Abstract
The DR-70® (FDP) test was the first cancer test cleared by USFDA for monitoring colorectal cancer (CRC) since Carcinoembryonic Antigen (CEA) in 1982. Conservatively, 50% of biopsy-positive CRC patients have negative CEA values. DR-70 and CEA values were compared for 113 CRC monitoring patients. Total concordance rates for DR-70 and CEA were 0.665 and 0.686, respectively. CRC patient pairs were grouped based on their CEA value to deduce DR-70's effectiveness at monitoring patients with low CEA values. DR-70 had 12% to 100% greater positive concordance rates than CEA in this group. DR-70 is a welcome new option for CRC patients.
INTRODUCTION
Awareness of the importance of CRC screening[ Citation 1 ] and early treatment has risen,[ Citation 2 ] but CRC is presently still a significant health concern in the United States.[ Citation 3 ] In 2007, the Surveillance, Epidemiology and End Results (SEER) program estimated there were 153,760 new colorectal cancer patients[ Citation 4 ] with a five year survival prediction of 50%. CRC accounts for approximately 10.6% of all new cancer cases and approximately 10% of all cancer deaths in the United States.[ Citation 5 ] Colorectal cancer (CRC) remains the second leading cause of cancer death in the United States,[ Citation 5 ] despite a reported decrease in colorectal cancer mortality over the past forty years. This decreased rate is related to increased screening, intervention, and monitoring programs.
Monitoring programs have emerged as an important tool in enhancing survival in post-operative CRC patients.[ Citation 6 ] For CRC, approximately half of all patients treated will experience disease recurrence.[ Citation 7 ] Curative retreatment options exist, and retreatment options are applied with a modest decrease in CRC mortality (approximately 10–15%).[ Citation8-10 ] However, in order to enhance the survival benefit of CRC monitoring programs, “the availability of sensitive and specific tests to identify recurrences at a treatable stage” needs improvement.[ Citation 6 ]
The AMDL-ELISA DR-70 (FDP) test (AMDL Diagnostics, Inc., Tustin, CA) is the first new in vitro diagnostic cancer test to be cleared by the US FDA for monitoring colorectal cancer (CRC) since January 14, 1982 when Carcinoembryonic Antigen (CEA) was approved. CEA has been in routine usage for many years as a blood test for monitoring CEA, but it also has well known limitations that are related to the nature of the tumor marker.[ Citation 6 ] CEA has been characterized as an oncofetal marker, which implies that it is only present during cancer progression or normal embryogenesis. Evidence exists that contradicts its classification as a pure oncofetal marker; as there are reports of this antigen's presence in healthy organs and its elevation due to benign conditions that affect the liver,[ Citation11-14 ] lungs,[ Citation 15 ] and the gastrointestinal system.[ Citation 16 ] Also, CEA is not a good target for a blood test because CEA is normally firmly attached to cancer cells due to its role as an adhesion molecule.[ Citation 15 ] In contrast, the DR-70 (FDP) antigen is freely diffusible in the blood.
For many CRC patients with biopsy confirmed cancer, CEA levels are not measurable above the physiological background. Data was taken directly from three independent studies[ Citation17-19 ] and presented directly in Table below to assess the need for an additional CRC monitoring tool. Approximately 50% of all CRC patients with low CEA values can not use the CEA test to monitor their cancer because their CEA levels fall below the manufacturer's defined physiological background level. In the table below, CEA Low Responders is the term given for biopsy positive cancer patients with low CEA values. All of the numbers in the table have been published in the referenced papers.
TABLE 1 Literature Based Definition of the % of CRC Patients Not Able to Use CEA
The DR-70 test measures both Fibrin and Fibrinogen Degradation Products (referred to collectively as FDP in this paper) in human serum samples. Measuring multiple FDP species prevents the DR-70 (FDP) immunoassay from underestimating the cancer-related levels of FDP.[ Citation 20 ] Refer to Figure for a schematic describing how the DR-70 (FDP) assay measures FDP generated from all of the major cancer induced FDP production pathways. Researchers have established a strong link between increased FDP levels and cancer.[ Citation20-22 ] This strong link is based on multiple factors including: a cancer-caused redirection of the coagulation cascade and a cancer-related increase in proteolysis within tumors as they grow and metastasize.
FIGURE 1 Cancer Elevates FDP Levels Through Two Pathways: Coagulation and Fibrinolysis. The AMDL-ELISA DR-70® (FDP) test measures the FDP produced by multiple pathways, unlike other FDP assays which only measure one pathway or one pathway product. Researchers have established that cancer causes elevated levels of both urokinase-type plasminogen activator (u-PA)[ Citation23-25 ] and tissue factor (TF).[ Citation26-28 ] Both the u-PA and TF pathways effect the production of FDP in cancer cells. The u-PA pathway (1A and 1B) activates plasmin by transforming plasminogen, the inactive precursor of plasmin, into functional plasmin.[ Citation23-25 ] The TF pathway (2) alters the extrinsic coagulation system causing an activation of thrombin.[ Citation26-29 ] Thrombin (3) converts Fibrinogen to Fibrin.[ Citation 30 ] The type of FDP produced will be different depending upon which of the two substrates is digested by plasmin. When fibrinogen is the substrate for plasmin (4), fragments D and E are the end products with fragments X and Y as intermediate products in this digestion. When fibrin is the substrate of plasmin (5), D-dimer is the end product. As a result of either pathway (4) or (5), cancer will cause an elevation in FDP levels as measured by the DR-70 (FDP). Tests that measure only one of the individual FDP species, i.e., D-dimer tests, will miss up to half of the FDP generated as a result of cancer physiology.
![FIGURE 1 Cancer Elevates FDP Levels Through Two Pathways: Coagulation and Fibrinolysis. The AMDL-ELISA DR-70® (FDP) test measures the FDP produced by multiple pathways, unlike other FDP assays which only measure one pathway or one pathway product. Researchers have established that cancer causes elevated levels of both urokinase-type plasminogen activator (u-PA)[ Citation23-25 ] and tissue factor (TF).[ Citation26-28 ] Both the u-PA and TF pathways effect the production of FDP in cancer cells. The u-PA pathway (1A and 1B) activates plasmin by transforming plasminogen, the inactive precursor of plasmin, into functional plasmin.[ Citation23-25 ] The TF pathway (2) alters the extrinsic coagulation system causing an activation of thrombin.[ Citation26-29 ] Thrombin (3) converts Fibrinogen to Fibrin.[ Citation 30 ] The type of FDP produced will be different depending upon which of the two substrates is digested by plasmin. When fibrinogen is the substrate for plasmin (4), fragments D and E are the end products with fragments X and Y as intermediate products in this digestion. When fibrin is the substrate of plasmin (5), D-dimer is the end product. As a result of either pathway (4) or (5), cancer will cause an elevation in FDP levels as measured by the DR-70 (FDP). Tests that measure only one of the individual FDP species, i.e., D-dimer tests, will miss up to half of the FDP generated as a result of cancer physiology.](/cms/asset/6bfcb213-5301-4f53-97c3-56a600e6ee0b/ljii_a_462252_o_f0001g.jpg)
Because the DR-70 test uses a different tumor marker than the CEA test, physicians have an additional blood test for monitoring CRC patients that may be superior to CEA for many of their patients with low CEA values. The purpose of this study is to determine if DR-70 is effective at monitoring CRC patients with low CEA levels.
EXPERIMENTAL
Description of the Clinical Samples
The samples for the serial monitoring study were retrospective banked samples that were collected blindly and without bias to include all patients with diagnosed colorectal cancer in the bank at the time of the collection. The serial monitoring samples for this study were obtained from two retrospective sample banks. Forty-eight serial sets were obtained from Geffen Cancer Center in Vero Beach, FL and sixty-four serial sets were from the serum banks at MD Anderson Cancer Center in Houston, TX. Institutional Review Board Approval for use of the samples and informed consent were available for each patient sample set.
Clinical information detailing the status of each patient's disease was collected at the time of each sample draw. The clinical diagnoses included Duke's Stage, grade and type (colon or rectal cancer). None of the patients had a history of malignancy within the past five years of the initial sample draw other than colorectal cancer. A breakdown of the patient series is presented in Table . The average number of observations per patient is 4.0.
TABLE 2 Patient Observation Series
Of the original 113 patients, one had to be dropped from further analysis due to incomplete clinical records. The 112 evaluable subjects in this CRC monitoring cohort consisted of 44 males and 68 females. The average age of the male patients was 65 while female patients averaged 62 years. The overall average age was 63 years. There was no significant difference between the average age of the males and the females in this cohort based on a student's t-test analysis for the determination of variances [t = 1.41, p = 0.163 (unequal variances)].
The ethnic composition of the cohort is shown in Table . Approximately 88% percent of the cohort was Caucasian; approximately 6% of African-American background; approximately 4% of Hispanic descent; and approximately 2% of Asian decent.
TABLE 3 Ethnic Distribution
Table presents the Dukes stage of the disease at time of diagnosis for 111 of the 112 evaluable serial patients. One patient's chart did not contain information related to the stage at time of diagnosis.
TABLE 4 Stage of Cancer at Time of Diagnosis
Table demonstrates the relationship between Dukes Stage at diagnosis and the presence of metastases. As the Stage of the disease progressed, the percentage of patients with metastases increased.
TABLE 5 Distribution of Metastases by Stage at Diagnosis
Statistical Analysis Plan for Association Between DR-70® (FDP)/AIA-Pack™ CEA and Disease Status
The initial objective of this analysis is to determine the overall positive, negative and total concordance values of the DR-70 (FDP) and CEA assays.[ Citation 31 ] Then, the CRC patients will be grouped based on their CEA values to evaluate the effectiveness of the DR-70® (FDP) assay at monitoring CRC patients with low CEA values; defined as a CEA value of 30 or below.
Defining the Clinical Sample Set
Serial samples were taken from 112 colon cancer patients resulting in a total of 445 paired observations in which a DR-70 (FDP) reading and a determination of disease progression were obtained. In total, there were also 445 paired observations in which an AIA-Pack™ CEA Assay reading and a determination of disease progression were obtained. The sequential draws covered an average longitudinal period of at least nine months. Progression of the DR-70 (FDP) value or AIA-Pack CEA Assay value in the serial monitoring set was evaluated as a percentage change between the current and previous readings (Y). The minimum percentage to specify disease progression in either assay was determined to be 15%, as will be described in detail later. Clinical disease progression (D) was determined by the Subject's physician based on their office procedures and clinical laboratory based analyses that were the standard of care during the time of the monitoring period.
Monitoring Cases for Response to Therapy
Subjects in the serial monitoring cohort were followed after surgery and or after various types of therapy including chemotherapy and radiation therapy. The response to therapy was evaluated using information provided in the records by the clinicians based on the results of clinical examinations and imaging results (i.e., bone scans, CT scans, magnetic resonance imaging studies, radiography, or ultrasound).
Response to therapy is defined as follows:
Complete response (CR) or no evidence of disease (NED): The complete disappearance of all clinical and image-measurable disease as evidenced by the clinical exam and imaging or other diagnostic modalities as ordered by the physician. | |||||
Partial Response (PR): In patients with metastases at the time of the original draw, a noticeable reduction in the size of primary metastatic lesions or bone metastases demonstrating at least stabilization as observed on the bone scan. | |||||
Stable Disease (SD): No significant change in the size of primary metastatic lesions or no noticeable increase in the size of primary lesions or no new lesions as evidenced by the clinical exam and imaging or other diagnostic modalities as ordered by the physician. | |||||
Progressive Disease (PD): Clinical or imaging results that clearly indicate the presence of lesions not seen on previous examinations or a significant increase in the size of primary or metastatic lesions. | |||||
Definition of Outcome Measure
The outcome measure for this analysis is the determination of progression of disease from time point i (clinical visit i, i = 1 to n − 1) to a succeeding time point j (clinical visit j, j = i + 1 to n). In this analysis n is the number of clinical visits for which samples are collected from a Subject after diagnosis of colorectal cancer and prior to death, loss to follow up or remission of disease.
Let D ij represent the variable disease progression as measured above and allow D ij to have the values
Determining Values of D
Disease progression from visit i to visit j will be determined by the Subject's physician based on any or all of the following:
Examination of the subject for clinical signs and symptoms, including the results of laboratory tests that are current standard of care for the assessment of colorectal cancer disease status. | |||||
Examination of radiographic findings (imaging) that can be used for the assessment of colorectal cancer disease status. Radiographic findings include results from CAT scans, PET scans, MRI and X-Ray images. | |||||
Determination of Clinical Significance in Marker Value Change
To ensure that the change between the values of the test device over a time interval could not be attributed to assay variation, a 15% increase from the previous visit was determined to be the most appropriate threshold for significant % change for the determination of disease progression in the DR-70 (FDP) assay. The coefficient of variation (CV) used in the calculation for significant % change was based on an imprecision study following regulatory guidelines. In that precision study, the total CV over all runs, days, and intra-assay was computed for each control specimen analyzed. The highest CV values were observed for specimens with a low concentration of (0.21–0.42 µg/mL); however, in a study of cancer progression, such as the one being reported here, such samples constituted less than 5% of the measurements. Over 80% of the measurements had concentrations of 0.6 µg/mL or higher where the CV is lower. Therefore the CV values for the lower concentrations will not be used to determine the significant % change. If the CV values for the highest laboratory specimens with concentration of 1.31 (CV = 7.85) or 4.11 (CV = 7.14) µg/mL are averaged, the mean is 7.495%. The CV is given by the following formula.
A 15% increase from the previous visit was also determined as the most appropriate threshold for significant % change for the determination of disease progression using the AIA-Pack™ CEA Assay from TOSOS Bioscience. The same evaluation, as above, was used with the CV listed in the AIA-Pack™ CEA Assay product insert.
Definition of Significance in Marker Value Change
Let δ equal the significant change in marker value for either assay, which has been determined at 15% for either assay, as described above. Let x i be the value of the test device obtained from the assay of a blood sample drawn from the Subject at visit i and x j be the value of the test device obtained from the assay of a blood sample drawn from the Subject at visit j.
Define Y ij as
Determining the Association Between D and Y
With D ij and Y ij defined above for either assay, a 2 × 2 contingency table can be constructed for the analysis of this data. The contingency table has the format of Table . In this table the variable a represents the number of (Y ij , D ij ) pairs that have the value of 1 for both Y ij and D ij . The variable b represents the number of (Y ij , D ij ) pairs that have the value 1 for Y ij and 0 for D ij . The variable c represents the number of (Y ij , D ij ) pairs that have the value 0 for Y ij and 1 for D ij . Lastly variable d represents the number of (Y ij , D ij ) pairs that have the value of 0 for both Y ij and D ij . The accrued values of a, b, c , and d are determined over all serial interval values of Y ij and D ij . The sum of a, b , c and d is the total number of all (Y ij , D ij ) pairs for all Subjects. This sum is designated N in Table .
TABLE 6 Model Contingency Table for D and Y
From Table , sensitivity and specificity are computed as follows:
From Table , concordance values are computed as follows:
Justification of Sample Size
Given the above assumptions and calculations, the minimum sample size for this study was determined to be 70 subjects with an average of 3 draws each.[ Citation 31 ] The samples are retrospective banked samples collected blindly and unbiased. Out of a total of 445 evaluable observations, there were 112 evaluable patient serial sets with an average of 4 draws each.
RESULTS
General Effectiveness of DR-70 (FDP) or CEA for CRC Monitoring
The clinical trial results were tabulated, as described above. The results for the DR-70 (FDP) test immediately follow in Table and the results for CEA are found in Table . In addition, the following interpretations are provided: Positive Concordance (C+), Negative Concordance (C−), Total Concordance (C), Sensitivity, and Specificity.
TABLE 7 Clinical Disease Status vs. AMDL-ELISA DR-70 (FDP)
TABLE 8 Clinical Disease Status vs. the TOSOH AIA-PACK CEA
Based on these data the concordances for the DR-70 (FDP) vs. Clinical Disease Status are:
Based on these data the concordances for the TOSOH AIA-PACK CEA vs. Clinical Disease Status are:
Effectiveness of DR-70 (FDP) Test in CRC Monitoring Patients with Low CEA Values
After grouping the CRC patients’ sample pairs in ascending order based on their CEA values, the following relationships were revealed. Positive concordance values of CRC patients measured with DR-70 or CEA in patient groups relative to their CEA values are provided in Figure . Negative concordances of CRC patients measured with DR-70 or CEA in groups relative to their CEA values are provided in Figure .
FIGURE 2 Positive Concordance for DR-70 or CEA Grouped by CEA values. Positive progression patient sample pairs were grouped in ascending order based on the CEA value. The % Concordance for DR-70 relative to the clinical findings was graphed in blue for each group. The % Concordance for CEA relative to the clinical findings was graphed in red for each group. Forty-six (46) of the 135 total positive progression patient pair values fell in the groups containing CEA values of 30 or less.
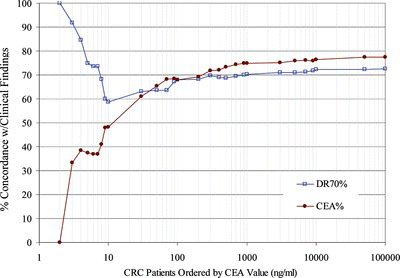
FIGURE 3 Negative Concordance for DR-70 or CEA Grouped by CEA values. Negative progression patient sample pairs were grouped in ascending order based on the CEA value. The % Concordance for DR-70 relative to the clinical findings was graphed in blue for each group. The % Concordance for CEA relative to the clinical findings was graphed in red for each group. There were a total of 199 negative progression patient pair values.
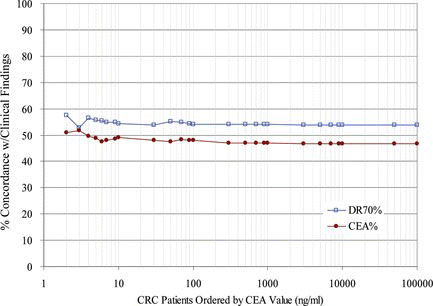
In Figure , DR-70 had between 12% and 100% greater positive concordance rates than CEA for CRC monitoring patients with low CEA values. In contrast, the negative concordance values of DR-70 and CEA showed less than 10% difference for all CRC patient groups in the trial, as depicted in Figure .
DISCUSSION
Overall, the results from this trial support the assertion that the AMDL-DR-70 (FDP) test is as good at monitoring CRC patients as CEA. Based on the results presented in Table and Table , the total concordance values for DR-70 and CEA are 0.665 and 0.686, respectively. The total concordance values for DR-70 and CEA differed by only 3.2% in this clinical trial.
In addition, Figure suggests that DR-70 is more effective at monitoring patients whose CEA values are 30 or less. Forty-six (46) of 135 positive progression patient pair values fell in the groups containing CEA values of 30 or less. In this group, DR-70 had between 12% and 100% greater positive concordance rates than CEA for CRC monitoring patients. The negative concordance rates were about the same for both assays across all groups when ordered by the CEA value of the patient. The difference between the negative concordance rates of these assays was less than 10% for all patient groups relative to their CEA values. Additional trials are planned to examine this same sub-group to verify the value of the test for patients with low CEA values.
The results of this trial suggest that DR-70 could have a positive impact on mortality that is associated with CRC recurrence. All of the patients in the reported trial were either post-surgery with no adjuvant therapy or post- therapy, as was described in the methods section. This holds clinical significance because disease progression in these patients would be described as disease recurrence. As reported in the introduction, approximately half of all CRC patients treated will experience disease recurrence. An additional and improved tool for the monitoring of disease recurrence could profoundly impact the mortality rate that is associated with CRC recurrence. Future studies could assess the impact of the DR-70 test on clinical outcomes in a longer term, prospective trial.
FDP has been shown to be valuable as a tumor marker in a number of different cancers.[ Citation32-43 ] FDP levels correlate with cancer occurrence, stage, progression and prognosis. Among these studies, the DR-70 (FDP) assay was used to detect FDP levels in 7,839 patients and the DR-70 (FDP) assay results consistently correlated with either the positive detection or positive progression of a variety of cancers.
Researchers have established a strong link between increased FDP levels and cancer which is based on multiple factors including: a cancer-caused redirection of the coagulation cascade[ Citation44-49 ] and a cancer-related increase in proteolysis within tumors as they grow[ Citation 50 , Citation 51 ] and metastasize.[ Citation52-56 ] Clinical studies reveal that measuring FDP levels, either with the DR-70 (FDP) test or with other related tests, has significant diagnostic value for a variety of cancers. These studies demonstrate that FDP levels correlate with the cancer stage[ Citation41-43 , Citation57-60 ] and with the cancer progression,[ Citation 41 , Citation 59 , Citation 61 ] as quantified by the number of lymph node metastases. Clinical research efforts have shown that pretreatment measurements of FDP levels have prognostic significance for post-treatment survival.[ Citation 33 , Citation 60 , Citation62-66 ] In addition to survival prognoses, pretreatment FDP values may be used to indicate when adjuvant systemic treatments are required for surgical Subjects.[ Citation 64 , Citation 66 ]
Cancer is a disease that is characterized by disregulation at the cellular level. As cancer progresses, the cellular disregulation spreads to the systems level. The coagulation system is one of the first systems affected by cancer-related processes. As referenced in Figure , the coagulation pathway may be inappropriately activated in cancer patients either by the activation of the coagulation pathway alone, the fibrinolysis pathway alone, or both pathways simultaneously. Coagulation may be increased due to elevated levels of tissue factor (TF),[ Citation 62 , Citation 67 ] which acts through the extrinsic coagulation system. Alternatively, the fibrinolysis pathway may be mistakenly activated in cancer patients through elevations in the levels of urokinase-type plasminogen activator (u-PA)[ Citation 44 ] that activates the protease plasmin. Disregulation of the coagulation system has important adverse affects on cancer patients because the coagulation system plays dual roles in homeostasis and immunity. As the cancer-related disregulation of the coagulation system increases, these clots can lead to heart attack, stroke, or pulmonary embolism. Other FDP-related tests have been helpful in predicting survival outcome based on the often fatal consequences of blood clots in cancer patients.[ Citation 59 , Citation 62 , Citation 67 ] As the utility of the DR-70 (FDP) assay becomes more widely known, DR-70 should be adapted to help patients with a variety of cancers in different clinical settings.
CONCLUSIONS
The DR-70 test appears to have additional benefits in monitoring CRC patients with low CEA values. DR-70 had between a 12% and a 100% greater positive concordance rate than CEA for CRC monitoring patients with low CEA values. Given that 50% is a conservative estimate of CRC patients with low CEA values, physicians and patients could significantly benefit from this new option for monitoring CRC cancer.
REFERENCES
- et al. Promoting early detection tests for colorectal carcinoma and adenomatous polyps: a framework for action: the strategic plan of the National Colorectal Cancer Roundtable . Cancer 2002 , 95 , 1618 – 1628 .
- Ladabaum , U. ; Song , K. Projected national impact of colorectal cancer screening on clinical and economic outcomes and health services demand . Gastroenterology 2005 , 129 , 1151 – 1162 .
- Hill , L.B. ; O'Connell , J.B. ; Ko , C.Y. Colorectal cancer: epidemiology and health services research . Surg. Oncol. Clin. N. Am. 2006 , 15 , 21 – 37 .
- Ries , L.A.G. ; Eisner , M.P. ; Kosary , C.L. SEER Cancer Statistics Review. National Cancer Institute http://seer.cancer.gov/csr/1975_2002/ (1975–2002) .
- American Cancer Society. Colorectal cancer facts & figures, special edition. Atlanta , Georgia : American Cancer Society , 2007.
- Anthony , T. Colorectal cancer follow-up in 2005 . Surg. Oncol. Clin. N. Am. viii (2006 ), 15 , 175 – 193 .
- Graffner , H. ; Hultberg , B. ; Johansson , B. ; Moller , T. ; Petersson , B.G. Detection of recurrent cancer of the colon and rectum . J. Surg. Oncol. 1985 , 28 , 156 – 159 .
- Renehan , A.G. ; Egger , M. ; Saunders , M.P. ; O'Dwyer , S.T. Mechanisms of improved survival from intensive followup in colorectal cancer: a hypothesis . Br. J. Cancer 2005 , 92 , 430 – 433 .
- et al. . Efficacy and cost of risk-adapted follow-up in patients after colorectal cancer surgery: a prospective, randomized and controlled trial . Eur. J. Surg. Oncol. 2002 , 28 , 418 – 423 .
- et al. . Role of follow-up in management of local recurrences of colorectal cancer: a prospective, randomized study . Dis. Colon. Rectum. 1998 , 41 , 1127 – 1133 .
- et al. . Immunohistochemical localization of CEA, CA19-9 and DU-PAN-2 in hepatitis C virus-infected liver tissues . Histopathology 2002 , 40 , 472 – 479 .
- Maestranzi , S. ; Przemioslo , R. ; Mitchell , H. ; Sherwood , R.A. The effect of benign and malignant liver disease on the tumour markers CA19-9 and CEA . Ann. Clin. Biochem. 1998 , 35 ( Pt 1 ), 99 – 103 .
- et al. . Carcinoembryonic antigen (CEA) in serum and bile of colorectal cancer patients with or without detectable liver metastases . Anticancer. Res. 1994 , 14 , 1409 – 1412 .
- Bell , H. ; Orjasaeter , H. Five years’ follow-up of patients with elevated carcinoembryonic antigen (CEA) and alcoholic liver disease, with special reference to mortality rate and development of malignancy . Hepatogastroenterology 1983 , 30 , 140 – 142 .
- et al. . CEA levels in serum and BAL in patients suffering from lung cancer: correlation with individuals presenting benign lung lesions and healthy volunteers . Med. Oncol. 2007 , 24 , 219 – 225 .
- Loewenstein , M.S. ; Zamcheck , N. Carcinoembryonic antigen (CEA) levels in benign gastrointestinal disease states . Cancer 1978 , 42 , 1412 – 1418 .
- Ladenson , J.H. ; McDonald , J.M. ; Landt , M. ; Schwartz , M.K. ( Washington University Case Conference ). Colorectal carcinoma and carcinoembryonic antigen (CEA) . Clin. Chem. 1980 , 26 , 1213 – 1220 .
- Wang , J.Y. ; Tang , R. ; Chiang , J.M. Value of carcinoembryonic antigen in the management of colorectal cancer . Dis. Colon. Rectum. 1994 , 37 , 272 – 277 .
- et al. . Preoperative carcinoembryonic antigen level as an independent prognostic factor in colorectal cancer: Taiwan experience . Jpn. J. Clin. Oncol. 2000 , 30 , 12 – 16 .
- Okholm , M. ; Iversen , L.H. ; Thorlacius-Ussing , O. ; Ejlersen , E. ; Boesby , S. Fibrin and fibrinogen degradation products in plasma of patients with colorectal adenocarcinoma . Dis. Colon. Rectum. 1996 , 39 , 1102 – 1106 .
- et al. . Fibrinogen catabolism within the procoagulant VX-2 tumor of rabbit lung in vivo: Effluxing fibrin(ogen) fragments contain antiangiogenic activity . J. Lab. Clin. Med. 2004 , 143 , 241 – 254 .
- et al. . Relationships among tumor burden, tumor size, and the changing concentrations of fibrin degradation products and fibrinolytic factors in the pleural effusions of rabbits with VX2 lung tumors . J. Lab. Clin. Med. 2006 , 147 , 27 – 35 .
- Baker , E.A. ; Bergin , F.G. ; Leaper , D.J. Plasminogen activator system, vascular endothelial growth factor, and colorectal cancer progression . Mol. Pathol. 2000 , 53 , 307 – 312 .
- et al. . Effects of urokinase receptor occupancy on plasmin generation and proteolysis of basement membrane by human tumor cells . Blood 1991 , 78 , 479 – 487 .
- et al. . Plasma urokinase receptor levels in patients with colorectal cancer: relationship to prognosis . J. Natl. Cancer Inst. 1999 , 91 , 869 – 874 .
- et al. . Expression of tissue factor and vascular endothelial growth factor is associated with angiogenesis in colorectal cancer . Am. J. Hematol. 2002 , 69 , 247 – 254 .
- et al. . Tissue factor expression in human colorectal carcinoma: correlation with hepatic metastasis and impact on prognosis . Cancer 2000 , 88 , 295 – 301 .
- et al. . Tissue factor expression and metastatic potential of colorectal cancer . Thromb. Haemost. 1998 , 80 , 894 – 898 .
- Versteeg , H.H. ; Spek , C.A. ; Peppelenbosch , M.P. ; Richel , D.J. Tissue factor and cancer metastasis: the role of intracellular and extracellular signaling pathways . Mol. Med. 2004 , 10 , 6 – 11 .
- et al.. Commutators of PAR-1 signaling in cancer cell invasion reveal an essential role of the Rho–Rho kinase axis and tumor microenvironment. Oncogene 2005, 24, 8240–8251.
- Pepe , M. The Statistical Evaluation of Medical Tests for Classification and Prediction . In Oxford Statistical Science Series 31 ; New York : Oxford University Press , 2003 .
- et al. . Ovarian Carcinoma: Clinical Validity by Simultaneous Determination of Fibrin Degradation Products with the DR-70™ Immunoassay and CA-125 . German J. Obstetrics and Gynocology 2006 , 66 , 68 – 75 .
- Li , X. ; Qiao , Z. ; Long , X. ; Wei , J. ; Cheng , Y. Serum concentration of AMDL DR-70 for the diagnosis and prognosis of carcinoma of the tongue . Br. J. Oral. Maxillofac. Surg. 2005 , 43 , 513 – 515 .
- et al. . The new DR-70 immunoassay detects cancer of the gastrointestinal tract: a validation study . Aliment Pharmacol. Ther. 2004 , 20 , 983 – 987 .
- et al. . Clinical performance of the AMDL DR-70 immunoassay kit for cancer detection . J. Immunoassay 1998 , 19 , 63 – 72 .
- Ding , L. ; Ping , S. ; Jingmei , Y. Application of tumor marker of DR-70(R) in the diagnosis of malignant tumors . Chongqing Med. J. 1999 , 28 , 1 – 3 .
- et al. . Sensitivity & Specificity of DR-70 Lung Cancer Immunoassay . Analytical Letters 1999 , 32 , 1351 – 1362 .
- et al. . D-dimer–can it be a marker for malignant gastric lesions? Acta. Oncol. 2004 , 43 , 770 – 771 .
- Rucker , P. ; Antonio , S.M. ; Braden , B. Elevated Fibrinogen-Fibrin Degradation Products (FDP) in Serum of Colorectal Cancer Patients . Analytical Letters 2004 , 37 , 2965 – 2976 .
- Lee , K.-H. ; Cho , D. ; Kim , K.-M. ; Kim , S.-M. ; Lee , D.-J. Meaning of the DR-70™ Immunoassay for Patients with the Malignant Tumor . Immune Network 2006 , 6 , 43 – 51 .
- Xu , G. ; Zhang , Y.L. ; Huang , W. Relationship between plasma D-dimer levels and clinicopathologic parameters in resectable colorectal cancer patients . World J. Gastroenterol. 2004 , 10 , 922 – 923 .
- Oya , M. ; Akiyama , Y. ; Yanagida , T. ; Akao , S. ; Ishikawa , H. Plasma D-dimer level in patients with colorectal cancer: its role as a tumor marker . Surg. Today 1998 , 28 , 373 – 378 .
- et al. . Plasma fibrin D-dimer levels correlate with tumour volume, progression rate and survival in patients with metastatic breast cancer . Br. J. Cancer 2002 , 86 , 389 – 395 .
- et al. . Elevated urokinase-type plasminogen activator receptor expression in a colon cancer cell line is due to a constitutively activated extracellular signal-regulated kinase-1-dependent signaling cascade . Oncogene 1997 , 14 , 2563 – 2573 .
- et al. . Plasminogen/plasmin regulates c-fos and egr-1 expression via the MEK/ERK pathway . Biochem. Biophys. Res. Commun. 2005 , 329 , 237 – 245 .
- Dvorak , H.F. Thrombosis and cancer . Hum. Pathol. 1987 , 18 , 275 – 284 .
- Esumi , N. ; Fan , D. ; Fidler , I.J. Inhibition of murine melanoma experimental metastasis by recombinant desulfatohirudin, a highly specific thrombin inhibitor . Cancer Res. 1991 , 51 , 4549 – 4556 .
- Saksela , O. ; Rifkin , D.B. Cell-associated plasminogen activation: regulation and physiological functions . Annu. Rev. Cell. Biol. 1988 , 4 , 93 – 126 .
- et al. . Tumor-associated urokinase-type plasminogen activator: biological and clinical significance . Biol. Chem. Hoppe Seyler 1992 , 373 , 611 – 622 .
- et al. . Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing . Lab. Invest. 1987 , 57 , 673 – 686 .
- et al. . Angiogenic activity of fibrin degradation products is located in fibrin fragment E . J. Pathol. 1992 , 168 , 47 – 53 .
- et al. . Diverse functions of protease receptor tissue factor in inflammation and metastasis . Immunol. Res. 2000 , 21 , 289 – 292 .
- Desrosiers , R.R. ; Cusson , M.H. ; Turcotte , S. ; Beliveau , R. Farnesyltransferase inhibitor SCH-66336 downregulates secretion of matrix proteinases and inhibits carcinoma cell migration . Int. J. Cancer 2005 , 114 , 702 – 712 .
- et al. . Vascular permeability factor, fibrin, and the pathogenesis of tumor stroma formation . Ann. N. Y. Acad. Sci. 1992 , 667 , 101 – 111 .
- Gasic , G.J. Role of plasma, platelets, and endothelial cells in tumor metastasis . Cancer Metastasis Rev. 1984 , 3 , 99 – 114 .
- Yan , L. ; Kumagai , S.G. ; McGuire , M.H. ; Yee , J.A. Protease activity and invasion of matrigel by the osteosarcoma-derived OSPR cell line. Biochem. Soc. Trans. 1994, 22, 18S.
- et al. . Serum levels of vascular endothelial growth factor dependent on the stage progression of lung cancer . Chest 2000 , 118 , 948 – 951 .
- et al. . Comparison of plasma D-dimer and thrombus precursor protein in patients with operable breast cancer as a potential predictor of lymph node metastasis . Blood Coagul Fibrinolysis 2004 , 15 , 9 – 13 .
- Oya , M. ; Akiyama , Y. ; Okuyama , T. ; Ishikawa , H. High preoperative plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer . Jpn. J. Clin. Oncol. 2001 , 31 , 388 – 394 .
- et al. . Plasma D-dimer levels in operable breast cancer patients correlate with clinical stage and axillary lymph node status . J. Clin. Oncol. 2000 , 18 , 600 – 608 .
- et al. . Plasma D-dimer levels show correlation with number of lymph node metastases in patients with esophageal cancer . J. Am. Coll. Surg. 2006 , 202 , 139 – 145 .
- et al. . Circulating D-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma . Cancer 2004 , 101 , 77 – 82 .
- Unsal , E. ; Atalay , F. ; Atikcan , S. ; Yilmaz , A. Prognostic significance of hemostatic parameters in patients with lung cancer . Respir. Med. 2004 , 98 , 93 – 98 .
- Buccheri , G. ; Torchio , P. ; Ferrigno , D. Plasma levels of D-dimer in lung carcinoma: clinical and prognostic significance . Cancer 2003 , 97 , 3044 – 3052 .
- et al. . HER-2 gene amplification correlates with higher levels of angiogenesis and lower levels of hypoxia in primary breast tumors . Clin. Cancer Res. 2004 , 10 , 4083 – 4088 .
- Pavey , S.J. ; Hawson , G.A. ; Marsh , N.A. Impact of the fibrinolytic enzyme system on prognosis and survival associated with non-small cell lung carcinoma . Blood Coagul Fibrinolysis 2001 , 12 , 51 – 58 .
- et al. . Tissue factor-positive microparticles: cellular origin and association with coagulation activation in patients with colorectal cancer . Thromb Haemost 2007 , 97 , 119 – 123 .