . In addition, summary statistics on other moving average periods are shown in Supplemental Table S2. All cases and controls had an address in the conterminous United States. Cases had addresses mainly located in Pennsylvania (PA), with only 24 (2.7%) listed outside of PA. Similarly, most control addresses were in PA (96.2%).
TABLE 1. Summary Statistics on Characteristics of Cases and Controls, Air Pollution Measurements for 7–10 Years Prior, and Median Household Income. Cases and Controls Were Matched at a One-To-Many Ratio by Age Category, Biological Sex at Birth, Race Category, Month, and Year of Encounter at Penn State Hershey Medical Center
illustrates the ORs and 95% CIs for incident sarcoidosis and air pollutant exposure estimates for moving averages of 1, 3, 6, and 10 years prior to the encounter date as well as for 7–8, 8–10, and 7–10 years prior to the encounter date. None of the associations with air pollutant exposure estimates were statistically significantly different from their value under the null hypothesis of one.
FIGURE 1. Adjusted odds ratios (ORs) for the association between incident sarcoidosis and air pollutants particulate matter <2.5 μm (PM2.5), ozone (O3), and PM2.5 elemental carbon (EC) for all moving average periods examined.
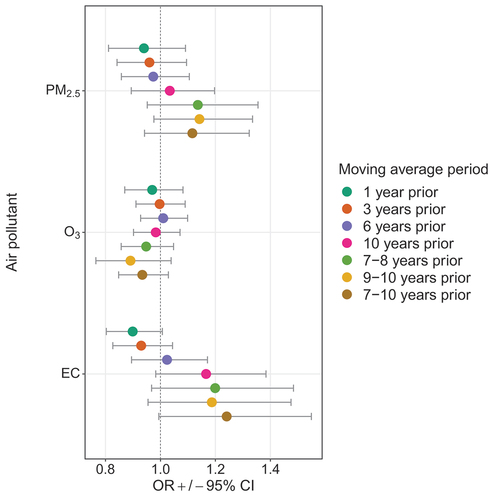
Moving averages from 1 to 10 years prior
ORs for PM2.5 increased monotonically as the moving average period rose from 1 to 10 years, ranging from 0.94 [0.81–1.09] for 1 year prior to 1.03 [0.89–1.20] for 10 years prior. For O3, this pattern was not observed and ORs for moving averages did not change monotonically, ranging from 0.97 [0.87–1.08] for 1 year prior to 1.01 [0.93–1.1] for 6 years prior to the encounter date. Associations with EC exhibited a similar pattern but were larger than those for PM2.5, with ORs displaying an upward trend from 0.90 [0.8–1.01] for 1 year prior to 1.17 [0.98–1.38] for 10 years prior.
Moving averages 7–8, 9–10, and 7–10 years prior
ORs for PM2.5 for 7–8, 9–10 and 7–10 years prior were 1.14 [0.95–1.36], 1.14 [0.98–1.34], and 1.12 [0.94–1.32], respectively. The ORs for O3 for these time-periods were all less than one and ranged from 0.89 [0.77–1.04] for the moving average 9–10 years prior to 0.95 [0.87–1.05] for 7–8 years prior. For EC, in each of the three moving average periods above, the ORs were above one, with the strongest association for a moving average period of 7–10 years prior (1.24 [0.99–1.55]). Taken together, our results suggest a long-term effect of EC (with a relevant exposure window of 4 years) on incident sarcoidosis combined with (and occurring after) a long latency period (7 years).
Discussion
Our study reports on the relationship between PM2.5, O3, and EC and the odds of incident sarcoidosis in central Pennsylvania from 2005–2018. During this time period, air pollution concentrations were low to moderate compared to US EPA air quality standards, and steadily decreasing after 2012. Despite this trend, and though not statistically significant, an increasing trend in the odds of sarcoidosis for longer moving averaging periods was noted for PM2.5 and especially for EC. For PM2.5 and EC, ORs were highest for moving averages representing 9–10 and 7–10 years prior, respectively. In summary, our results suggest a long-term effect of EC (with a relevant exposure window of 4 years) on incident sarcoidosis combined with a long latency period (7 years), and thus long-term exposure to TRAP may be an elevated risk factor for sarcoidosis.
The findings of the present analysis are consistent with a study by Rice et al. (Citation2019) on interstitial lung abnormalities using Framingham Heart Study data. Among healthy participants, investigators found that 5-year estimated exposures to EC were associated with CT findings of interstitial lung abnormalities as well as progression of these abnormalities (Rice et al. Citation2019). Although Rice et al. (Citation2019) did not specifically identify sarcoidosis, sarcoidosis might present as an interstitial lung abnormality (Criado et al. Citation2010). One postulated mechanism for the effects of EC on sarcoidosis involves its ability to induce inflammation via oxidative stress, especially in the lungs (Neophytou et al. Citation2013; Salvi et al. Citation1999). Further, Tripathy et al. (Citation2021) demonstrated that long-term exposures to PM2.5 and black carbon (a proxy measure of EC) in humans were associated with inflammatory immune responses. Given these results, it is conceivable that PM2.5 and/or EC may be exaggerating the immune response resulting in enhanced risk of sarcoidosis.
It is not immediately clear why effects of EC might elicit such a long latency period. This may be due in part to 1) reduced exposure error for longer-term averages, 2) residual or uncontrolled confounding by other factors including perhaps anti-inflammatory effects of TRAP nitrogen monoxide), or 3) may be a feature of a currently unknown biological mechanism.
Previous investigators reported sarcoidosis patients with complicated disease courses may benefit from earlier interventions in order to address related co-morbidities (Bourbonnais and Samavati Citation2008; Nagai et al. Citation2008). However, given the unconfirmed etiology of sarcoidosis, knowledge regarding the specific circumstances or actions for early intervention remains incomplete. Results of this analysis demonstrate possible associations which, if replicated in other populations, might motivate avenues for early interventions such as: 1) air pollution exposure mitigation and 2) early deployment of health screening programs to communities with high levels of EC, efforts that may provide a public health benefit through early diagnosis and management of sarcoidosis.
Strengths of our study include the highly-spatially and temporally resolved information on PM2.5, O3, and EC levels available across a long, multi-year time period. This enabled characterization of exposure estimates at the residential (street address) level and with respect to a given encounter date. This encounter date was used to calculate moving averages prior to this date. The one-to-many matching ratio provides a control population that resembles the source population in central Pennsylvania to the extent possible. Another strength is that outcome misclassification is reduced by our requirement that cases possessed two ICD codes separated by at least one year, and that confounding is minimized by our use of matching on several criteria. In addition, confounding by socio-economic position was controlled using US Census tract household income data.
Limitations of this study include the potential for selection bias being induced due to controls not being fully representative of the source population, as in any case-control study. This may be especially exaggerated given that data from a single institution were used; however, Penn State Health is one of only a few predominant medical providers in the central Pennsylvania region. In any investigation using ICD codes to identify cases and controls there is also the possibility of misdiagnosis. Further, air pollution was characterized utilizing the patient’s address at the time of first encounter at Penn State Medical Center and, if patients had recently moved, would not represent participants’ address history or residential mobility. In addition, residential exposure estimates do not fully characterize personal exposure to air pollutants. Further, our data were not available on where participants spent their time or on air pollutants other than those examined. It should be noted that data on parameters, such as tobacco smoking, personal hobbies, occupation type, and indoor pollutants such as mold were not available. It is noteworthy that despite our inclusion of socioeconomic position as a confounder, residual confounding by occupation type remains possible as data on occupation type were not available. It should be noted that tobacco smokers exhibit reduced clearance of inhaled particles and thus exposure to atmospheric PM including constituents such as EC and tobacco smoking may act in concert with these particles in eliciting exaggerated immune responses, although it was not possible to control for tobacco smoking itself or as an effect modifier in our analyses.
Conclusions
Exposure to EC may contribute to increased odds of incident sarcoidosis over long time periods and may present an opportunity for intervention. Future studies need to confirm these results across larger geographical regions and in different populations. Future studies also need to investigate dose-response patterns, including evaluation of whether a threshold exists, for effects of EC on sarcoidosis, as well as confirm our findings regarding the length of the latency period (7 years) and the time window of vulnerability (4 years).
Authors’ contributions
JDY: Analyzed and interpreted the data and was a major contributor in writing the manuscript. AW: Was a major contributor in data acquisition, study design, and writing the manuscript. GTF: Substantial contributions in conception of the project and study design. DG: Substantial contributions in conception of the project and study design. MB: Contributed to acquiring data, study design, interpretation of results, and manuscript editing. MFH: Substantial contributions in conception of the project, study design, and interpretation of results. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was reviewed and approved by the Institutional Review Board at Pennsylvania State University Human Subjects Protection Office (STUDY00019450).
Supplemental Material
Download MS Word (33.3 KB)Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15287394.2024.2369255.
Additional information
Funding
References
- Alexeeff, S. E., B. A. Coull, A. Gryparis, H. Suh, D. Sparrow, P. S. Vokonas, and J. Schwartz. 2011. “Medium-Term Exposure to Traffic-Related Air Pollution and Markers of Inflammation and Endothelial Function.” Environmental Health Perspectives 119 (4): 481–486. https://doi.org/10.1289/ehp.1002560.
- Arkema, E. V., and Y. C. Cozier. 2020. “Sarcoidosis Epidemiology: Recent Estimates of Incidence, Prevalence and Risk Factors.” Current Opinion in Pulmonary Medicine 26 (5): 527–534. https://doi.org/10.1097/MCP.0000000000000715.
- Baughman, R. P., S. Field, U. Costabel, R. G. Crystal, D. A. Culver, M. Drent, M. A. Judson, and G. Wolff. 2016. “Sarcoidosis in America. Analysis Based on Health Care Use.” Annals of the American Thoracic Society 13 (8): 1244–1252. https://doi.org/10.1513/AnnalsATS.201511-760OC.
- Baughman, R. P., A. S. Teirstein, M. A. Judson, M. D. Rossman, H. Yeager, E. A. Bresnitz, L. DePalo, et al. 2001. “Clinical Characteristics of Patients in a Case Control Study of Sarcoidosis.” American Journal of Respiratory and Critical Care Medicine 164 (10): 1885–1889. https://doi.org/10.1164/ajrccm.164.10.2104046.
- Bennett, D., E. Bargagli, R. M. Refini, and P. Rottoli. 2019. “New Concepts in the Pathogenesis of Sarcoidosis.” Expert Review of Respiratory Medicine 13 (10): 981–991. https://doi.org/10.1080/17476348.2019.1655401.
- Bhatnagar, A. 2022. “Cardiovascular Effects of Particulate Air Pollution.” Annual Review of Medicine 73 (1): 393–406. https://doi.org/10.1146/annurev-med-042220-011549.
- Bourbonnais, J. M., and L. Samavati. 2008. “Clinical Predictors of Pulmonary Hypertension in Sarcoidosis.” The European Respiratory Journal 32 (2): 296–302. https://doi.org/10.1183/09031936.00175907.
- Brito-Zerón, P., R. Pérez-Álvarez, and M. Ramos-Casals. 2022. “Sarcoidosis.” Medicina Clínica 159 (4): 195–204. https://doi.org/10.1016/j.medcli.2022.03.009.
- Brugge, D., J. L. Durant, and C. Rioux. 2007. “Near-Highway Pollutants in Motor Vehicle Exhaust: A Review of Epidemiologic Evidence of Cardiac and Pulmonary Health Risks.” Environmental Health: A Global Access Science Source 6 (1): 23. https://doi.org/10.1186/1476-069X-6-23.
- Celada, L. J., C. Hawkins, and W. P. Drake. 2015. “The Etiologic Role of Infectious Antigens in Sarcoidosis Pathogenesis.” Clinics in Chest Medicine 36 (4): 561–568. https://doi.org/10.1016/j.ccm.2015.08.001.
- Cheng, I., J. Yang, C. Tseng, J. Wu, S. Shariff-Marco, S. L. Park, S. M. Conroy, et al. 2022. “Traffic-Related Air Pollution and Lung Cancer Incidence: The California Multiethnic Cohort Study.” American Journal of Respiratory and Critical Care Medicine 206 (8): 1008–1018. https://doi.org/10.1164/rccm.202107-1770OC.
- Chung, C. P., P. Rohan, S. Krishnaswami, and M. L. McPheeters. 2013. “A Systematic Review of Validated Methods for Identifying Patients with Rheumatoid Arthritis Using Administrative or Claims Data.” Vaccine [Internet] 31 (Suppl 10): K41–K61. https://doi.org/10.1016/j.vaccine.2013.03.075.
- Colboc, H. B., D. P. Moguelet, V. Weil, R. P. Frochot, L. Emmanuel, C. Francès, C. Jouanneau, C. Bernaudin, J.-F. Bachmeyer, and M. Daudon. 2016. “Detection of Silica and Calcium Carbonate Deposits in Granulomatous Areas of Skin Sarcoidosis by Μfourier Transform Infrared Spectroscopy and Field Emission Scanning Electron Microscopy Coupled with Energy Dispersive X-Ray Spectroscopy Analysis.” Comptes Rendus Chimie 19 (11–12): 1631–1641. https://doi.org/10.1016/j.crci.2016.05.007.
- Colboc, H. B., P. Moguelet, D. Bazin, C. Bachmeyer, V. Frochot, R. Weil, E. Letavernier, C. Jouanneau, M. Daudon, and J. F. Bernaudin. 2019. “Physicochemical Characterization of Inorganic Deposits Associated with Granulomas in Cutaneous Sarcoidosis.” Journal of the European Academy of Dermatology and Venereology: JEADV 33 (1): 198–203. https://doi.org/10.1111/jdv.15167.
- Cooper, D., and S. Suau. 2023. “Sarcoidosis.” Immunology and Allergy Clinics of North America 43 (3): 583–591. https://doi.org/10.1016/j.iac.2022.10.011.
- Cornejo, C. M., P. Haun, J. English, and M. Rosenbach. 2019. “Immune Checkpoint Inhibitors and the Development of Granulomatous Reactions.” Journal of the American Academy of Dermatology 81 (5): 1165–1175. https://doi.org/10.1016/j.jaad.2018.07.051.
- Criado, E., M. Sánchez, J. Ramírez, P. Arguis, T. M. de Caralt, R. J. Perea, and A. Xaubet. 2010. “Pulmonary Sarcoidosis: Typical and Atypical Manifestations at High-Resolution CT with Pathologic Correlation.” Radiographics 30 (6): 1567–1586. https://doi.org/10.1148/rg.306105512.
- Crowley, L. E., R. Herbert, J. M. Moline, S. Wallenstein, G. Shukla, C. Schechter, G. S. Skloot, et al. 2011. ““Sarcoid like” Granulomatous Pulmonary Disease in World Trade Center Disaster Responders.” American Journal of Industrial Medicine 54 (3): 175–184. https://doi.org/10.1002/ajim.20924.
- Dockery, D. W., C. A. Pope, X. Xu, J. D. Spengler, J. H. Ware, M. E. Fay, B. G. Ferris, and F. E. Speizer. 1993. “An Association Between Air Pollution and Mortality in Six U.S. Cities.” New England Journal of Medicine 329 (24): 1753–1759. https://doi.org/10.1056/NEJM199312093292401.
- Duvall, C., N. Pavlovic, N. S. Rosen, A. L. Wand, J. M. Griffin, D. R. Okada, H. Tandri, et al. 2023. “Sex and Race Differences in Cardiac Sarcoidosis Presentation, Treatment and Outcomes.” Journal of Cardiac Failure 29 (8): 1135–1145. https://doi.org/10.1016/j.cardfail.2023.03.022.
- Ehsanifar, M., Z. Yavari, and M. Rafati. 2022. “Exposure to Urban Air Pollution Particulate Matter: Neurobehavioral Alteration and Hippocampal Inflammation.” Environmental Science and Pollution Research International 29 (33): 50856–50866. https://doi.org/10.1007/s11356-022-19367-9.
- Graff, P., J. Larsson, I. L. Bryngelsson, P. Wiebert, and P. Vihlborg. 2020. “Sarcoidosis and Silica Dust Exposure Among Men in Sweden: A Case–Control Study.” British Medical Journal Open 10 (9): e038926. https://doi.org/10.1136/bmjopen-2020-038926.
- Grunewald, J., J. C. Grutters, E. V. Arkema, L. A. Saketkoo, D. R. Moller, and J. Müller-Quernheim. 2019. “Sarcoidosis.” Nat Rev Dis Prim 5 (1): 45. https://doi.org/10.1038/s41572-019-0096-x.
- Hayashi, F., T. Kido, N. Sakamoto, Y. Zaizen, M. Ozasa, M. Yokoyama, H. Yura, et al. 2020. “Pneumoconiosis with a Sarcoid-Like Reaction Other Than Beryllium Exposure: A Case Report and Literature Review.” Medicina (Kaunas) 56 (11): 630. https://doi.org/10.3390/medicina56110630.
- Hena, K. M. 2020. “Sarcoidosis Epidemiology: Race Matters.” Frontiers in Immunology 11:537382. https://doi.org/10.3389/fimmu.2020.537382.
- Iannuzzi, M. C., B. A. Rybicki, and A. S. Teirstein. 2007. “Sarcoidosis.” New England Journal of Medicine 357 (21): 2153–2165. https://doi.org/10.1056/NEJMra071714.
- Jonsson, E., B. Järvholm, and M. Andersson. 2019. “Silica Dust and Sarcoidosis in Swedish Construction Workers.” Occupational Medicine 69 (7): 482–486. https://doi.org/10.1093/occmed/kqz118.
- Kushima, H., Y. Kinoshita, H. Ishii, and M. Fujita. 2020. “Tattoo-Induced Systemic Sarcoidosis.” BMJ Case Reports 13 (8): e237723. https://doi.org/10.1136/bcr-2020-237723.
- Lane, K. J., J. I. Levy, A. P. Patton, J. L. Durant, W. Zamore, and D. Brugge. 2023. “Relationship Between Traffic-Related Air Pollution and Inflammation Biomarkers Using Structural Equation Modeling.” Science of the Total Environment 870:161874. https://doi.org/10.1016/j.scitotenv.2023.161874.
- Lanki, T., R. Hampel, P. Tiittanen, S. Andrich, R. Beelen, B. Brunekreef, J. Dratva, et al. 2015. “Air Pollution from Road Traffic and Systemic Inflammation in Adults: A Cross-Sectional Analysis in the European ESCAPE Project.” Environmental Health Perspectives 123 (8): 785–791. https://doi.org/10.1289/ehp.1408224.
- Lei, J., R. Chen, C. Liu, Y. Zhu, X. Xue, Y. Jiang, S. Shi, Y. Gao, H. Kan, and J. Xuan. 2023. “Fine and Coarse Particulate Air Pollution and Hospital Admissions for a Wide Range of Respiratory Diseases: A Nationwide Case-Crossover Study.” International Journal of Epidemiology 52 (3): 715–726. https://doi.org/10.1093/ije/dyad056.
- Llanos, O., and N. Hamzeh. 2019. “Sarcoidosis.” The Medical Clinics of North America 103 (3): 527–534. https://doi.org/10.1016/j.mcna.2018.12.011.
- Marcoval, J., J. Mañá, A. Moreno, I. Gallego, Y. Fortuño, and J. Peyrí. 2001. “Foreign Bodies in Granulomatous Cutaneous Lesions of Patients with Systemic Sarcoidosis.” Archives of Dermatology 137 (4): 427–430. https://www.ncbi.nlm.nih.gov/pubmed/11295921.
- Mumby, S., K. F. Chung, and I. M. Adcock. 2019. “Transcriptional Effects of Ozone and Impact on Airway Inflammation.” Frontiers in Immunology 10:1610. https://doi.org/10.3389/fimmu.2019.01610.
- Murray, C. J. L., A. Y. Aravkin, P. Zheng, C. Abbafati, K. M. Abbas, M. Abbasi-Kangevari, F. Abd-Allah, et al. 2020. “Global Burden of 87 Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019.” Lancet 396 (10258): 1223–1249. https://doi.org/10.1016/S0140-6736(20)30752-2.
- Nagai, S., T. Handa, Y. Ito, K. Ohta, M. Tamaya, and T. Izumi. 2008. “Outcome of Sarcoidosis.” Clinics in Chest Medicine 29 (3): 565–574. https://doi.org/10.1016/j.ccm.2008.03.006.
- Neophytou, A. M., J. E. Hart, J. M. Cavallari, T. J. Smith, D. W. Dockery, B. A. Coull, E. Garshick, and F. Laden. 2013. “Traffic-Related Exposures and Biomarkers of Systemic Inflammation, Endothelial Activation and Oxidative Stress: A Panel Study in the US Trucking Industry.” Environmental Health: A Global Access Science Source 12 (1): 105. https://doi.org/10.1186/1476-069X-12-105.
- Newman, L. S., C. S. Rose, E. A. Bresnitz, M. D. Rossman, J. Barnard, M. Frederick, M. L. Terrin, et al. 2004. “A Case Control Etiologic Study of Sarcoidosis: Environmental and Occupational Risk Factors.” American Journal of Respiratory and Critical Care Medicine 170 (12): 1324–1330. https://doi.org/10.1164/rccm.200402-249OC.
- Nunes, H., D. Bouvry, P. Soler, and D. Valeyre. 2007. “Sarcoidosis.” Orphanet Journal of Rare Diseases 2 (1): 46. https://doi.org/10.1186/1750-1172-2-46.
- Perlman, S. E., S. Friedman, S. Galea, H. P. Nair, M. Eros-Sarnyai, S. D. Stellman, J. Hon, and C. M. Greene. 2011. “Short-Term and Medium-Term Health Effects of 9/11.” The Lancet 378 (9794): 925–934. https://doi.org/10.1016/S0140-6736(11)60967-7.
- Pirozzi, C. S., D. L. Mendoza, Y. Xu, Y. Zhang, M. B. Scholand, and R. P. Baughman. 2018. “Shortterm Particulate Air Pollution Exposure Is Associated with Increased Severity of Respiratory and Quality of Life Symptoms in Patients with Fibrotic Sarcoidosis.” International Journal of Environmental Research Public Health 15 (6): 1077. https://doi.org/10.3390/ijerph15061077.
- Puett, R. C., J. E. Hart, J. D. Yanosky, D. Spiegelman, M. Wang, J. A. Fisher, B. Hong, and F. Laden. 2014. “Particulate Matter Air Pollution Exposure, Distance to Road, and Incident Lung Cancer in the Nurses’ Health Study Cohort.” Environmental Health Perspectives 122 (9): 926–932. https://doi.org/10.1289/ehp.1307490.
- Puett, R. C., J. D. Yanosky, M. A. Mittleman, J. Montresor-Lopez, R. A. Bell, T. L. Crume, D. Dabelea, et al. 2019. “Inflammation and Acute Traffic-Related Air Pollution Exposures Among a Cohort of Youth with Type 1 Diabetes.” Environment International 132:105064. https://doi.org/10.1016/j.envint.2019.105064.
- Rice, M. B., W. Li, J. Schwartz, Q. di, I. Kloog, P. Koutrakis, D. R. Gold, et al. 2019. “Ambient Air Pollution Exposure and Risk and Progression of Interstitial Lung Abnormalities: The Framingham Heart Study.” Thorax 74 (11): 1063–1069. https://doi.org/10.1136/thoraxjnl-2018-212877.
- Rossides, M., P. Darlington, S. Kullberg, and E. V. Arkema. 2023. “Sarcoidosis: Epidemiology and Clinical Insights.” Journal of Internal Medicine 293 (6): 668–680. https://doi.org/10.1111/joim.13629.
- Rybicki, B. A., M. Major, J. Popovich, M. J. Maliarik, and M. C. Iannuzzi. 1997. “Racial Differences in Sarcoidosis Incidence: A 5-Year Study in a Health Maintenance Organization.” American Journal of Epidemiology 145 (3): 234–241. https://doi.org/10.1093/oxfordjournals.aje.a009096.
- Salvi, S., A. Blomberg, B. Rudell, F. Kelly, T. Sandström, S. T. Holgate, and A. Frew. 1999. “Acute Inflammatory Responses in the Airways and Peripheral Blood After Short-Term Exposure to Diesel Exhaust in Healthy Human Volunteers.” American Journal of Respiratory and Critical Care Medicine 159 (3): 702–709. https://doi.org/10.1164/ajrccm.159.3.9709083.
- Seaton, A. 2020. “Silica Dust and Sarcoidosis.” Occupational Medicine 70 (2): 139. https://doi.org/10.1093/occmed/kqaa016.
- Sève, P., Y. Pacheco, F. Durupt, Y. Jamilloux, M. Gerfaud-Valentin, S. Isaac, L. Boussel, et al. 2021. “Sarcoidosis: A Clinical Overview from Symptoms to Diagnosis.” Cells 10 (4): 766. https://doi.org/10.3390/cells10040766.
- Shkirkova, K., K. Lamorie-Foote, M. Connor, A. Patel, G. Barisano, H. Baertsch, Q. Liu, T. E. Morgan, C. Sioutas, and W. J. Mack. 2020. “Effects of Ambient Particulate Matter on Vascular Tissue: A Review.” Journal of Toxicology and Environmental Health, Part B 23 (7): 319–350. https://doi.org/10.1080/10937404.2020.1822971.
- Sin, D. D., D. Doiron, A. Agusti, A. Anzueto, P. J. Barnes, B. R. Celli, G. J. Criner, et al. 2023. “Air Pollution and COPD: GOLD 2023 Committee Report.” The European Respiratory Journal 61 (5): 2202469. https://doi.org/10.1183/13993003.02469-2022.
- Spagnolo, P., G. Rossi, R. Trisolini, N. Sverzellati, R. P. Baughman, and A. U. Wells. 2018. “Pulmonary Sarcoidosis.” The Lancet Respiratory Medicine 6 (5): 389–402. https://doi.org/10.1016/S2213-2600(18)30064-X.
- Thurston, G. D., H. Kipen, I. Annesi-Maesano, J. Balmes, R. D. Brook, K. Cromar, S. de Matteis, et al. 2017. “A Joint ERS/ATS Policy Statement: What Constitutes an Adverse Health Effect of Air Pollution? An Analytical Framework.” The European Respiratory Journal 49 (1): 1600419. https://doi.org/10.1183/13993003.00419-2016.
- Tripathy, S., A. L. Marsland, E. J. Kinnee, B. J. Tunno, S. B. Manuck, P. J. Gianaros, and J. E. Clougherty. 2021. “Long-Term Ambient Air Pollution Exposures and Circulating and Stimulated Inflammatory Mediators in a Cohort of Midlife Adults.” Environmental Health Perspectives 129 (5): 57007. https://doi.org/10.1289/EHP7089.
- US Census Bureau American Communities Survey. 2017. “B19013 Median Household Income in the Past 12 Months (In 2017 Inflation-Adjusted Dollars).” 2017: ACS 5-Year Estimates Detailed Tables.
- Valeyre, D., A. Prasse, H. Nunes, Y. Uzunhan, P. Y. Brillet, and J. Müller-Quernheim. 2014. “Sarcoidosis.” Lancet (London, England) 383 (9923): 1155–1167. https://doi.org/10.1016/S0140-6736(13)60680-7.
- Vargas, V. M. F., F. M. R. da Silva Júnior, T. D. Silva Pereira, C. S. D. Silva, and M. V. Coronas. 2023. “A Comprehensive Overview of Genotoxicity and Mutagenicity Associated with Outdoor Air Pollution Exposure in Brazil.” Journal of Toxicology and Environmental Health, Part B 26 (3): 172–199. https://doi.org/10.1080/10937404.2023.2175092.
- Wanat, K. A., and M. Rosenbach. 2015. “Cutaneous Sarcoidosis.” Clinics in Chest Medicine 36 (4): 685–702. https://doi.org/10.1016/j.ccm.2015.08.010.
- Weuve, J., E. E. Bennett, L. Ranker, K. Z. Gianattasio, M. Pedde, S. D. Adar, J. D. Yanosky, and M. C. Power. 2021. “Exposure to Air Pollution in Relation to Risk of Dementia and Related Outcomes: An Updated Systematic Review of the Epidemiological Literature.” Environmental Health Perspectives 129 (9): 96001. https://doi.org/10.1289/EHP8716.
- Yanosky, J. D., J. Fisher, D. Liao, D. Rim, R. Vander Wal, W. Groves, and R. C. Puett. 2018. “Application and Validation of a Line-Source Dispersion Model to Estimate Small Scale Traffic-Related Particulate Matter Concentrations Across the Conterminous US.” Air Quality, Atmosphere, & Health 11 (6): 741–754. https://doi.org/10.1007/s11869-018-0580-6.
- Yanosky, J. D., C. J. Paciorek, F. Laden, J. E. Hart, R. C. Puett, D. Liao, and H. H. Suh. 2014. “Spatio-Temporal Modeling of Particulate Air Pollution in the Conterminous United States Using Geographic and Meteorological Predictors.” Environmental Health: A Global Access Science Source 13 (1): 63. https://doi.org/10.1186/1476-069X-13-63.
