 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The application of biosolids, manure, and slurry onto agricultural soils and the growing use of treated wastewater in agriculture result in the introduction of human and veterinary pharmaceuticals to the environment. Once in the soil environment, pharmaceuticals may be taken up by crops, resulting in consequent human exposure to pharmaceutical residues. The potential side effects of pharmaceuticals administered in human medicine are widely documented; however, far less is known regarding the risks that arise from incidental dietary exposure. The aim of this study was to evaluate human exposure to pharmaceutical residues in crops and assess the associated risk to health for a range of pharmaceuticals frequently detected in soils. Estimated concentrations of carbamazepine, oxytetracycline, sulfamethoxazole, trimethoprim, and tetracycline in soil were used in conjunction with plant uptake and crop consumption data to estimate daily exposures to each compound. Exposure concentrations were compared to Acceptable Daily Intakes (ADIs) to determine the level of risk. Generally, exposure concentrations were lower than ADIs. The exceptions were carbamazepine, and trimethoprim and sulfamethoxazole under conservative, worst-case scenarios, where a potential risk to human health was predicted. Future research therefore needs to prioritize investigation into the health effects following exposure to these compounds from consumption of contaminated crops.
Introduction
The quantity and diversity of pharmaceuticals being administered to humans and animals is increasing and will continue to rise as population growth persists and as new pharmaceutical treatments continue to be developed (Arnold et al. Citation2013; Carter et al. Citation2014; NHSBSA Citation2019). Although pharmaceuticals are critical in the alleviation and prevention of disease, as these compounds are designed to induce beneficial biological effects at low concentrations, these agents also possess the innate capability to act as potent environmental contaminants (Arnold et al. Citation2013; A. B. Boxall Citation2004; Boxall et al. Citation2006). This is of particular concern as (1) the lifecycle of a pharmaceutical compound does not terminate at the time of dose administration, and (2) there are numerous pathways by which both human and veterinary pharmaceuticals may be released into the natural environment (Bartolo, Azzopardi, and Serracino-Inglott Citation2021; A. B. Boxall Citation2004; Chung et al. Citation2019; Kostopoulou and Nikolaou Citation2008; Mennigen et al. Citation2011; Prosser and Sibley Citation2015).
Current wastewater treatment (WWT) processes fail to completely remove all pharmaceuticals from the influent (Carter et al. Citation2018; Wang and Wang Citation2016). Persistent and relatively sorptive pharmaceuticals display a tendency toward partition onto activated sludge, which then settles out (Prosser and Sibley Citation2015; Vinayagam et al. Citation2022). This waste by-product is then often further treated, for example, by anaerobic digestion, before being applied to agricultural land as a fertilizer (Mordechay et al. Citation2021; Oberoi et al. Citation2022; Shahriar et al. Citation2021; Wu et al. Citation2012), a practice that is expected to increase in the future (Carter et al. Citation2014). Persistent and water-soluble compounds might, on the other hand, pass through the treatment process and then be emitted in the effluent water (Prosser and Sibley Citation2015), which many countries use for irrigation (Garduño-Jiménez et al. Citation2023; Mordechay et al. Citation2018).
Globally, approximately 5.6 × 109 m3 of treated wastewater (TWW) is used each year for irrigation (Mordechay et al. Citation2018), with rising water insecurity further driving demand (Carter et al. Citation2014). TWW is supplied continually to irrigated soils (Lees et al. Citation2016; Palli et al. Citation2019), whereas biosolid applications occur periodically in line with growing seasons (Mordechay et al. Citation2018). Once in the soil, some pharmaceuticals might persist for months to years (Boxall et al. Citation2006; Samarasinghe et al. Citation2021).
Veterinary pharmaceuticals have also been detected in soils, chiefly due to the application of manure and slurry from treated livestock (Boxall et al. Citation2006; Keerthanan et al. Citation2021; Kumar, Gupta, Baidoo, et al. Citation2005; Prosser and Sibley Citation2015). With antibiotics readily added to feedstuffs in a bid to promote livestock growth (Kumar, Gupta, Baidoo, et al. Citation2005; Prosser and Sibley Citation2015), manure was found to contain antibiotics at concentrations as high as 216 mg/L (Kumar, Gupta, Chander, et al. Citation2005). It is, therefore, not surprising that multiple investigators have documented the occurrences of a large number and wide range of types of pharmaceuticals in various soil environments across the world (Albero et al. Citation2018; Aus Der Beek et al. Citation2016; Keerthanan et al. Citation2021; Samarasinghe et al. Citation2021).
Recognition that pharmaceuticals are released into, and occur in, soil environments has raised concerns over the potential for plants to take up these compounds (Pullagurala et al. Citation2018; Wu et al. Citation2012). Several investigators have demonstrated the ability of plants to take up pharmaceuticals from their surroundings (Boxall et al. Citation2006; Carter et al. Citation2014, Citation2018; Keerthanan et al. Citation2021; Kumar, Gupta, Baidoo, et al. Citation2005; Madikizela, Ncube, and Chimuka Citation2018; Mordechay et al. Citation2018; Sabourin et al. Citation2012; Wu et al. Citation2012). The observed uptake of pharmaceuticals into plants has raised concerns over potential impacts on human health (Boxall et al. Citation2006; Duarte, Oldenkamp, and Ragas Citation2022; Egbeyemi et al. Citation2023; Shahriar et al. Citation2021). The consumption of contaminated crops has been recognized as a primary pathway of human exposure to pharmaceuticals in the environment (Keerthanan et al. Citation2021; Liu et al. Citation2020). It is of interest that Schapira et al. (Citation2020) reported that carbamazepine was detected in the urine of up to 84% of healthy Israeli individuals attributed to consumption of crops that had unknowingly been contaminated by TWW irrigation. It is well established that direct administration of pharmaceuticals to humans results in side effects. Investigation by Pirmohamed et al. (Citation2004) suggested that adverse drug reactions represent up to 1 in 16 hospital admissions, or 4% of hospital bed capacity. However, it has also been noted that consumption of pharmaceuticals via contaminated crops might also pose a risk to human health (Egbeyemi et al. Citation2023; Keerthanan et al. Citation2021; Liu et al. Citation2020; Meffe et al. Citation2021), yet the potential toxicological impacts in humans inadvertently consuming pharmaceutical residues via crops are still to be established.
Since the publication by Boxall et al. (Citation2006), the first notable study to quantify the human health risk posed by pharmaceutical residues in crops, various investigators have consistently projected relatively low concentrations of pharmaceuticals in crops and, as such, deemed there to be no appreciable human health risk (Boxall et al. Citation2006; Carter et al. Citation2014; Castaño-Trias et al. Citation2024; Liu et al. Citation2020; Meffe et al. Citation2021; Ponce-Robles et al. Citation2022; Prosser and Sibley Citation2015; Sunyer-Caldú, Quintana, and Diaz-Cruz Citation2023). Despite the Comprehensive European Food Consumption Database highlighting the age-based variation in consumption patterns (EFSA Citation2018), risk assessments have not considered these age-based consumption statistics in estimations of dietary intakes.
Although early experimental work in this field focused on a restricted number of compounds and crops, recent reviews have expanded assessments to include more than 50 different compounds (Sunyer-Caldú, Quintana, and Diaz-Cruz Citation2023). Published literature has also been documented to facilitate investigation of a broader spectrum of compounds (Prosser and Sibley Citation2015). However, with some 200 plant species consumed by humans (FAO Citation1999), approximately 2000 active pharmaceutical ingredients authorized for use in the UK (Burns et al. Citation2018) and the potential for combined exposure from both veterinary and human use, previous risk studies might not provide a comprehensive or accurate assessment of the real risks to human health.
Human health risk assessment studies, to date, have also not completely exploited the wealth of literature on the uptake of pharmaceuticals into plants. However, Sleight et al. (Citation2023) recently performed a systematic review of plant uptake data for pharmaceuticals and developed a single database of plant uptake factors (UFs). The UTOPIC database holds standardized UFs that are unique to both plant species and pharmaceuticals, necessary for the investigation of the role of crop type in risk assessment (Sleight, Boxall, and Toet Citation2023). This database provides a useful resource for improved human health risk assessments for pharmaceuticals released to soil environments and accumulated by plants.
The aim of this study therefore was to use the UTOPIC database alongside comprehensive consumption statistics for vegetables and fruits, to assess the level of risk posed to human health by exposure to pharmaceutical residues in crops. Published uptake factors, specific to pharmaceutical and crop type, were collated and employed to predict environmental concentrations of a range of commonly detected pharmaceuticals in different crop types. This study addressed the following specific research questions: (1) Do residues of human or veterinary pharmaceuticals in crops pose a risk to human health? (2) Is crop type a driver of the potential human health risk from pharmaceutical residues in crops? (3) Does potential risk level vary between age groups?
Methods
Human health risks associated with exposures to carbamazepine, sulfamethoxazole, oxytetracycline, trimethoprim, and tetracycline residues in crops were assessed. These five human and veterinary pharmaceuticals were primarily selected for investigation based upon data availability as these drugs were identified as having the most data available in the published literature on accumulation into plants. Principally, these pharmaceuticals were the most frequently studied compounds in the UTOPIC database that also held published acceptable daily intakes (ADIs) (Prosser and Sibley Citation2015; Schwab et al. Citation2005; Sleight, Boxall, and Toet Citation2023) and sufficient usage data to estimate environmental exposure concentrations according to European Medicine Agency Guidelines (EMA Citation2016, Citation2018).
Data collection – consumption, crop uptakes, and toxicological thresholds
Human pharmaceutical consumption rates were estimated using 2018 National Health Service community prescribing statistics for England (NHSBSA Citation2019), while consumption rates for veterinary pharmaceuticals were determined from maximum treatments for intensively reared livestock detailed in the 2021 NOAH Compendium prescribing datasheets (NOAH Citation2021).
Uptake factors (UFs) were obtained from the UTOPIC MS Access database (Sleight, Boxall, and Toet Citation2023). This unique database used a novel systematic review process to comprehensively collate and standardize UFs from published research and facilitate identification of both pharmaceutical-specific and crop-specific UFs for this study. Maximum, minimum, and median UFs were extracted for each combination of pharmaceutical and crop type under investigation (grains and grain-based products; fruit, vegetables, and their products; starchy roots and tubers; and other crops) to ensure UFs were representative of the large range of published values held within the database. For any combination of crop type and pharmaceutical without a published UF, a mean was obtained from the available crop types. The database contains data for a range of exposure pathways, including from spiked soil, spiked irrigation water, biosolids, and manure. In this study, only UFs from studies using spiked soils were used due to limited data availability for other exposure pathways and a lack of standardization in experimental methodology and data reporting limiting the validity of comparisons between studies using different exposure pathways.
For each pharmaceutical, risk to health was assessed using the ADI, which is defined as the amount of pharmaceuticals that a human might consume over their lifespan without experiencing adverse toxicological effects (Carter et al. Citation2014; Prosser and Sibley Citation2015; Schwab et al. Citation2005). ADI values are widely available in published literature; however, there is a marked variation in calculation methods and published values (Schwab et al. Citation2005; Sinclair et al. Citation2006). For the present study, ADIs were extracted from the two extensive compendia of ADIs reported by Prosser and Sibley (Citation2015) and Schwab et al. (Citation2005) and, as per the recommendation of Sinclair et al. (Citation2006) for a conservative assessment of risk, the lowest magnitude ADI value was selected if the two sources presented conflicting values. The ADIs (μg/kg/day) were derived from a combination of a lowest possible dose, and a series of uncertainty or safety factors (SF) to account for uncertainties in the calculation, using Equation 1 (Prosser and Sibley Citation2015) or Equation 2 (Schwab et al. Citation2005)
where LTD is the lowest therapeutic dose (mg/day); 70 is the average bodyweight of adults (kg); SFa was comprised of three safety factors of 10 for all pharmaceuticals in this study (Prosser and Sibley Citation2015); the point of departure (POD) (lowest doses resulting in pharmacological effects in human clinical trials, mg/kg/day); 1000 is a mass conversion factor; and SF1–5 are uncertainty factors, selected based upon the individual POD (Schwab et al. Citation2005). PODs were either the lowest observed effect level (LOEL) or the lowest observed adverse effect level (LOAEL). For more details on the values used in the derivation of ADIs using EquationEquation 1(1)
(1) and EquationEquation 2
(2)
(2) , see (Prosser and Sibley Citation2015) and (Schwab et al. Citation2005), respectively. EquationEquation 2
(2)
(2) was not used for the derivation of ADIs for both oxytetracycline and tetracycline, but rather published values were obtained directly from the World Health Organization (FAO and WHO Citation1998). A full list of ADIs employed in this study and their sources is provided in .
Table 1. ADI and PEC values used in the calculation of human health risk from pharmaceutical residues in crops
Crop consumptions were estimated using level one of the Comprehensive European Food Consumption Database (EFSA Citation2018). Daily consumption statistics (g/kg BW/day) were extracted for grains and grain-based products; fruit and fruit products; vegetables and vegetable products; starchy roots and tubers; legumes, nuts, and oilseeds; and herbs, spices, and condiments were extracted for adolescents (10–17 years old), adults (18–64 years old), elderly (65–74 years old), and very elderly (>75 years old). All other subpopulations in the database were deemed unrepresentative of the 70 kg average bodyweight used in the derivation of ADIs. Consumptions were then combined into the five crop types of interest: grains and grain-based products; fruit, vegetables, and their products (fruit and fruit products with vegetables and vegetable products); starchy roots and tubers; and other crops (legumes, nuts, and oilseeds with herbs, spices, and condiments).
Estimation of concentration in soils
Worst-case scenario predicted environmental concentrations of pharmaceuticals based upon human (PEChuman) and veterinary (PECveterinary) use were estimated using EMA guidelines (EMA (Citation2016) (for human-use) and EMA (Citation2018) (for veterinary-use)). Concentrations of pharmaceuticals in soil resulting from the treatment of intensively reared livestock were determined. The calculations were based upon the highest daily dose (mg/kg BW/day) and the longest treatment length (days) to provide a conservative estimation of exposure. When dosage data were only reported for the salt form of a pharmaceutical, these were converted into the non-salt form using a molecular mass correction. Worst-case PECs resulting from human use of pharmaceuticals were estimated for all compounds, however a PEC for the veterinary use of carbamazepine was not estimated as it is not used as a livestock medicine. Estimated removal during wastewater treatment for each compound (Fsludge) was taken from Singer et al. (Citation2016).
Concentration in the sludge (PECsludge, mg/kg) was then estimated using Equation 3, and, finally, concentration in the soil (PEChuman, mg/kg) estimated using Equation 4
where Mppd is mass of pharmaceutical consumed per person per day (mg), calculated from mass used in England in 2018 (NHSBSA Citation2019), population of England in 2018 (55,977,178) and using 365 days in year; Fexr is fraction of pharmaceutical excreted, as estimated by (Singer et al. Citation2016); Asludge is sludge application rate to land (0.5 kg/m2/year); Dsoil is soil mixing depth (0.2 m); and RHOsoil is bulk density of soil (1700 kg/m3). The exact data used to calculate PEChuman values have been summarized in Table S1.
Equation 5 was used to estimate PECveterinary values (mg/kg) for all intensive livestock treatments in the NOAH Compendium (Citation2021) that contain a pharmaceutical of interest where D is the maximum daily dose (mg/kg BW/day−), obtained from (NOAH Citation2021); Ad is the maximum treatment length (days), obtained from (NOAH Citation2021); BW is animal bodyweight (kg), P is animal turnover rate (place/year), 170 is the EU nitrogen spreading limit (kg/N/ha); Fh is fraction of herd treated, 1500 is bulk density of dry soil (kg/m3); 10000 is the area of 1 ha (m2/ha); 0.05 is the depth of penetration into soil (m); Ny is yearly nitrogen production per place (kg of N place/year); and H is a housing factor (EMA Citation2016). See (EMA Citation2016) for the livestock-specific variables and selection criteria. The maximum (worst-case) PECveterinary value was then selected for each pharmaceutical. A summary of the treatments (NOAH Citation2021) associated with each worst-case PECveterinary value are located in Table S2.
Potential degradation of the pharmaceuticals within the soil was estimated using Equations 6–8 (EMA Citation2016).
Where PECsoil 1 year is the predicted environmental concentration in soil 1 year after spreading (mg/kg). PECsoil initial is the PEChuman or PECveterinary value calculated in EquationEquations 4(4)
(4) and Equation5
(5)
(5) . DT50 is the half-life of pharmaceuticals in soil (days), determined from the literature (Table S4, Table S5). Fs is the fraction degraded in soil 1 year after application, and PECsoilplateau is the predicted environmental concentration in soil at plateau concentration (mg/kg).
Estimation of concentration in crops
The product of UF and PEC was used to predict the concentration of each pharmaceutical within each crop type (CC, mg/kg). For each crop type, this was repeated applying the minimum, median, and maximum uptake factor.
Estimation of human exposure
Human exposures (HE, mg/kg BW/day) were estimated using Equation 9 where Ccrop is daily human consumption of crop type (g/kg BW/day), calculated from the European Food Safety Authority consumption statistics (EFSA Citation2018); 1000 is a mass conversion factor; and CC is the predicted concentration of pharmaceuticals in the crop (mg/kg).
Risk assessment
Level of risk was assessed by comparing the ratio of HE to ADI, a common approach taken when risk assessing biological compounds (Burns et al. Citation2018; Schwab et al. Citation2005; Sinclair et al. Citation2006; Topaz et al. Citation2020). HE values were converted into μg/kg BW/day before being divided by the pharmaceutical-specific ADI to derive a risk quotient (RQ) for each combination of crop type and pharmaceutical at a minimum, median, and maximum risk scenario (Equation 10).
The acceptable risk threshold was set at a risk quotient of 1, where human exposure is equal to the ADI. The greater the magnitude above this threshold, the greater the risk to health.
Results
Acceptable daily intakes, predicted environmental concentrations, and uptake factors
A summary of the ADIs, PECs, and UFs used to calculate risk is given in , with associated raw data found in the Supplemental Data. ADIs were available for all pharmaceuticals and ranged from 2.9 μg/kg BW/day (carbamazepine) to μg/kg BW/day (oxytetracycline) ().
Worst-case PECs resulting from human use of pharmaceuticals were estimated for all compounds, however a PEC for the veterinary use of carbamazepine was not estimated as it is not used as a livestock medicine. PEChuman values ranged from 1 × 10−3 mg/kg (tetracycline) to 7.03 × 10−2 mg/kg (carbamazepine). Maximum daily consumption was estimated as 2.01 mg/kg (carbamazepine) and excretion ranged between 20% (sulfamethoxazole) to 100% (oxytetracycline). PECveterinary values were consistently greater than their human counterparts, ranging from 0.18 mg/kg (tetracycline) to 15 mg/kg (sulfamethoxazole). Leporine treatments resulted in the highest PECs, producing the worst-case PEC for two of the four veterinary pharmaceuticals (sulfamethoxazole and trimethoprim), while treatments for weaner pigs and cattle over 2 years old produced the remaining worst-case PECs (oxytetracycline and tetracycline, respectively).
UFs for carbamazepine into grains and grain-based products, sulfamethoxazole into other crops, trimethoprim into other crops, tetracycline into grains and grain-based products, tetracycline into starchy roots and tubers, and tetracycline into other crops were not held in the UTOPIC database and were, therefore, substituted with the mean of the rest of the available data. The maximum and minimum UF values highlighted the large variation in UF values published in the literature (). UFs ranged from a minimum value of 4.2 × 10−4 (sulfamethoxazole into fruit, vegetables, and their products) to a maximum of 1.56 × 102 (sulfamethoxazole into fruit, vegetables, and their products). Median UFs had a smaller range, from 1.85 × 10−3 (sulfamethoxazole into grains and grain-based products) to 12.6 (carbamazepine into fruit, vegetables, and their products).
Table 2. Median, Minimum, and Maximum UFs Used in the Calculation of Risk from Pharmaceutical Residues in Crops. UFs Were Obtained from the UTOPIC Database (Sleight, Boxall, and Toet Citation2023), with Their Source Publication Referenced. ** For Any Combination of Crop Type and Pharmaceuticals without Published UF Data, a Mean Average Was Taken from All Crop Types with Available UFs
Predicted human exposure to pharmaceutical residues
On comparison of adolescent (10–17 years-old), adult (18–64 years-old), elderly (65–74 years-old), and very elderly (>75 years-old) dietary exposures to pharmaceutical residues in crops, all age groups were predicted to be exposed to comparable concentrations of pharmaceuticals (). Humans were predicted to be exposed to pharmaceutical concentrations between 2.04 × 10−4 μg/kg−BW/day (human tetracycline, adults) and 17.2 μg/kg−BW/day (carbamazepine, adolescents), with adolescent, adult, elderly, and very elderly exposure concentrations of the same order of magnitude for each pharmaceutical.
Figure 1a–1e. Median predicted daily exposure of adolescents (10–17 years old), adults (18–64 years old), elderly (65–74 years old), and very elderly (>75 years old) to pharmaceutical residues in crops. Error bars represent maximum and minimum exposure scenarios derived using maximum and minimum plant uptake factors, respectively.
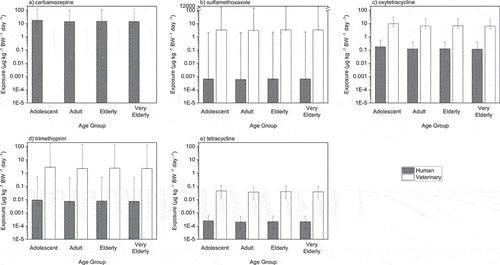
For all pharmaceuticals administered in both a human and veterinary context, veterinary usage was predicted to result in greater exposure. Exposures to all human-use pharmaceuticals were predicted to be below ADI, with the exception of carbamazepine. Veterinary-use pharmaceutical exposures were predicted to be between 54 (oxytetracycline) and 5.04 × 103 -fold (sulfamethoxazole) greater than their human-use counterparts. Exposure over the ADI was projected with the veterinary use of sulfamethoxazole and trimethoprim, with a maximum risk scenario pointing toward human exposure of up to 1.15 × 104 μg/kg−BW/day (sulfamethoxazole, very elderly) and 1.63 × 102 μg/kg−BW/day (trimethoprim, adolescents).
Do residues of human or veterinary pharmaceuticals in crops pose a risk to human health?
Age-specific risk quotients associated with the exposure to different human and veterinary pharmaceutical residues in crops are illustrated in . Risk quotients ranged from 2.5 × 10−5 (human tetracycline, adults) to 2.01 × 103 (veterinary sulfamethoxazole, elderly and very elderly), with similar risk quotients projected for all age groups. For compounds used in both veterinary and human treatments, the veterinary risk quotients were always greater than the human risk quotients. For the human-use pharmaceuticals, median risk quotients were below the acceptable risk threshold for all compounds except carbamazepine. Risk quotients for the veterinary use of oxytetracycline and tetracycline were also below the acceptable risk threshold, with median risk quotients for veterinary use of these tetracycline antibiotics predicted to be (2.08 × 10−1−3.15 × 10−1) for oxytetracycline and (6.42 × 10−3−7.84 × 10−3) for tetracycline.
Figure 2a-2e. Median risk from pharmaceutical residues in crops to adolescents (10–17 years old), adults (18–64 years old), elderly (65–74 years old); and very elderly (>75 years old). Error bars represent maximum and minimum risk scenarios derived using maximum and minimum UFs, respectively.
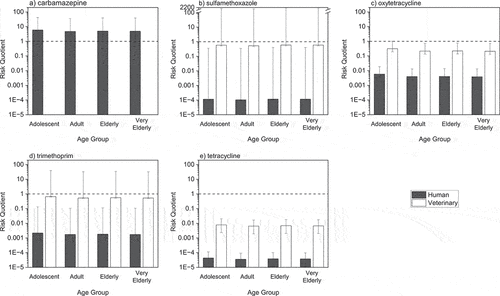
In all age groups, exposures to veterinary-use trimethoprim and sulfamethoxazole were predicted to exceed the acceptable risk threshold, suggesting all ages are subject to an unacceptable level of risk from these pharmaceuticals. However, the ADI values were only exceeded under a maximum risk scenario for these compounds. Under a maximum risk scenario, risk quotients associated with these pharmaceuticals were projected up to 3.88 × 101 (adolescents, trimethoprim) and 2.02 × 103 (very elderly, sulfamethoxazole).
Is crop type a driver in the human health risk from pharmaceutical residues in crops?
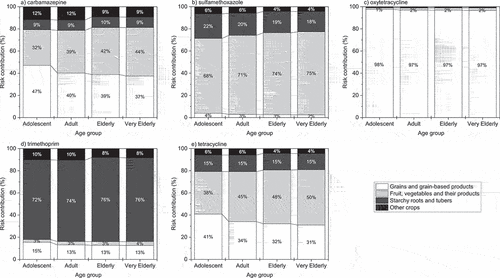
The relative contributions of grains and grain-based products; fruit, vegetables, and their products; starchy roots and tubers; and other crops, to health risk associated with pharmaceutical residues in crops ranged from 0.04% (starchy roots and tubers, oxytetracycline, adolescents) to 97.7% (grains, and grain-based products, oxytetracycline, adolescents) (a−3e). Pharmaceutical residues within fruit, vegetables, and their products were the greatest contributors to health risk from sulfamethoxazole across all age groups and tetracycline risk in all age groups except adolescents. Grains and grain-based products were the largest contributors to risk from oxytetracycline, whilst starchy roots and tubers were the most significant contributors to risk for trimethoprim. When comparing age-based risk, little variation was observed in the make-up of risk between age groups.
Discussion
Over the past decade, the occurrence of human and veterinary pharmaceuticals in the environment has been widely documented, resulting in concerns over the uptake of such substances by crops intended for consumption and potential impact on human health (Keerthanan et al. Citation2021). This study also predicted pharmaceutical residues to contaminate human diets, with pharmaceutical residues indicated to be present in crops consumed by adolescents, adults, elderly, and very elderly. Human exposure to oxytetracycline and tetracycline was predicted to be below the acceptable risk threshold in all scenarios, including when considering maximum uptake and maximum risk. This supports the general consensus that, although present, residues of these pharmaceuticals in crops are insufficiently concentrated to pose a threat to human health (Boxall et al. Citation2006; Carter et al. Citation2014; Castaño-Trias et al. Citation2024; Ponce-Robles et al. Citation2022; Prosser and Sibley Citation2015). Contrary to these findings, however, this assessment estimated human exposure concentrations to pose an unacceptable risk to adolescents, adults, elderly, and very elderly for residues of carbamazepine, trimethoprim, and sulfamethoxazole in crops resulting from their use in human and veterinary treatments. However, it is important to recognize that our estimations of risk are likely to be conservative as these calculations assume that an individual’s plant diet is derived entirely from systems receiving carbamazepine, trimethoprim, and sulfamethoxazole.
Carbamazepine is an anticonvulsant medication used predominantly in the treatment of epilepsy and neuropathic pain. Carbamazepine had the greatest total mass prescribed of the compounds assessed. This meant that, despite having a relatively low excretion factor (0.26), the predicted environmental concentration of carbamazepine was the highest of the compounds investigated in this study. In addition, carbamazepine is known to be persistent in soils, with the DT50 values ranging between 40 and >1000 days (Table S4). The persistency of carbamazepine in soils infers that there is potential for carbamazepine concentrations to increase over time with successive applications of sludge or contaminated irrigation water, even when applications are temporally separated (Equationequations 5(5)
(5) -Equation7
(7)
(7) ). The ADI of carbamazepine was also the lowest of the compounds assessed. The relatively high predicted environmental concentration combined with the known affinity for uptake into crops and relatively low ADI led carbamazepine to have the highest risk quotient of the compounds assessed.
Trimethoprim and sulfamethoxazole are frequently administered in combination to treat urinary tract, respiratory system, and gastrointestinal infections in humans (Wishart et al. Citation2018). However, the use of these antibiotics may also produce deleterious side effects such as harmful bone marrow depression, which manifests as blood cell disorders including thrombocytopenia, leukopenia, or megaloblastic anemia and, more rarely, severe adverse reactions resulting in death (FDA Citation2012). As such, the levels of exposure predicted in this study should not be ignored.
Present veterinary usage estimates for sulfamethoxazole and trimethoprim are indicative of consumption over the ADI of up to 1.15 × 104 μg/kg−BW/day and 1.63 × 102 μg/kg−BW/day, respectively, compared to prior studies where exposures to these pharmaceuticals were predicted to be below the ADI and presented no undue health risk (Boxall et al. Citation2006; Prosser and Sibley Citation2015). This discrepancy in findings may be explained by the fact that the present study included comprehensive use of UFs and consumption data in its calculations of risk quotients, whereas previous investigations were limited by only considering uptake and consumption data pertaining to a few crops.
Pharmaceutical concentrations in soils were consistently predicted to be considerably larger as a result of veterinary use than as a result of human use. This reflects data availability, as veterinary PECs assume all animals are treated, whereas human PEC calculations were based upon prescription data. There are also differences in metabolism and absorption efficiency between humans and livestock. Approximately 30% of the carbamazepine administered orally to humans is excreted unaltered (Zhang, Geißen, and Gal Citation2008), while up to 90% of veterinary antibiotics are excreted unchanged into the environment (Kumar, Gupta, Chander, et al. Citation2005). The PECveterinary values estimated in this study were also markedly higher than historic predictions. For example, Boxall et al. (Citation2006) estimated veterinary use of oxytetracycline and trimethoprim to yield soil concentrations of 3.05 × 102 μg/kg and 0.5 μg/kg, respectively, whereas larger concentrations of 1.3 mg/kg and 3.03 mg/kg were predicted in this study. These comparatively high concentrations might constitute a function of the conservative, worst-case scenario method used in this study to calculate PECveterinary values as, based upon EMA guidelines (Citation2016), it was assumed that 100% of all veterinary pharmaceutical residues might be released into the environment. Pharmaceutical usage has also significantly increased since these earlier predictions were made (NHSBSA Citation2019), therefore some rise in soil concentrations was expected (Boxall et al. Citation2006).
Previously investigators established that the pharmaceutical uptake process in crops is a composite procedure, unique to both the concerned plant species and pharmaceutical substance (Carter et al. Citation2014, Citation2018; Wu et al. Citation2012), and the large range in UFs obtained from the UTOPIC database is indicative of this unique nature of uptake (all UFs used in this study are found in ). When comparing the uptake of tetracycline and oxytetracycline, median UFs were of the same order of magnitude within all crop types except grains and grain-based products. Comparable UFs were expected for these two chemically similar, broad-spectrum tetracycline antibiotics, as investigators showed that pharmaceutical substance and crop species are both important controllers of the pharmaceutical uptake process in crops (Carter et al. Citation2014, Citation2018; Wu et al. Citation2012). The lack of conformity of oxytetracycline and tetracycline uptake by grains and grain-based products may be a result of limited data availability. Although the UTOPIC database has comprehensively collated the uptake data for the first time, there are still wide gaps in the research and, of the five study pharmaceuticals, tetracycline data were most limited (). These data gaps also indicate that, with rising concern surrounding pharmaceuticals in the environment (Keerthanan et al. Citation2021), further research is warranted to improve understanding in this field.
Human health risk from pharmaceutical residues in crops was demonstrated to be comparably complex. Not only was the list of contributing factors varied, including the concentration of pharmaceuticals within the soil, crop uptake, consumption, and ADI, but the relative importance of each factor as a driver of risk was also dependent upon both the type of crop and the pharmaceutical involved. For example, the presentation of carbamazepine as the pharmaceutical with the largest median risk quotient was primarily driven by the potentially toxic combination of high uptake and high consumption in fruit, vegetables, and their products and grains and grain-based products. The median UFs associated with the uptake of carbamazepine into all crop types were greater than all other UFs () while, at the same time, fruit vegetables and their products were the most consumed crop type in all age groups except adolescents (Table S3). However, the health risk presented by sulfamethoxazole is more likely a symptom of the high soil concentrations predicted to arise from its veterinary usage. The PECveterinary value for sulfamethoxazole (15.1 mg/kg) was the largest PEC of the study, while its PEChuman value (3.03 × 10−3 mg/kg) was the second lowest PEC of the study. This was reflected in the associated risk quotients, with only veterinary use of sulfamethoxazole pointing toward an unacceptable level of risk to health ().
The importance of each crop type as a driver of risk from pharmaceutical exposure varied considerably between pharmaceuticals. Over all, fruit vegetables and their products and grains and grain-based products were found to contribute the most to risk, while starchy roots and tubers and other crops contributed the least. (). No single crop type was individually found to pose a disproportional risk to human health. This contrasts with previous findings into the significance of plant species in pharmaceutical uptake and risk, where it was indicated that crops that grow below ground pose a greater threat to human health than crops that grow above ground (Boxall et al. Citation2006; Carter et al. Citation2014). Exposure concentrations used in these previous investigations, however, were not always representative of natural soil environments, using uniform concentrations to spike soils, rather than calculating PECs for each pharmaceutical.
Figure 3a-3e. Relative contribution of grains and grain-based products; fruit, vegetables and their products; starchy roots and tubers; and other crops to the median risk posed by pharmaceutical residues in crops to adolescents (10–17 years old), adults (18–64 years old), elderly (65–74 years old), and very elderly (>75 years old).
No age group was deemed to be at an elevated level of risk from exposure to pharmaceutical residues, with adolescents, adults, elderly, and very elderly each found to be at comparable levels of exposure risk from contaminated crops. This agrees with evidence from human trials that demonstrated the presence of pharmaceuticals in human diets at all stages of adulthood, and indicated that the potential health risks associated with the consumption of pharmaceutical residues do not increase with age (Paltiel et al. Citation2016; Schapira et al. Citation2020). Schapira et al. (Citation2020) noted no significant relationship between age and carbamazepine concentration in the urine of Israeli adults not actively ingesting pharmaceuticals, suggesting that carbamazepine residues in the TWW used in Israeli agriculture pose an equal risk level to consumers of all ages. The results from the present investigation indicate that human trials might demonstrate similar observations for a wide selection of pharmaceuticals.
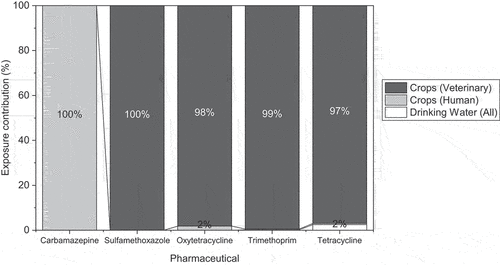
Keerthanan et al. (Citation2021) suggested that crop consumption may be the most important pathway through which humans become exposed to pharmaceuticals. Comparison of predicted human exposures to pharmaceuticals via crops (estimated in this study) and via drinking water (estimated by Webb et al. Citation2003) supports this and demonstrates crop consumption to be the predominant exposure pathway (). Drinking water contamination was predicted to expose humans to lower concentrations than crop consumption for all pharmaceuticals, with veterinary pharmaceuticals in crops responsible for >99% of the total predicted exposure to all pharmaceuticals, excluding carbamazepine, which is not used in a veterinary context (). Thus far, drinking water has been the focal point of the majority of environmental exposure research (Keerthanan et al. Citation2021), although this permits the placement of crop contamination and consumption at the forefront of future research.
Figure 4. Relative contributions of human and veterinary pharmaceutical residues in drinking water (Webb et al. Citation2003); human pharmaceutical residues in crops; and veterinary pharmaceutical residues in crops to the environmental exposure of humans to carbamazepine, sulfamethoxazole, oxytetracycline, trimethoprim, and tetracycline.
Regional differences in dietary consumption also need to be considered. This study estimated dietary consumption from the Comprehensive European Food Consumption Database (EFSA Citation2018). Whilst these data are applicable as a best estimate for risk assessment at the European scale, dietary consumption is likely to be highly variable on both regional and individual scales. A study of four European countries (Denmark, France, Italy, and the Czech Republic) found clear geographic variability in consumption of fruit and vegetables between countries, with mean fruit intake ranging from 118 to 199 g/day and vegetable consumption ranging from 95 to 239 g/day (Mertens et al. Citation2019). Variations in consumption were also identified within countries according to socio-economic factors such as age, gender, and educational level. Potential risks posed by consumption of contaminated crops might vary according to these differences in dietary consumption, alongside regional differences in pharmaceuticals prescribed and consumed, WWT, and agricultural practice. This investigation presents our best estimate of potential human health risks associated with consumption of contaminated crops at the European scale, but further study is required to understand the exposure risk to sub-populations at a finer scale.
The exposure pathway of pharmaceuticals to crops also needs to be carefully considered in future risk assessments. Pharmaceutical uptake by crops and associated potential risk to human health may vary depending on the source of pharmaceuticals, including from manure, biosolids, or contaminated irrigation water. The route of entry of antibiotics into agricultural soils through irrigation water or manure amendment was noted to exhibit important implications for behavior and resultant availability of antibiotics in agricultural soils (Albero et al. Citation2018). An assessment of the influence of agronomic practices on exposure and human health risks of antibiotics in commercially grown vegetable crops found that irrigation with contaminated water and fertilization with manure were the most important factors driving uptake, but that crop type, productivity, and growing time also played a significant role (Tadić et al. Citation2021). Alongside the exposure pathway, the timing and frequency of application are important. In England, Wales, and Northern Ireland, treated sludge cannot be applied to land within 10 months of harvest if crops are normally in direct contact with soil and are eaten raw (Defra Citation2018). The mean DT50 of carbamazepine in soil was found to be 210 days (Table S5), indicating that concentrations of carbamazepine may increase over time with repeated sludge applications. This emphasizes the importance of potential long-term considerations in future risk assessments.
Further research is warranted to address the gaps in UF data indicated by the present study (). Of some 2000 pharmaceuticals in use in the UK (Burns et al. Citation2018), only the 5 pharmaceuticals with the greatest availability of data were investigated; however, UFs were not available across the range of crops consumed in the human diet, with fruits, vegetables, and their products the only crop type with a full complement of UFs. Furthermore, the wide disparity between minimum and maximum UFs obtained from the literature was indicative of the lack of experimental protocols and variable quality in data in this field (Fantke, Arnot, and Doucette Citation2016). To provide high-quality uptake data, Fantke et al. (Citation2016) described the merits of setting standardized parameters in uptake experiments. It is also vital that future studies on pharmaceutical uptake into crops clearly state the crop part analyzed (e.g. roots, stem, leaves), such that the risk for edible parts of crops may be evaluated. Burns et al. (Citation2018) also recommended the use of relevant pharmaceutical data, such as prescription dispensing statistics and metabolism rates, to prioritize study compounds based upon potential release into soils.
Whilst investigating the pharmaceutical compounds with the greatest data availability in terms of plant uptake enabled a comprehensive evaluation of potential risk, these compounds are not necessarily the priority in terms of potential human health risk. Compounds may be better prioritized in terms of their affinity for uptake, with the UTOPIC database suggesting propranolol, norfloxacin, diclofenac, and metronidazole have the potential to accumulate in crops. Compounds might also be prioritized in terms of consumption data or associated predicted environmental concentrations. Alternatively, compounds may be prioritized in terms of their potential adverse effects. For example, many cytotoxic drugs used to treat cancer are teratogenic and therefore may be of greater concern, even with lower uptake potential or lower predicted or measured environmental concentrations. The need for a comprehensive, compound-specific assessment of cytotoxic pharmaceuticals and compounds with allergenic potential was emphasized by Schwab et al. (Citation2005). A comprehensive prioritization of pharmaceuticals in terms of their occurrence, persistence, bioaccumulation potential, and potential environmental and human health risks was presented by Castaño-Trias et al. (Citation2024). However, this paper indicates the need for more experimental data on pharmaceutical concentrations in edible parts of crops in order to reach a more accurate risk assessment.
While the risk from carbamazepine, trimethoprim, and sulfamethoxazole has been predicted, these predictions assume that individuals are receiving all of their food from land contaminated with these compounds. In reality, this is not likely to be the case as humans may be consuming food materials from different sources with different degrees of contamination. This risk assessment also does not account for the potential effects of processing and cooking on concentrations of pharmaceutical contaminants in crops. Whilst some fruit and vegetable products may be consumed raw, many might usually only be consumed after cooking. Investigations into the effects of boiling and frying on the concentrations of heavy metals in string beans and potatoes found that cooking processes generally decreased concentrations of contaminants in these vegetables (Perelló et al. Citation2008). Similarly, a study of the effects of washing and boiling on concentrations of persistent organic pollutants (PCBs, PCDDs, and PCDFs) in spinach found that cooking reduced concentrations of these contaminants by 21–61% compared to initial concentrations (Tsutsumi et al. Citation2002). It is likely that cooking processes might also act to reduce concentrations of pharmaceutical contaminants in vegetable crops; however, presently no apparent such data are available.
It is also possible that pharmaceutical residues may be metabolized within plants following uptake. A hydroponic study of tomato plants exposed to carbamazepine showed that at least 33% of the carbamazepine taken up into the plants was transformed into a range of 11 transformation products (Riemenschneider et al. Citation2017). Similarly, metabolites were found to account for a significant proportion of caffeine uptake concentrations in lettuce (Chuang et al. Citation2018). It is conceivable that concentrations of pharmaceuticals in crops may be diminished by metabolic processes in plants; however, the potential toxicity of respective metabolites also needs to be carefully evaluated, as concentrations of metabolites may exceed concentrations of parent compounds. A study of the occurrence of antibiotics in commercially grown vegetable crops found that concentrations of metabolites exceeded the concentrations of parent compounds in 73% of the total samples (Tadić et al. Citation2021).
More study is also required to explore the efficacy of ADIs as a protective measure against long-term, low-level pharmaceutical exposure. ADIs might often result in an overestimation of risk (Carter et al. Citation2014), with the various uncertainty or safety factors employed in their calculations (see EquationEquation 1(1)
(1) and EquationEquation 2
(2)
(2) ) often not applicable for a large proportion of the general population, and responsible for artificially low ADI values and inflated risk quotients (Carter et al. Citation2014). The ADI approach also does not account for contraindications. This might enhance the risk for specific groups of individuals who may be vulnerable to low-level exposure to pharmaceuticals that are contraindicated for specific life stages such as during pregnancy (Daughton Citation2008).
This risk assessment considered the potential risk posed by each pharmaceutical in isolation; however, these compounds may exist in the environment in a complex mixture of contaminants. Potential interactions between pharmaceuticals and the possibility that these may enhance health risks were not considered (Daughton Citation2008; Pomati et al. Citation2006). Pomati et al. (Citation2006) demonstrated that a mixture of 13 pharmaceuticals, including both sulfamethoxazole and carbamazepine, affected cell physiology and morphology and inhibited human embryonic stem cell growth when at concentrations relevant to polluted surface waters, suggesting that, assuming additivity, pharmaceutical residues might exert a combined effect on human health. With trimethoprim and sulfamethoxazole known to interact with 1,006 and 1,339 other pharmaceuticals, respectively (Wishart et al. Citation2018). Toxicant-induced loss of tolerance (TILT) or “multiple chemical sensitivity” (MCS) describes a syndrome of allergy-like symptoms that may be induced by repeated low-level exposure to chemicals. This might decrease an individual’s tolerance to chemicals even at low concentrations, which might not affect the majority of the population (Masri et al. Citation2021). Further research is required to explore whether concurrent exposure enhances human health risk beyond the level projected in this study. Biomonitoring studies, such as those used to assess the human health implications associated with the use of carbamazepine-contaminated TWW in Israeli agriculture (Paltiel et al. Citation2016; Schapira et al. Citation2020), demonstrated an applicable approach that might confirm current levels of human exposure to pharmaceutical residues as well as elucidate the resultant health threat.
This assessment considered the potential risk posed to human health through the dietary consumption of crops containing pharmaceutical residues. The presence of pharmaceuticals in the environment may also incur indirect risks to human health. For example, there is growing concern around the development of antimicrobial resistance genes in the environment as the concentrations of antibiotics in WWT exert a selection pressure on bacteria (Larsson and Flach Citation2022). Human health may also be indirectly affected by the presence of pharmaceuticals in the environment due to potential impacts on crop health affecting plant development, yield, and nutrient composition (Carter et al. Citation2015). As such, the potential risk posed to human health by pharmaceuticals in the environment is multifaceted.
Conclusions
This study aimed to estimate the level of risk posed by pharmaceutical residues within crops consumed in human diets. Published uptake factors (UFs) and comprehensive consumption statistics were used to determine dietary pharmaceutical exposure. Associated risks to human health were examined in relation to predetermined ADIs. It was evident that pharmaceuticals may be consumed as part of the human diet, with the consumption of crops contaminated with the residues of veterinary pharmaceuticals the dominant exposure pathway. Carbamazepine, oxytetracycline, sulfamethoxazole, trimethoprim, and tetracycline were each demonstrated to be taken up by all major crop types consumed in the diets of adolescents, adults, elderly, and very elderly, exposing all age groups to their potentially harmful residues. However, the levels of exposure predicted were such that, at present, only human usage of carbamazepine, and veterinary usage of trimethoprim and sulfamethoxazole may pose an unacceptable level of risk to human health.
The driving mechanisms of risk were also investigated, with risk observed to be complex, specific to both the type of crop and pharmaceutical concerned. No type of crop was identified to disproportionately drive health risk, and no marked relationship was exhibited between age and calculated risk quotient. The present study conducted a highly conservative assessment of risk, potentially overestimating the current risk posed by dietary pharmaceutical residues. However, the expected rise in the use of TWW for irrigation and biosolids for fertilizer and the possibility of concurrent exposure to multiple pharmaceuticals displays the potential to amplify risk beyond the level predicted in this study, increasing its future relevance.
The limited availability of relevant high-quality plant uptake data indicated a clear direction for further research. To improve understanding and increase confidence in findings, coordinated uptake investigations are needed for the collation of more comprehensive uptake data, while further exploration is also warranted into the adverse health effects that arise from sustained dietary exposure to multiple pharmaceutical residues. Based upon projected individual exposure concentrations, as well as the likelihood for concurrent exposure, this study demonstrated the pertinence of prioritizing investigation into the adverse health effects of exposure to carbamazepine, trimethoprim, and sulfamethoxazole in ensuing human health risk assessments.
Author contribution statement
Kirsten Earl: Conceptualization, Data Curation, Methodology, Formal Analysis, Investigation, Writing – Original Draft, Writing – Review & Editing. Alistair Boxall: Conceptualization, Writing – Review & Editing, Supervision, Project administration. Harriet Sleight: Conceptualization, Investigation, Writing – Original Draft, Writing – Review & Editing, Visualisation, Supervision. Nahum Ashfield: Methodology, Investigation, Writing – Review & Editing.
Supplemental Material
Download MS Word (88.2 KB)Acknowledgments
This work was supported by the Natural Environment Research Council ACCE DTP under grant number NE/S00713X/1.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data is included in the supplemental files or is available publicly.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15287394.2024.2371418.
Additional information
Funding
References
- Albero, B., J. L. Tadeo, M. Escario, E. Miguel, and R. A. Pérez. 2018. “Persistence and Availability of Veterinary Antibiotics in Soil and Soil-Manure Systems.” Science of the Total Environment 643:1562–1570. https://doi.org/10.1016/j.scitotenv.2018.06.314.
- Arnold, K. E., A. B. A. Boxall, A. R. Brown, R. J. Cuthbert, S. Gaw, T. H. Hutchinson, S. Jobling, et al. 2013. “Assessing the Exposure Risk and Impacts of Pharmaceuticals in the Environment on Individuals and Ecosystems.” Biology Letters 9 (4): 20130492. https://doi.org/10.1098/rsbl.2013.0492.
- Aus Der Beek, T., F. A. Weber, A. Bergmann, S. Hickmann, I. Ebert, A. Hein, and A. Küster. 2016. “Pharmaceuticals in the Environment—Global Occurrences and Perspectives.” Environmental Toxicology and Chemistry 35 (4): 823–835. https://doi.org/10.1002/etc.3339.
- Bartolo, N. S., L. M. Azzopardi, and A. Serracino-Inglott. 2021. “Pharmaceuticals and the Environment.” Early Human Development 155:105218. https://doi.org/10.1016/j.earlhumdev.2020.105218.
- Boxall, A. B. 2004. “The Environmental Side Effects of Medication: How Are Human and Veterinary Medicines in Soils and Water Bodies Affecting Human and Environmental Health?” EMBO Reports 5 (12): 1110–1116. https://doi.org/10.1038/sj.embor.7400307.
- Boxall, A. B. A., P. Johnson, E. J. Smith, C. J. Sinclair, E. Stutt, and L. S. Levy. 2006. “Uptake of Veterinary Medicines from Soils into Plants.” Journal of Agricultural and Food Chemistry 54 (6): 2288–2297. https://doi.org/10.1021/jf053041t.
- Burns, E. E., L. J. Carter, J. Snape, J. Thomas-Oates, and A. B. Boxall. 2018. “Application of Prioritization Approaches to Optimize Environmental Monitoring and Testing of Pharmaceuticals.” Journal of Toxicology and Environmental Health, Part B 21 (3): 115–141. https://doi.org/10.1080/10937404.2018.1465873.
- Carter, L. J., E. Harris, M. Williams, J. J. Ryan, R. S. Kookana, and A. B. A. Boxall. 2014. “Fate and Uptake of Pharmaceuticals in Soil–Plant Systems.” Journal of Agricultural and Food Chemistry 62 (4): 816–825. https://doi.org/10.1021/jf404282y.
- Carter, L. J., M. Williams, C. Böttcher, and R. S. Kookana. 2015. “Uptake of Pharmaceuticals Influences Plant Development and Affects Nutrient and Hormone Homeostases.” Environmental Science and Technology 49 (20): 12509–12518. https://doi.org/10.1021/acs.est.5b03468.
- Carter, L. J., M. Williams, S. Martin, S. P. Kamaludeen, and R. S. Kookana. 2018. “Sorption, Plant Uptake and Metabolism of Benzodiazepines.” Science of the Total Environment 628-629:18–25. https://doi.org/10.1016/j.scitotenv.2018.01.337.
- Castaño-Trias, M., S. Rodríguez-Mozaz, P. Verlicchi, and G. Buttiglieri. 2024. “Selection of Pharmaceuticals of Concern in Reclaimed Water for Crop Irrigation in the Mediterranean Area.” Journal of Hazardous Materials 466:133538. https://doi.org/10.1016/j.jhazmat.2024.133538.
- Chitescu, C. L., A. I. Nicolau, and A. A. M. Stolker. 2013. “Uptake of Oxytetracycline, Sulfamethoxazole and Ketoconazole from Fertilised Soils by Plants.” Food Additives and Contaminants Part A-Chemistry Analysis Control Exposure & Risk Assessment 30 (6): 1138–1146. https://doi.org/10.1080/19440049.2012.725479.
- Chuang, Y. H., C. H. Liu, R. Hammerschmidt, W. Zhang, S. A. Boyd, and H. Li. 2018. “Metabolic Demethylation and Oxidation of Caffeine During Uptake by Lettuce.” Journal of Agricultural and Food Chemistry 66 (30): 7907–7915. https://doi.org/10.1021/acs.jafc.8b02235.
- Chung, B. Y., S. M. Choi, T. H. Roh, D. S. Lim, M. Y. Ahn, Y. J. Kim, H. S. Kim, and B.-M. Lee. 2019. “Risk Assessment of Phthalates in Pharmaceuticals.” Journal of Toxicology & Environmental Health A 82 (5): 351–360. https://doi.org/10.1080/15287394.2019.1598053.
- Daughton, C. 2008 Pharmaceuticals As Environmental Pollutants: The Ramifications for Human Exposure.” In International Encyclopedia of Public Health, edited by K. Heggenhougen, and S. Quah, 66–102. Oxford: Academic Press. https://doi.org/10.1016/B978-012373960-5.00403-2.
- Defra. 2018. Sewage Sludge in Agriculture: Code of Practice for England, Wales and Northern Ireland. Department for Environment, Food & Rural Affairs. www.gov.uk/government/publications/sewage-sludge-in-agriculture-code-of-practice
- Duarte, D. J., R. Oldenkamp, and A. M. J. Ragas. 2022. “Human Health Risk Assessment of Pharmaceuticals in the European Vecht River.” Integrated Environmental Assessment and Management 18 (6): 1639–1654. https://doi.org/10.1002/ieam.4588.
- EFSA. 2018. The EFSA Comprehensive European Food Consumption database, Version 2 [Online], European Food Safety Authority. Accessed October 9, 2021. https://data.europa.eu/data/datasets/the-efsa-comprehensive-european-food-consumption-database?.
- Egbeyemi, M. M., S. A. Lateef, S. J. Akinsete, M. O. Omobowale, and T. A. Ewemoje. 2023. “Health Risk Assessment for Uptake and Accumulation of Pharmaceuticals in Jute Mallow (Corchorus olitorius) Irrigated with Treated Hospital Wastewater.” Environmental Monitoring and Assessment 195 (8): 956. https://doi.org/10.1007/s10661-023-11565-3.
- EMA. 2016. Guideline on Environmental Impact Assessment for Veterinary Medicinal Products in Support of the Vich Guidelines Gl6 and Gl38 [Online], European Medicines Agency. Accessed October 9, 2021. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-environmental-impact-assessment-veterinary-medicinal-products-support-vich-guidelines-gl6_en.pdf.
- EMA. 2018. Guideline on the Environmental Risk Assessment of Medicinal Products for Human Use – Draft [Online], European Medicines Agency. Accessed February 3, 2022. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-environmental-risk-assessment-medicinal-products-human-use-revision-1_en.pdf.
- Fantke, P., J. A. Arnot, and W. J. Doucette. 2016. “Improving Plant Bioaccumulation Science Through Consistent Reporting of Experimental Data.” Journal of Environmental Management 181:374–384. https://doi.org/10.1016/j.jenvman.2016.06.065.
- FAO. 1999. Women: Users, Preservers and Managers of Agrobiodiversity. Rome, Italy: Food and Agriculture Organisation of the United Nations.
- FAO & WHO. 1998. Evaluation of Certain Veterinary Drug Residues in Food: Forty-Seventh Report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization.
- FDA. 2012. FDA Approved Drug Products: Sulfamethoxazole/Trimethoprim Oral Suspension. Silver Spring, Maryland: U.S. Food and Drug Administration.
- Garduño-Jiménez, A.-L., J.-C. Durán-Álvarez, C. A. Ortori, S. Abdelrazig, D. A. Barrett, and R. L. Gomes. 2023. “Delivering on Sustainable Development Goals in Wastewater Reuse for Agriculture: Initial Prioritization of Emerging Pollutants in the Tula Valley, Mexico.” Water Research 238:119903. https://doi.org/10.1016/j.watres.2023.119903.
- Hawker, D. W., R. Cropp, and M. Boonsaner. 2013. “Uptake of Zwitterionic Antibiotics by Rice (Oryza Sativa L.) in Contaminated Soil.” Journal of Hazardous Materials 263:458–466. https://doi.org/10.1016/j.jhazmat.2013.09.066.
- Holling, C. S., J. L. Bailey, B. En Heuvel, and C. A. Kinney. 2012. “Uptake of Human Pharmaceuticals and Personal Care Products by Cabbage (Brassica campestris) from Fortified and Biosolids-Amended Soils.” Journal of Environmental Monitoring 14 (11): 3029–3036. https://doi.org/10.1039/c2em30456b.
- Keerthanan, S., C. Jayasinghe, J. K. Biswas, and M. Vithanage. 2021. “Pharmaceutical and Personal Care Products (PPCPs) in the Environment: Plant Uptake, Translocation, Bioaccumulation, and Human Health Risks.” Critical Reviews in Environmental Science and Technology 51 (12): 1221–1258. https://doi.org/10.1080/10643389.2020.1753634.
- Kipper, K., K. Herodes, M. Lillenberg, L. Nei, E. Haiba, and S. V. Litvin. 2010. “Plant Uptake of Some Pharmaceuticals Commonly Present in Sewage Sludge Compost.” ICBEE 2010 - 2nd International conference on chemical, biological and environmental engineering, proceedings, Cairo, Egypt, 261–264.
- Knight, E. R., L. J. Carter, and M. J. Mclaughlin. 2018. “Bioaccumulation, Uptake, and Toxicity of Carbamazepine in Soil–Plant Systems.” Environmental Toxicology and Chemistry 37 (4): 1122–1130. https://doi.org/10.1002/etc.4053.
- Kostopoulou, M., and A. Nikolaou. 2008. “Analytical Problems and the Need for Sample Preparation in the Determination of Pharmaceuticals and Their Metabolites in Aqueous Environmental Matrices.” TrAC Trends in Analytical Chemistry 2 (11): 1023–1035. https://doi.org/10.1016/j.trac.2008.09.011.
- Kumar, K., S. C. Gupta, S. Baidoo, Y. Chander, and C. J. Rosen. 2005. “Antibiotic Uptake by Plants from Soil Fertilized with Animal Manure.” Journal of Environmental Quality 34 (6): 2082–2085. https://doi.org/10.2134/jeq2005.0026.
- Kumar, K., S. C. Gupta, Y. Chander, and A. K. Singh. 2005. “Antibiotic Use in Agriculture and Its Impact on the Terrestrial Environment.” Advances in Agronomy 87:1–54 doi:https://doi.org/10.1016/S0065-2113(05)87001-4.
- Larsson, D. G. J., and C.-F. Flach. 2022. “Antibiotic Resistance in the Environment.” Nature Reviews Microbiology 20 (5): 257–269. https://doi.org/10.1038/s41579-021-00649-x.
- Lee, D. B., H. S. Kim, C. Lee, and K. H. Kim. 2018. “Effect of Soil Organic Matter Content on Uptake of Four Veterinary Antibiotics by Pepper.” Soil Science 183 (5): 188–194. https://doi.org/10.1097/SS.0000000000000244.
- Lees, K., M. Fitzsimons, J. Snape, A. Tappin, and S. Comber. 2016. “Pharmaceuticals in Soils of Lower Income Countries: Physico-Chemical Fate and Risks from Wastewater Irrigation.” Environment International 94:712–723. https://doi.org/10.1016/j.envint.2016.06.018.
- Li, Y., J. He, H. Qi, H. Li, S. A. Boyd, and W. Zhang. 2020. “Impact of Biochar Amendment on the Uptake, Fate and Bioavailability of Pharmaceuticals in Soil-Radish Systems.” Journal of Hazardous Materials 398:122852. https://doi.org/10.1016/j.jhazmat.2020.122852.
- Li, Y., J. B. Sallach, W. Zhang, S. A. Boyd, and H. Li. 2019. “Insight into the Distribution of Pharmaceuticals in Soil-Water-Plant Systems.” Water Research 152:38–46. https://doi.org/10.1016/j.watres.2018.12.039.
- Liu, X., C. Liang, X. Liu, F. Zhao, and C. Han. 2020. “Occurrence and Human Health Risk Assessment of Pharmaceuticals and Personal Care Products in Real Agricultural Systems with Long-Term Reclaimed Wastewater Irrigation in Beijing, China.” Ecotoxicology and Environmental Safety 190:110022. https://doi.org/10.1016/j.ecoenv.2019.110022.
- Madikizela, L. M., S. Ncube, and L. Chimuka. 2018. “Uptake of Pharmaceuticals by Plants Grown Under Hydroponic Conditions and Natural Occurring Plant Species: A Review.” Science of the Total Environment 636:477–486. https://doi.org/10.1016/j.scitotenv.2018.04.297.
- Masri, S., C. S. Miller, R. F. Palmer, and N. Ashford. 2021. “Toxicant-Induced Loss of Tolerance for Chemicals, Foods, and Drugs: Assessing Patterns of Exposure Behind a Global Phenomenon.” Environmental Sciences Europe 33 (1): 65. https://doi.org/10.1186/s12302-021-00504-z.
- Meffe, R., A. De Santiago-Martín, G. Teijón, V. Martínez Hernández, I. López-Heras, L. Nozal, and I. De Bustamante. 2021. “Pharmaceutical and Transformation Products During Unplanned Water Reuse: Insights into Natural Attenuation, Plant Uptake and Human Health Impact Under Field Conditions.” Environment International 157:106835. https://doi.org/10.1016/j.envint.2021.106835.
- Mennigen, J. A., P. Stroud, J. M. Zamora, T. W. Moon, and V. L. Trudeau. 2011. “Pharmaceuticals As Neuroendocrine Disruptors: Lessons Learned from Fish on Prozac.” Journal of Toxicology and Environmental Health B 14 (5–7): 387–412. https://doi.org/10.1080/10937404.2011.578559.
- Mertens, E., A. Kuijsten, M. Dofková, L. Mistura, L. D’addezio, A. Turrini, C. Dubuisson, et al. 2019. “Geographic and Socioeconomic Diversity of Food and Nutrient Intakes: A Comparison of Four European Countries.” European Journal of Nutrition 5 (4): 1475–1493. https://doi.org/10.1007/s00394-018-1673-6.
- Mordechay, E. B., V. Mordehay, J. Tarchitzky, and B. Chefetz. 2021. “Pharmaceuticals in Edible Crops Irrigated with Reclaimed Wastewater: Evidence from a Large Survey in Israel.” Journal of Hazardous Materials 416:126184. https://doi.org/10.1016/j.jhazmat.2021.126184.
- Mordechay, E. B., J. Tarchitzky, Y. Chen, M. Shenker, and B. Chefetz. 2018. “Composted Biosolids and Treated Wastewater as Sources of Pharmaceuticals and Personal Care Products for Plant Uptake: A Case Study with Carbamazepine.” Environmental Pollution 232:164–172. https://doi.org/10.1016/j.envpol.2017.09.029.
- NHSBSA. 2019. Prescription Cost Analysis – England 2019 [Online], National Health Service Business Services Authority. Accessed February 18, 2021 2022. https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/2018.
- NOAH. 2021. NOAH Compendium [Online], National Office of Animal Health. Accessed October 8, 2021. https://www.noahcompendium.co.uk/home.
- Oberoi, A. S., K. C. Surendra, D. Wu, H. Lu, J. W. C. Wong, and S. Kumar Khanal. 2022. “Anaerobic Membrane Bioreactors for Pharmaceutical-Laden Wastewater Treatment: A Critical Review.” Bioresource Technology 361:127667. https://doi.org/10.1016/j.biortech.2022.127667.
- Palli, L., F. Spina, G. C. Varese, M. Vincenzi, M. Aragno, G. Arcangeli, N. Mucci, D. Santianni, S. Caffaz, and R. Gori. 2019. “Occurrence of Selected Pharmaceuticals in Wastewater Treatment Plants of Tuscany: An Effect-Based Approach to Evaluate the Potential Environmental Impact.” International Journal of Hygiene and Environmental Health 222 (4): 717–725. https://doi.org/10.1016/j.ijheh.2019.05.006.
- Paltiel, O., G. Fedorova, G. Tadmor, G. Kleinstern, Y. Maor, and B. Chefetz. 2016. “Human Exposure to Wastewater-Derived Pharmaceuticals in Fresh Produce: A Randomized Controlled Trial Focusing on Carbamazepine.” Environmental Science & Technology 50 (8): 4476–4482. https://doi.org/10.1021/acs.est.5b06256.
- Perelló, G., R. Martí-Cid, J. M. Llobet, and J. L. Domingo. 2008. “Effects of Various Cooking Processes on the Concentrations of Arsenic, Cadmium, Mercury, and Lead in Foods.” Journal of Agricultural and Food Chemistry 56 (23): 11262–11269. https://doi.org/10.1021/jf802411q.
- Pirmohamed, M., S. James, S. Meakin, C. Green, A. K. Scott, T. J. Walley, K. Farrar, B. K. Park, and A. M. Breckenridge. 2004. “Adverse Drug Reactions as Cause of Admission to Hospital: Prospective Analysis of 18 820 Patients.” British Medical Journal 329 (7456): 15–19. https://doi.org/10.1136/bmj.329.7456.15.
- Pomati, F., S. Castiglioni, E. Zuccato, R. Fanelli, D. Vigetti, C. Rossetti, and D. Calamari. 2006. “Effects of a Complex Mixture of Therapeutic Drugs at Environmental Levels on Human Embryonic Cells.” Environmental Science & Technology 40 (7): 2442–2447. https://doi.org/10.1021/es051715a.
- Ponce-Robles, L., L. Benelhadj, A. J. García-García, F. Pedrero-Salcedo, P. A. Nortes-Tortosa, J. Albacete, and J. J. Alarcón. 2022. “Risk Assessment for Uptake and Accumulation of Pharmaceuticals by Baby Leaf Lettuce Irrigated with Reclaimed Water Under Commercial Agricultural Activities.” Journal of Environmental Management 324:116321. https://doi.org/10.1016/j.jenvman.2022.116321.
- Prosser, R. S., and P. K. Sibley. 2015. “Human Health Risk Assessment of Pharmaceuticals and Personal Care Products in Plant Tissue Due to Biosolids and Manure Amendments, and Wastewater Irrigation.” Environment International 75:223–233. https://doi.org/10.1016/j.envint.2014.11.020.
- Pullagurala, V. L. R., S. Rawat, I. O. Adisa, J. A. Hernandez-Viezcas, J. R. Peralta-Videa, and J. L. Gardea-Torresdey. 2018. “Plant Uptake and Translocation of Contaminants of Emerging Concern in Soil.” Science of the Total Environment 636:1585–1596. https://doi.org/10.1016/j.scitotenv.2018.04.375.
- Riemenschneider, C., B. Seiwert, M. Moeder, D. Schwarz, and T. Reemtsma. 2017. “Extensive Transformation of the Pharmaceutical Carbamazepine Following Uptake into Intact Tomato Plants.” Environmental Science & Technology 51 (11): 6100–6109. https://doi.org/10.1021/acs.est.6b06485.
- Sabourin, L., P. Duenk, S. Bonte-Gelok, M. Payne, D. R. Lapen, and E. Topp. 2012. “Uptake of Pharmaceuticals, Hormones and Parabens into Vegetables Grown in Soil Fertilized with Municipal Biosolids.” Science of the Total Environment 431:233–236. https://doi.org/10.1016/j.scitotenv.2012.05.017.
- Samarasinghe, S. V. A. C., K. Krishnan, R. J. Aitken, R. Naidu, and M. Megharaj. 2021. “Persistence of the Parabens in Soil and Their Potential Toxicity to Earthworms.” Environmental Toxicology and Pharmacology 83:103574. https://doi.org/10.1016/j.etap.2020.103574.
- Schapira, M., O. Manor, N. Golan, D. Kalo, V. Mordehay, N. Kirshenbaum, R. Goldsmith, B. Chefetz, and O. Paltiel. 2020. “Involuntary Human Exposure to Carbamazepine: A Cross-Sectional Study of Correlates Across the Lifespan and Dietary Spectrum.” Environment International 143:105951. https://doi.org/10.1016/j.envint.2020.105951.
- Schwab, B. W., E. P. Hayes, J. M. Fiori, F. J. Mastrocco, N. M. Roden, D. Cragin, R. D. Meyerhoff, J. Vincent, and P. D. Anderson. 2005. “Human Pharmaceuticals in US Surface Waters: A Human Health Risk Assessment.” Regulatory Toxicology and Pharmacology 42 (3): 296–312. https://doi.org/10.1016/j.yrtph.2005.05.005.
- Shahriar, A., J. W. Tan, P. Sharma, D. Hanigan, P. Verburg, K. Pagilla, and Y. Yang. 2021. “Modeling the Fate and Human Health Impacts of Pharmaceuticals and Personal Care Products in Reclaimed Wastewater Irrigation for Agriculture.” Environmental Pollution 276:116532. https://doi.org/10.1016/j.envpol.2021.116532.
- Sinclair, C. J., A. B. A. Boxall, S. A. Parsons, and M. R. Thomas. 2006. “Prioritization of Pesticide Environmental Transformation Products in Drinking Water Supplies.” Environmental Science & Technology 40 (23): 7283–7289. https://doi.org/10.1021/es0603507.
- Singer, H. P., A. E. Wössner, C. S. Mcardell, and K. Fenner. 2016. “Rapid Screening for Exposure to “Non-target” Pharmaceuticals from Wastewater Effluents by Combining HRMS-Based Suspect Screening and Exposure Modeling.” Environmental Science & Technology 50 (13): 6698–6707. https://doi.org/10.1021/acs.est.5b03332.
- Sleight, H., A. B. A. Boxall, and S. Toet. 2023. “Uptake of Pharmaceuticals by Crops: A Systematic Review and Meta-Analysis.” Environmental Toxicology and Chemistry 42 (10): 2091–2104. https://doi.org/10.1002/etc.5700.
- Sun, Y. M., Y. J. Guo, M. M. Shi, T. L. Qiu, M. Gao, S. L. Tian, and X. M. Wang. 2021. “Effect of Antibiotic Type and Vegetable Species on Antibiotic Accumulation in Soil-Vegetable System, Soil Microbiota, and Resistance Genes.” Chemosphere 263:128099. https://doi.org/10.1016/j.chemosphere.2020.128099.
- Sunyer-Caldú, A., G. Quintana, and M. S. Diaz-Cruz. 2023. “Factors Driving PPCPs Uptake by Crops After Wastewater Irrigation and Human Health Implications.” Environmental Research 237:116923. https://doi.org/10.1016/j.envres.2023.116923.
- Tadić, Đ., M. J. Bleda Hernandez, F. Cerqueira, V. Matamoros, B. Piña, and J. M. Bayona. 2021. “Occurrence and Human Health Risk Assessment of Antibiotics and Their Metabolites in Vegetables Grown in Field-Scale Agricultural Systems.” Journal of Hazardous Materials 401:123424. https://doi.org/10.1016/j.jhazmat.2020.123424.
- Topaz, T., A. Boxall, Y. Suari, R. Egozi, T. Sade, and B. Chefetz. 2020. “Ecological Risk Dynamics of Pharmaceuticals in Micro-Estuary Environments.” Environmental Science & Technology 54 (18): 11182–11190. https://doi.org/10.1021/acs.est.0c02434.
- Tsutsumi, T., T. Iida, T. Hori, R. Nakagawa, K. Tobiishi, T. Yanagi, Y. Kono, et al. 2002. “Recent Survey and Effects of Cooking Processes on Levels of PCDDs, PCDFs and Co-PCBs in Leafy Vegetables in Japan.” Chemosphere 46 (9–10): 1443–1449. https://doi.org/10.1016/S0045-6535(01)00275-2.
- Uddin, M., J. W. Chen, X. L. Qiao, R. Tian, and M. H. Zhu. 2020. “Insight into Dynamics and Bioavailability of Antibiotics in Paddy Soils by in situ Soil Moisture Sampler.” Science of the Total Environment 703:135562. https://doi.org/10.1016/j.scitotenv.2019.135562.
- Vinayagam, V., S. Murugan, R. Kumaresan, M. Narayanan, M. Sillanpää, N. Viet, D. Vo, O. S. Kushwaha, P. Jenis, and P. Potdar. 2022. “Sustainable Adsorbents for the Removal of Pharmaceuticals from Wastewater: A Review.” Chemosphere 300:134597. https://doi.org/10.1016/j.chemosphere.2022.134597.
- Wang, J., and S. Wang. 2016. “Removal of Pharmaceuticals and Personal Care Products (PPCPs) from Wastewater: A Review.” Journal of Environmental Management 182:620–640. https://doi.org/10.1016/j.jenvman.2016.07.049.
- Webb, S., T. Ternes, M. Gibert, and K. Olejniczak. 2003. “Indirect Human Exposure to Pharmaceuticals via Drinking Water.” Toxicology Letters 142 (3): 157–167. https://doi.org/10.1016/S0378-4274(03)00071-7.
- Wishart, D. S., Y. D. Feunang, A. C. Guo, E. J. Lo, A. Marcu, J. R. Grant, T. Sajed, D. Johnson, C. Li, and Z. Sayeeda. 2018. “Drugbank 5.0: A Major Update to the Drugbank Database for 2018.” Nucleic Acids Research 46 (D1): D1074–D1082. https://doi.org/10.1093/nar/gkx1037.
- Wu, C., A. L. Spongberg, J. D. Witter, and B. M. Sridhar. 2012. “Transfer of Wastewater Associated Pharmaceuticals and Personal Care Products to Crop Plants from Biosolids Treated Soil.” Ecotoxicology and Environmental Safety 85:104–109. https://doi.org/10.1016/j.ecoenv.2012.08.007.
- Yu, X. L., X. X. Liu, H. Liu, J. H. Chen, and Y. Sun. 2019. “The Accumulation and Distribution of Five Antibiotics from Soil in 12 Cultivars of Pak Choi.” Environmental Pollution 254:113115. https://doi.org/10.1016/j.envpol.2019.113115.
- Zhang, Y., S.-U. Geißen, and C. Gal. 2008. “Carbamazepine and Diclofenac: Removal in Wastewater Treatment Plants and Occurrence in Water Bodies.” Chemosphere 73 (8): 1151–1161. https://doi.org/10.1016/j.chemosphere.2008.07.086.
