Abstract
We recently found that chemokine-driven peritoneal cell aggregation is the primary mechanism of postoperative adhesion in a mouse model. To investigate this in humans, paired samples of peritoneal lavage fluid were obtained from seven patients immediately after incision (preoperative) and before closure (postoperative), and were assayed for the presence of 27 cytokines and chemokines using multiplex beads assay. As a result, IL-6 and CCL5 showed the most striking increase during operation. Recombinant CCL5 or lavage fluid induced chemotaxis of human peripheral blood mononuclear cells. We propose that CCL5 is possibly involved in the mechanism of postoperative adhesion in humans.
INTRODUCTION
Postoperative adhesions occur in the majority of patients following laparotomy and laparoscopy.[ Citation 1 , Citation 2 ] Treatment of adhesions can be expensive, and in some cases, life-threatening. In spite of the large number of surgical operations performed daily, the mechanism of peritoneal adhesion is not well understood. Previous reports have shown that peritoneal injury is triggered by leakage of plasma proteins, followed by formation of fibrinous deposits and proliferation of fibroblasts.[ Citation 3 ] Rapid and transient influx of neutrophils into the peritoneal cavity also occurs, followed by the accumulation of mononuclear cells, usually macrophages.[ Citation 4 , Citation 5 ] CD4+ T cells and the T cell-derived proinflammatory cytokine interleukin (IL)-17 also play significant roles in peritoneal adhesion.[ Citation 6 ] Active roles of these cells in adhesion have been shown.[ Citation 7 , Citation 8 ] We previously found that peritoneal macrophages (PMF) form a large aggregated mass that packs the serosal side of the perforating ulcer with adhesion of visceral tissues in a colitis model in mice.[ Citation 9 ] Transcripts encoding chemokine C-C motif receptor 8 (CCR8) were upregulated in the aggregating cells, while other chemokine receptors were downregulated compared with naïve PMF. Of note, chemokine C-C motif ligand 1 (CCL1), the ligand for CCR8, was also produced by macrophages and mesothelial cells in response to inflammatory stimuli. The presence of CCL1-induced formation of cell aggregates by PMF and mesothelial cells in vitro, and neutralization of CCL1 prevented formation of peritoneal membranous adhesion in vivo. [ Citation 9 ] Thus, autoactivation of peritoneal macrophages via chemokines was the initial trigger for peritoneal adhesion. These results from the mouse system prompted us to investigate similar chemokine-/cytokine-driven mechanisms of peritoneal adhesion in humans. In this study, we collected peritoneal lavage fluid at the beginning and the end of laparotomy, and quantified the presence of 27 cytokines and chemokines in an effort to identify potential molecules that could be targeted to prevent adhesion formation in humans.
EXPERIMENTAL METHODS
Peritoneal Lavage Fluid and Exudate Collection
This study was performed with approval from the ethics committees of the National Center for Global Health and Medicine and Jichi Medical University. Seven patients (Table ) who had laparotomy for colorectal resection of colorectal cancer were recruited in this study with informed consent. These patients had not had a previous major laparotomy, and none of them suffered from inflammatory bowel disease. Immediately after incision at the beginning of the operation (preoperative) and before closure at the end (postoperative), 1 L of saline was poured into the peritoneal cavity, and as much fluid as possible was aspirated back into a bottle. The recovered fluid volume was approximately 777–934 mL [857 ± 76 mL, mean ± standard deviation (SD)]. After removal of visible fat debris, exudate cells were separated by centrifugation at 600 × g. Supernatant aliquots were maintained at −80°C until use, and were subjected to cytokine assays using the Bio-Plex Suspension Array system (Bio-Rad Japan, Tokyo, Japan). The assay tested for the presence of IL-1β, IL-1 receptor antagonist (IL-1Ra), IL-2, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, basic fibroblast growth factor (FGF basic), granulocyte-colony stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), platelet-derived growth factor (PDGF)-BB, vascular endothelial growth factor (VEGF), eotaxin (CCL11), IP-10 [chemokine C-X-C motif ligand 10 (CXCL10)], MCP-1 (CCL2), MIP1α (CCL3), MIP1β (CCL4), RANTES (CCL5), and IL-8 (CXCL8).
TABLE 1 Patient Demographics
Cell Culture
Preoperative peritoneal exudate cells from five patients (Cases 1–3, 6, and 7) were cultured in a 24-well plate at 5.8 × 106 cells/mL per well, with or without 100 ng/mL lipopolysaccharide (LPS; from Salmonella minnesota, Sigma-Aldrich, St. Louis, MO, USA) for 20 h. Supernatant of duplicated cultures was harvested and subjected to the assay described above.
Chemotaxis Assay
Peripheral blood mononuclear cells (PBMCs) obtained from a healthy volunteer using Ficoll-Paque Plus (GE Healthcare Japan, Tokyo, Japan) were prestained for 30 min at 37°C with 3 µM 2′,7′-bis(2-carboxyethyl)–5-(and-6)-carboxyfluorescein acetomethyl ester (BCECF-AM, Molecular Probes, Eugene, OR, USA) and then suspended at 2 × 106 cells/mL in Hank's balanced saline solution containing 0.5% bovine serum albumin and 20 mM HEPES. Chemotaxis assay was performed using a Chemo Tx-96 Chemotaxis plate (NeuroProbe, Inc., Gaithersburg, MD, USA), as follows. After washing, 65 µL of cell suspension was loaded onto the membrane plate and placed onto a flat-bottomed microtiter plate with 96 wells containing 30 µL of recombinant CCL5 (R & D Systems, Minneapolis, MN, USA) solution in each well or peritoneal lavage fluid diluted with phosphate buffered saline (1:1). The plate was then incubated at 37°C for 120 min, and cells that had undergone migration were collected. These collected cells were lysed with 0.1% triton X-100 and counted using a fluorescence microplate reader (FlexStation, Molecular Device Japan, Tokyo, Japan). Data were shown as the average of five scans. Anti-human CCL5 and anti-human CCL2 antibodies were purchased from R & D Systems.
Statistical Analysis
The results were statistically analyzed by the two-tailed paired t-test using Prism 4 software (GraphPad Software, Inc., La Jolla, CA, USA). When P values were less than 0.05, the results were considered as a significant difference.
RESULTS AND DISCUSSION
We measured the amounts of various cytokines and chemokines in the peritoneal fluid from patients prior to and following a colorectal operation in an effort to identify molecules that may be involved in the formation of adhesions in the gut. Many molecules were drastically increased in the peritoneal lavage fluid after the laparotomy procedure (Table ). The concentration of IL-6 was increased 11-fold, while that of IL-1β, PDGF, and IL-1Ra showed more than a fivefold increase. Furthermore, IFN-γ, IL-13, IL-4, IL-10, and VEGF were increased more than twofold in postoperative samples. When the percent composition of each cytokine in the total sample was evaluated, we observed that FGF basic, GM-CSF, and IL-6 were dominant in the preoperative samples. However, only IL-6 increased to account for more than 50% of the measured cytokines in the postoperative samples (Figure ). For the chemokine response, we observed that CCL5, CCL3, CCL2, CXCL8, and CCL4 were increased after the operation. However, in terms of absolute amount of protein, CCL5, CCL2, CXCL8, and CCL4 were the major secreted chemokines. As for percent composition, increases in CCL5 were the most obvious, while the relative amount of other chemokines did not significantly change, except for CXCL10 (Figure ). One patient, case 5 in Table , who underwent lobectomy of the liver with relatively long operation time was included in our study. Since this case might have different conditions of the peritoneal cavity from other cases, we reviewed individual data. However, this patient did not show a particular secretion pattern of CCL5, IL-6, or other cytokines and chemokines when compared with other cases (Figure ). Since we had found that the CCL1/CCR8 system is critical in the formation of peritoneal adhesions in mice,[ Citation 9 ] we attempted to measure the amount of CCL1 present in the samples, but the concentration in the original peritoneal lavage fluid was less than the sensitivity of the assay. We detected 10 pg/mL CCL1 after removal of albumin, with a 10-fold greater concentration of the fluid in the preoperative sample from one individual, but this patient had severe peritoneal adhesion and was therefore excluded from this study.
FIGURE 1 Relative amount of measured cytokines (A) and chemokines (B) listed in Table . Percentage was calculated by dividing the concentration of cytokines or chemokines (pg/mL) with sum of the concentrations (total concentration) of measured cytokines or chemokines (pg/mL). (C) Data from an individual case for CCL5 and IL-6 are shown. Blank bars, preoperative concentration; solid bars, postoperative concentration. The case number indicates the patient ID in Table (color figure available online).
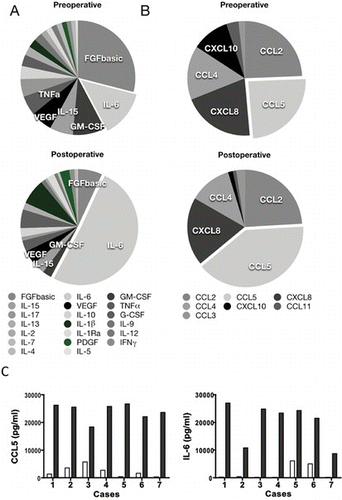
TABLE 2 Concentration of Cytokines and Chemokines (pg/mL) in Peritoneal Lavage Fluid
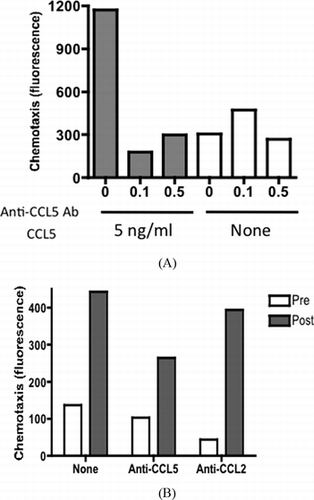
The majority of cells harvested from the peritoneal lavage fluid prior to the operation were CD33+CD14+ macrophage-type cells (data not shown) and lymphocytes as reported previously.[ Citation 10 ] In order to know how these cells respond to inflammatory stress that possibly occurs during colorectal operation, we tested if the treatment of these cells with LPS, a potent activator of macrophage type cells, would enhance the chemokine/cytokine response. As expected, secretion of inflammatory cytokines, which were upregulated during operation including TNF-α and IL-6, were significantly augmented in the presence of LPS (Figure ). In spite of the striking increase in CCL5 in the postoperative lavage samples, the amount secreted by cultured exudate cells was low and was not augmented by LPS. Similar results were obtained for CCL2 (data not shown). These results suggested that peritoneal exudate cells are not the origin of CCL5. Further, to support the inflammatory process that may be mediated by CCL5 in the peritoneal cavity, we tested PBMCs if they have chemotaxis potential in response to CCL5. As shown in Figure , recombinant CCL5 as well as peritoneal fluid was able to induce chemotaxis of PBMCs. Anti-CCL5 antibody but not anti-CCL2 antibody partially inhibited the chemotaxis induced by peritoneal lavage fluid.
FIGURE 2 TNF-α, IL-6, and CCL5 production by peritoneal exudate cells. Peritoneal exudate cells harvested from preoperative lavage fluid were cultured with (LPS) or without LPS (none). Culture supernatant was subjected to the assay for TNF-α (A), IL-6 (B), and CCL5 (C).
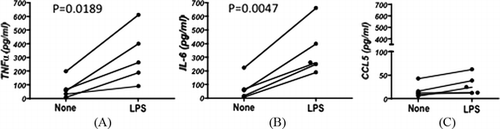
FIGURE 3 CCL5-driven chemotaxis of human PBMCs. (A) Chemotaxis of PBMCs induced by recombinant CCL5 was measured in the presence of indicated concentrations of anti-CCL5 antibody (mg/mL). (B) Pre- (blank) and postoperative (solid) peritoneal fluid from case 1 was tested for the chemotactic potential of human PBMCs in the presence of 0.1 mg/mL of anti-CCL5 antibody or anti-CCL2 antibody. Data are shown as an average of duplicated assay.
This study reports the comprehensive analysis of the secretion of cytokines and chemokines in the peritoneal cavity during laparotomy. Prior studies have investigated individual cytokines in peritoneal fluid after operation,[ Citation 11 ] but the total composition of cytokines present in the peritoneal cavity before and after operation has not been addressed until now. Importantly, assaying both the pre- and postoperative lavage fluid from the same individuals enabled us to quantify the cytokine levels under conditions close to the naïve status of each patient, as well as quantify the net secretion during the operation. Although the stage of the disease in the tested cases varied, the result was quite constant. For example, IL-1β, IL-6, IL-10, IL-12, IL-13, IL-15, and IL-17 were detected in the preoperative fluid. In combination with the high amount of GM-CSF, this array of cytokines may work to maintain the steady-state nature of the peritoneal cavity and its resident cells. After operation, increases in IL-6 were the most striking, though those of IL-13 and IL-15 were also statistically significant. A previous study reported that adhesion formation 48 h after laparoscopy was associated with a high concentration of IL-6.[ Citation 12 ] Though most studies have not addressed IL-13 and IL-15 under these conditions, they are most likely involved in the response to surgical stress in the peritoneal cavity. Since IL-15 is known to activate T cells, which are involved in adhesion formation,[ Citation 13 , Citation 14 ] it is possible that it could trigger the reaction of these cells to induce further immune responses. IL-13 may induce alternative (M2 type) activation of macrophages,[ Citation 15 ] and M2 type macrophages have reduced abilities to stimulate T cell proliferation and Th1 differentiation, with the production of large amounts of IL-10.[ Citation 16 ] Therefore, the balance of IL-15 and IL-13 may be critical for avoiding excess immune reactions. In the mouse, IL-17 is associated with adhesion formation[ Citation 6 ]; however, we found that in humans, the amount of IL-17 either decreased or remained unchanged after the operation. It is possible that IL-17 may not play a role in this process until a later time. The amount of IFN-γ was not very high in the peritoneal fluid prior to the operation, but the fact that it increased by 3.2-fold following laparotomy may support the report that recommends IFN-γ as a target for prevention of adhesion in a mouse model.[ Citation 17 ] Although differences were not statistically significant, elevation of IL-1Ra and IL-10 may be important, as both have antagonizing effects on the inflammatory response.
Large amounts of the chemokine CCL5 was produced during the laparotomy. CCL5 is a chemoattractant for T cells, monocytes/macrophages, eosinophils, basophils, natural killer cells, granulocytes, and dendritic cells,[ Citation 18 ] and therefore induce cascade reactions following immune responses in the peritoneal cavity together with CCL3, CCL4, and CXCL8, eventually leading to peritoneal adhesion. The exudate cells did not produce a significant amount of CCL5 during the overnight culture in the presence of LPS. This result suggests that peritoneal exudate cells may be not the source of CCL5 during operation. Indeed, human peritoneal mesothelial cells are reported to produce CCL5 in response to IL-1β or TNF-α, which results in significant up-regulation of CCL2 and CCL5 protein secretion.[ Citation 19 ] These results indicate it is possible that CCL5 levels present in a sample may be indicative of mesothelial cell injury.
In our previous study using a mouse model, inhibition of macrophage migration and aggregation was an effective way of preventing peritoneal adhesion. In mice, we found approximately 0.5–1.5 ng/mL CCL1 in 2 mL peritoneal lavage fluid in mice with peritoneal adhesion, which was undetectable in our ELISA system in the peritoneal cavities of naïve mice.[ Citation 9 ] This indicated that around 0.09 ng/g body weight (mouse body weight was estimated as 22 g) of CCL1 was secreted in mice when adhesion was induced. In contrast, in humans, only 0.00017 ng/g (body weight was estimated as 60 kg) of CCL1 was detected as mentioned above. Although we failed to detect a significant amount of CCL1 during the operation in humans, which needs further study, we did observe dramatic changes in CCL5 instead. CCL5 was produced at the level of 0.04 ng/g body weight in humans after laparotomy, which was comparable to the level of CCL1 in mice. Thus, we postulate that CCL5 may be a component of a chemokine system unique to the human peritoneal cavity. Importantly, both CCL1 in mice and CCL5 in humans are primarily produced by mesothelial cells. CCL5 induces recruitment of a wide range of cells including monocytes, T cells, eosinophils, mast cells, and basophils to sites of inflammation.[ Citation 18 ] Indeed, we confirmed the chemoattractant activity of peritoneal lavage fluid, which was partially mediated by CCL5. In addition to its chemotactic activity, CCL5 promotes angiogenesis and cancer metastasis,[ Citation 18 ] which may be related to the formation of postoperative adhesion. In addition, increase of CCL5 may promote postoperative cancer cell dissemination in the peritoneal cavity. Since major cytokines in the peritoneal cavity are produced by exudate cells, blocking their migration to mesothelial cells may be an effective way to prevent peritoneal adhesion. However, it is necessary to only block molecules that function specifically in peritoneal exudate and mesothelial cells, without affecting systemic immunity against infection, tissue repair, or coagulation and fibrinolytic systems. As such, inhibition of chemokines that specifically recruit immune cells may be more suitable for inhibiting adhesion than blocking major cytokines and chemokines. Although further investigation is required, we propose that targeting CCL5 may prevent postoperative peritoneal adhesion as well as cancer metastasis.
CONCLUSION
During laparotomy, acute secretion of chemokines and cytokines, which are involved in the recruitment and activation of macrophages, was induced. IL-6 and CCL5 showed the most striking increase. Increase of CCL5 is the major primary response to surgical stress in the peritoneal cavity and is possibly involved in the mechanism of postoperative adhesion in humans.
ACKNOWLEDGMENTS
This work was supported by grants and contracts from the program Grants-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science; Research on Publicly Essential Drugs and Medical Devices from the Japan Health Sciences Foundation and Organization and the Ministry of Health, Labor, and Welfare; and a Grant for International Health Research (21-101) from the Ministry of Health, Labour and Welfare.
Notes
a Data are shown as an average of seven cases with a standard deviation.
*Statistically significant difference (P < 0.05) between pre- and postoperative samples.
REFERENCES
- Ellis , H. ; Moran , B. J. ; Thompson , J. N. ; Parker , M. C. ; Wilson , M. S. ; Menzies , D. ; McGuire , A. ; Lower , A. M. ; Hawthorn , R. J. ; O'Brien , F. ; Buchan , S. , Crowe , A. M. Adhesion-related hospital readmissions after abdominal and pelvic surgery: A retrospective cohort study . Lancet 1999 , 353 , 1476 – 1480 .
- Saed , G. M. ; Kruger , M. ; Diamond , M. P. Expression of transforming growth factor-beta and extracellular matrix by human peritoneal mesothelial cells and by fibroblasts from normal peritoneum and adhesions: Effect of Tisseel . Wound Repair Regen. 2004 , 12 , 557 – 564 .
- Holmdahl , L. Making and covering of surgical footprints . Lancet 1999 , 353 , 1456 – 1457 .
- Ellis , H. ; Harrison , W. ; Hugh , T. B. The healing of peritneum under normal and pathological conditions . Br. J. Surg. 1965 , 52 , 471 – 476 .
- Haney , A. F. Endometriosis, macrophages, and adhesions . Prog. Clin. Biol. Res. 1993 , 381 , 19 – 44 .
- Chung , D. R. ; Chitnis , T. ; Panzo , R. J. ; Kasper , D. L. ; Sayegh , M. H. ; Tzianabos , A. O. CD4 + T cells regulate surgical and postinfectious adhesion formation . J. Exp. Med. 2002 , 195 , 1471 – 1478 .
- Rodgers , K. E. ; di Zerega , G. S. Function of peritoneal exudate cells after abdominal surgery . J. Invest. Surg. 1993 , 6 , 9 – 23 .
- Ar'Rajab , A. ; Mileski , W. ; Sentementes , J. T. ; Sikes , P. ; Harris , R. B. ; Dawidson , I. J. The role of neutrophils in peritoneal adhesion formation . J. Surg. Res. 1996 , 61 , 143 – 146 .
- Hoshino , A. ; Kawamura , Y. I. ; Yasuhara , M. ; Toyama-Sorimachi , N. ; Yamamoto , K. ; Matsukawa , A. ; Lira , S. A. ; Dohi , T. Inhibition of CCL1-CCR8 interaction prevents aggregation of macrophages and development of peritoneal adhesions . J. Immunol. 2007 , 178 , 5296 – 5304 .
- Kubicka , U. ; Olszewski , W. L. ; Tarnowski , W. ; Bielecki , K. ; Zikowska , A. ; Wierzbicki , Z. Normal human immune peritoneal cells: Subpopulations and functional characteristics. Scand. J. Immunol. 1996, 44, 157–163.
- Cahill , R. A. ; Redmond , H. P. Cytokine orchestration in post-operative peritoneal adhesion formation . World J. Gastroenterol. 2008 , 14 , 4861 – 4866 .
- Cheong , Y. C. ; Laird , S. M. ; Shelton , J. B. ; Ledger , W. L. ; Li , T. C. ; Cooke , I. D. The correlation of adhesions and peritoneal fluid cytokine concentrations: A pilot study . Hum. Reprod. 2002 , 17 , 1039 – 1045 .
- Gutt , C. N. ; Hollander , D. ; Brier , C. H. ; Kim , Z. G. ; Lorenz , M. Influence of laparoscopy and laparotomy on systemic and peritoneal T lymphocytes in a rat model . Int. J. Colorectal Dis. 2001 , 16 , 216 – 220 .
- Tzianabos , A. O. ; Holsti , M. A. ; Zheng , X. X. ; Stucchi , A. F. ; Kuchroo , V. K. ; Strom , T. B. ; Glimcher , L. H. ; Cruikshank , W. W. Functional Th1 cells are required for surgical adhesion formation in a murine model . J. Immunol. 2008 , 180 , 6970 – 6976 .
- Mantovani , A. ; Sica , A. ; Locati , M. Macrophage polarization comes of age . Immunity 2005 , 23 , 344 – 346 .
- Xu , W. ; Schlagwein , N. ; Roos , A. ; van den Berg , T. K. ; Daha , M. R. ; van Kooten , C. Human peritoneal macrophages show functional characteristics of M-CSF-driven anti-inflammatory type 2 macrophages . Eur. J. Immunol. 2007 , 37 , 1594 – 1599 .
- Kosaka , H. ; Yoshimoto , T. ; Fujimoto , J. ; Nakanishi , K. Interferon-gamma is a therapeutic target molecule for prevention of postoperative adhesion formation . Nat. Med. 2008 , 14 , 437 – 441 .
- Levy , J. A. The unexpected pleiotropic activities of RANTES . J. Immunol. 2009 , 182 , 3945 – 3946 .
- Robson , R. L. ; McLoughlin , R. M. ; Witowski , J. ; Loetscher , P. ; Wilkinson , T. S. ; Jones , S. A. , Topley , N. Differential regulation of chemokine production in human peritoneal mesothelial cells: IFN-gamma controls neutrophil migration across the mesothelium in vitro and in vivo . J. Immunol. 2001 , 167 , 1028 – 1038 .