ABSTRACT
Very high levels of β-core fragment human chorionic gonadotrophin (βcf-hCG) are reported to potentially cause false negative results in point-of-care (POC)/over-the-counter (OTC) pregnancy tests. To investigate this further, women’s daily early morning urine samples, collected prior to conception and during pregnancy, were analysed for intact, free β-, and βcf-hCG. The proportion of βcf-hCG was found to be related to that of hCG produced and in circulation. Therefore, best practice for accuracy testing of POC/OTC pregnancy tests would be to test devices against clinical samples containing high levels of βcf-hCG as well as standards spiked with biologically relevant ratios.
Introduction
Human chorionic gonadotrophin (hCG) is a hormone produced in early pregnancy, detectable in body fluids soon after embryo implantation. Detection of hCG in urine is the established basis for home pregnancy tests. A variety of hCG isoforms are detectable in urine,[Citation1] including intact hCG, free α and β subunits of hCG, nicked forms, and the β-core fragment of hCG (βcf-hCG; a hCG breakdown product found only in urine).
Published studies have shown that very high levels of βcf-hCG may cause false negative results in point-of-care (POC) and over-the-counter (OTC) pregnancy tests.[Citation2–Citation5] This is due to the “hook effect” in assays for intact or free β-hCG, whereby βcf-hCG saturates β-hCG-specific antibodies, with the paring antibody unable to recognise the bound βcf-hCG, preventing formation of the antibody sandwich.
Gronowski et al. evaluated the susceptibility to βcf-hCG interference of three commercially available POC tests.[Citation1] The addition of 1,000,000 pmol/L of βcf-hCG to a urine sample containing 17,800 IU/L of hCG caused a change from a clear positive to a negative result with one device, a change to a negative/faint positive with a second device, and for a third device the positive result remained unchanged; 500,000 pmol/L of βcf-hCG gave a negative/faint positive result for the first two devices and a positive result for the third device. Nerenz et al. reported false negative results with 2/11 POC tests evaluated with a solution of intact hCG (500 pmol/L) spiked with 500,000 pmol/L of βcf-hCG.[Citation3] This study observed that as the concentration of hCG increases, the amount of βcf-hCG required to inhibit the positive signal must also increase. Another study by Nerenz et al. found that two OTC tests analysed showed a dose-dependent inhibition of the positive signal when a 500 pmol/L intact hCG sample was spiked with 500,000 and 1,000,000 pmol/L of βcf-hCG.[Citation4] However, these OTC devices still generated a clearly positive result with 1,000,000 pmol/L of βcf-hCG and were less susceptible to βcf-hCG interference than previously tested POC devices.
Collectively, these studies call for further testing and/or modification of POC/OTC pregnancy tests to ensure they are not susceptible to the excess βcf-hCG hook effect,[Citation6] but it is important that the testing conducted is representative of true clinical scenarios that the assays might encounter. The studies discussed above have clearly demonstrated the hook effect can lead to false negative results and this effect has also been observed in clinical samples. Therefore, it is extremely important that manufacturers validate their assays for potential βcf-hCG interference. Indeed, the most recent pregnancy tests cleared by the US Food and Drug Administration (FDA) have included validation data regarding βcf-hCG interference. However, there is no consensus on what methodology should be used for validation. Spiking samples that contain low levels of intact hCG with high levels of βcf-hCG, which is not observed clinically and thus not physiologically relevant, does not provide relevant information;[Citation7] testing should mimic the extreme clinical scenarios capable of producing false negatives, but should not extend to non-clinically relevant conditions.
In order to determine appropriate methodology for ensuring the accuracy of POC/OTC pregnancy tests in the presence of βcf-hCG, the clinical ratio of intact hCG to βcf-hCG found in the urine of pregnant women should be considered. Reported here are investigations undertaken to determine the ratio of intact hCG to βcf-hCG in early pregnancy urine samples, together with recommendations on how POC/OTC pregnancy test devices should be evaluated for accuracy in the presence of physiologically relevant βcf-hCG levels. The ratio of free β-hCG levels to intact hCG is also reported.
Experimental
As part of a previous study, 11 early morning urine samples (days 16–46 post-ovulation) were taken from 37 pregnant women, who had been collecting daily urine samples. Samples were also collected from women pre-conception to enable determination of the day of ovulation for each woman by identification of the luteinising hormone (LH) surge (AutoDELFIA quantitative LH assay; ovulation presumed as LH surge +1 day). This enabled accurate assignment of pregnancy duration for each volunteer. Intact, free β- and βcf-hCG were measured using AutoDELFIA immunoassays, using in-house reagents for the βcf-hCG assay and the Perkin Elmer assays for intact hCG and free β. Median levels and centile ranges by day of pregnancy were derived. A further seven urine samples were taken from each of 30 women at weekly intervals of 6–12 weeks from ovulation. These samples were used to determine the ratio of intact hCG:βcf-hCG.
Results
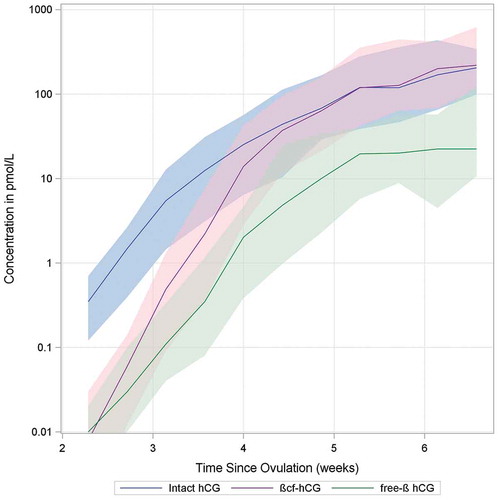
In urine samples from this population of pregnant women, βcf-hCG levels were lower than intact hCG levels in early pregnancy (median ratio βcf:intact hCG=0.64 for weeks 2–6 post-ovulation). However, after approximately week 5/6 of pregnancy (post-ovulation, determined by LH surge +1 day), βcf-hCG levels began to increase relative to hCG levels, and βcf-hCG became the more dominant urinary form (median ratio βcf:intact hCG = 2.84 for weeks 6–12 post-ovulation). Free β-hCG levels tracked those of intact hCG, albeit at a lower concentration for weeks 2–6 of pregnancy ().
Figure 1. Change in median urinary levels of intact hCG, free β-hCG, and β-core hCG over time, 2–6 weeks from ovulation (shaded areas within 10th and 90th percentiles).
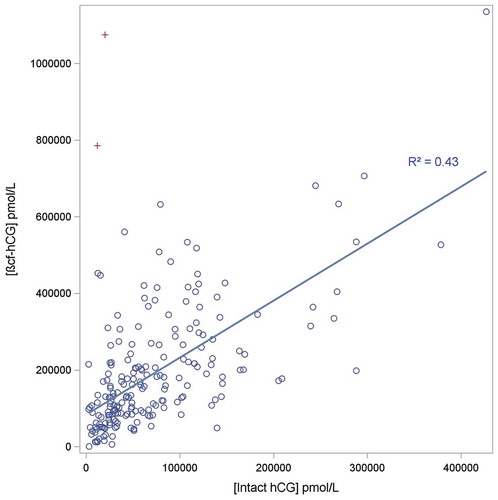
The ratio of βcf-hCG:intact hCG was calculated. The urine sample with the highest amount of βcf-hCG relative to hCG, with significantly large levels of βcf-hCG (>500,000 pmol/mL), was from a woman at 9 weeks’ post-conception and contained 11,693 pmol/mL intact hCG and 785,924 pmol/mL βcf-hCG (ratio 67.2). is a scatterplot of hCG levels relative to βcf-hCG in these urine samples 6–12 weeks’ post-ovulation. It demonstrates the positive correlation between βcf-hCG levels and intact hCG levels, but the R-squared value from the regression analysis was 0.72, indicating some variation is evident. However, examination of the residuals indicates that the relationship between these two forms is not linear as there is a bias to increasing βcf-hCG levels at higher intact hCG concentrations. This is consistent with the earlier finding that the ratio of βcf-hCG to intact hCG increases with gestational age.
Figure 2. Scatterplot of urinary levels of hCG relative to βcf-hCG levels in pregnant women between 6–12 weeks post ovulation. Regression line is shown (intercept set at 0), with points labelled as (+) not included in the regression analysis (the βcf-hCG levels for these two observations were outside the 99.9% prediction interval).
Discussion
These investigations demonstrate a positive correlation between βcf-hCG levels and intact hCG levels, which is expected given that βcf-hCG is a breakdown product of hCG produced in the kidneys and excreted in urine. The same is true for the comparison of free β-hCG vs. intact hCG. Previous studies have also reported that intact hCG urinary levels are higher than those of βcf-hCG in early pregnancy and that this situation reverses after ~5 weeks of pregnancy.[Citation1,Citation8] Data from a complete pregnancy found that from week 5 to delivery, the concentration of βcf-hCG is approximately 10-fold that of hCG.[Citation1] Data obtained from urine samples of 25 pregnant women across all three trimesters found that the pattern of intact hCG and βcf-hCG was highly variable between women.[Citation8] However, a correlation between intact hCG and βcf-hCG was observed, with a similar R-squared value for the regression analysis as that reported in this study (0.70 and 0.72, respectively). Although the results of this study are not directly comparable to the one reported here, as the samples used were collected from routine clinic visits, therefore, they were not necessarily first morning urine and were not collected daily for each woman, as in this study. In addition, dating of pregnancy relied on clinical records, and is thus less accurate than in this study (particularly in early pregnancy prior to dating scans), where gestational age was based on the day of ovulation (as determined by detection of the LH surge).
Device susceptibility to false negatives is more likely to be determined by the absolute concentration of all immune-reactive hCG species present in the sample rather than the ratio of intact hCG to individual interfering hCG variants. Therefore, when testing POC and OTC pregnancy tests for βcf-hCG hook effect, the βcf-hCG:intact hCG ratio should include samples with a considerable excess of βcf-hCG and be conducted on samples/standards with a high level of intact hCG. The findings here provide a basis for determining clinically relevant excess levels, i.e., ≤ 67-fold excess seen in this study. Previous studies have used ratios of βcf-hCG:hCG ≤1000-fold and unrealistic βcf-hCG concentrations for normal pregnancy; thus, their results should be interpreted with caution. Additionally, it is clear from these data that there is individual heterogeneity in isoform distribution within urine samples, thus validation on clinical samples with high levels of βcf-hCG is also important.
Based on the findings reported here, we would recommend the following methodology for testing of POC/OTC pregnancy test devices to ensure their accuracy in the presence of βcf-hCG. Pooled pregnant urine samples collected late in the first trimester, which already naturally contain high hCG and βcf-hCG levels, should be spiked to contain final concentrations of 0.5 μmol/L and 1 μmol/L of βcf-hCG. The existing concentration of βcf-hCG in the pooled sample should be determined using a quantitative measurement assay, and then the βcf-hCG standards should be produced by adding βcf-hCG up to the required concentrations of 0.5 µmol/L and 1 µmol/L (final βcf-hCG concentrations should be confirmed by a quantitative measurement assay). These pooled urine standards should then be tested using the device being investigated in three batches (≥10 devices in each), with the order of testing randomized and performed blinded. Devices should also be tested with a positive and negative urine sample, consisting of pooled pregnant urine from 9–12 weeks’ post-ovulation and pooled urine from non-pregnant women. Additionally, batches of devices should be tested with banked clinical samples collected from pregnant women late in the first trimester, which have been tested as having levels of βcf-hCG >90% centile. For testing of pregnancy test device accuracy, urinary samples with low levels of intact hCG should not be spiked with high levels of βcf-hCG, as this is physiologically inaccurate. Although the FDA has realized the potential for βcf-hCG to cause false negatives, the requirement to validate for potential interference has not been adopted by regulators in other countries. Therefore, it is important that manufacturers validate their tests beyond the regulations of these countries to ensure pregnancy tests remain equally effective for use in women in the late first trimester.
Conclusions
The proportion of βcf-hCG is related to that of hCG produced and in circulation. Accuracy testing of POC/OTC pregnancy tests should, therefore, be conducted with biologically relevant ratios of these hCG forms. Urinary samples with low levels of hCG should not be spiked with high levels of βcf-hCG, as this is unrepresentative, of clinical application. Due to the complexity in a variety of hCG isoforms present at the end of the first trimester, best practice would be to test devices against clinical samples containing high levels of βcf-hCG as well as validating using appropriate spiked standards.
Conflict of interest
This study was conducted by SPD Development Co. Ltd., the manufacturer of Clearblue home pregnancy and fertility tests. S. Johnson, S. Eapen, P. Smith and G. Warren are employees of SPD Development Co., Ltd. (Bedford UK), who funded this study. M. Zinaman has received consultancy from SPD Development Co., Ltd., not relating to this study.
Acknowledgments
Dr. Debra Scates, supported by integrated medhealth communication (imc), provided medical writing assistance in the development of this manuscript, supported by SPD Development Co. This study was funded and conducted by SPD Development Co. Ltd., the manufacturer of Clearblue home pregnancy and fertility tests. Data from this manuscript have been presented at the American College of Obstetricians and Gynecologists (ACOG) meeting, May 2–6, 2015, held in San Francisco and the Royal College of Obstetricians and Gynaecologists (RCOG) meeting, June 20‒22, 2016, held in Birmingham, UK.
Funding
This study was conducted by SPD Development Co. Ltd., the manufacturer of Clearblue home pregnancy and fertility tests.
Additional information
Funding
References
- Stenman, U.H.; Tiitinen, A.; Alfthan, H.; Valmu, L. The Classification, Functions and Clinical Use of Different Isoforms of HCG. Hum. Reprod. Update. 2006, 12(6), 769–784.
- Gronowski, A.M.; CervinskI, M.; Stenman, U.H.; Woodworth, A.; Ashby, L.; Scott, M.G. False-negative Results in Point-of-Care Qualitative Human Chorionic Gonadotropin (hCG) Devices due to Excess hCGβ Core Fragment. Clin. Chem. 2009, 55(7), 1389‒1394.
- Nerenz, R.D.; Song, H.; Gronowski, A.M. Screening Method to Evaluate Point-of-care Human Chorionic Gonadotropin (hCG) Devices for Susceptibility to the Hook Effect by hcg β core Fragment: Evaluation of 11 Devices. Clin. Chem. 2014, 60(4), 667‒674.
- Nerenz, R.D.; Gronowski, A.M. Point-of-care and Over-the-counter Qualitative Human Chorionic Gonadotropin (hCG) Devices Remain Susceptible to False-Negative Results Caused by Excess hCG β core Fragment. Clin. Chem. 2013, 59(11), 1672‒1674.
- Nerenz, R.D.; Butch, A.W.; Ashby, L.; Woldermarian, G.A.; Gronowski, A.M. Evaluation of Semi-quantitative Pregnancy Device for Susceptibility to Interference Caused by hCGβcf. Clin. Biochem. 2015, 48(12), 815–817.
- Nerenz, R.D.; Gronowski, A.M. Qualitative Point-of-care Human Chorionic Gonadotropin Testing: Can We Defuse This Ticking Time Bomb? Clin. Chem. 2015, 61(3), 483–486.
- Ledden, D.J.; Novamo, A.K.; Schulman, L.S. Evaluation of the CLINITEST Human Chorionic Gonadotropin (hCG) Pregnancy Test for Susceptibility to the Hook Effect by the hCG β Core Fragment. Clin. Chem. 2014, 60(12), 1578–1580.
- Nerenz, R.D.; Yarbrough, M.L.; Stenma, U.; Gronowski, A.M. Characterizing Urinary hCGβcf Patterns during Pregnancy. Clin. Biochem. 2016, 49(10–11), 777–781.
