?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Accurate gas samples containing tritiated molecules are essential for the development of tritium monitoring tools and to study tritium-induced reaction dynamics. We prepared gas samples that may contain any of the six hydrogen isotopologues by manometrically mixing high-purity homonuclear isotopologues and forming the remaining isotopologues by chemical equilibration. In order to independently verify the relative isotopologue concentrations to the manometrically derived composition and thus validate the accuracy of the produced gas samples, we measured the effective speed of sound (SoS) in the gas mixtures, which are highly sensitive to small deviations in the relative molar fractions due to the large difference in the individual SoSs. We found that deviations between the manometrically derived and measured SoSs are on a 0.1% level, demonstrating the accuracy of the sample production procedure and the suitability of SoS measurements for inline composition monitoring in tritium applications.
I. INTRODUCTION
The development and characterization of tritium monitoring devices is of great importance in the diverse field of tritium processing, e.g., process control and accountancy. This includes applications like exhaust processing in future fusion plants[Citation1] and the operation of the gaseous tritium source of the neutrino mass experiment KATRIN.[Citation2] For this purpose, highly accurate reference gases containing well-defined admixtures of tritium are essential. At the Tritium Laboratory Karlsruhe, the TRIHYDE facility has been commissioned, capable of fabricating gas samples that may contain any of the six hydrogen isotopologues with subpercent accuracy of the relative concentration.[Citation3]
The TRIHYDE facility produces these samples by precise manometric gas mixing of the homonuclear isotopologues (,
, and
), and consequently, the sample accuracy is limited by the available purity of the initial feed gases. The stable isotopologues are commercially available as reference gases; however, no certified isotopically pure tritium samples are available on a technical scale. In addition, the heteronuclear isotopologues HT and DT must be formed in situ because of their inherent decomposition to the chemical equilibrium ratio.[Citation4] In order to independently verify the accuracy of the TRIHYDE-produced samples, the relative isotopic ratio of individual samples was evaluated using inline composition analyses based on speed of sound (SoS) measurements.
This method is especially sensitive due to the wide range of the isotopologue SoSs (from m/s to
m/s at room temperature) and is also responsive to common impurities like helium, nitrogen, and argon. Furthermore, this technique can be used in line, offers real-time monitoring (
s), and is applicable in a wide pressure regime starting around 100 hPa. It should be noted, that because only a single property is measured, i.e., SoS in a mixture, there is ambiguity in the derived fractions if more than two constituents are present. In this case, a priori knowledge of the constituents is required for a correct composition analysis. Nonetheless, this technique can be used for detecting changes in composition and to cross check expected molar fractions in a gas sample.[Citation5]
In this paper, we compare the measured to the expected SoS in a gas mixture based on the isotope ratio given by TRIHYDE and evaluate this technique as a “quality flag” for the prepared reference gas samples containing tritiated molecules.
II. EXPERIMENT
II.A. Setup
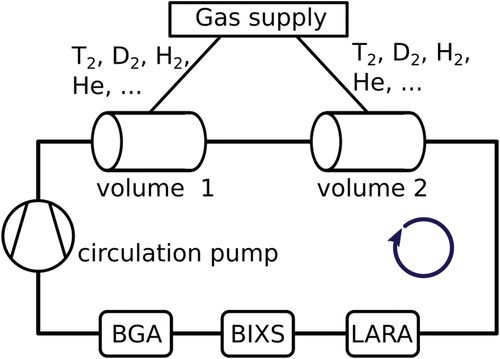
All gas mixtures used in this study were prepared by a manometric mixing principle using two volumes of about 0.8 L. gives a general overview of the mixing loop. Two vessels with accurately known volume (), temperature (
), and pressure (
, MKS Baratron 627D) were filled with the initial homonuclear isotopologues to the target pressure ratio. The gas sample was subsequently produced by circulating the gas using a metal bellows pump (MB601DC). In addition to the SoS measurements [Stanford Research Instruments binary gas analyzer (BGA)], various integrated analytical tools like the laser Raman systems (LARAs)[Citation6,Citation7] and beta-induced X-ray spectrometry (BIXS)[Citation8] enabled in situ composition monitoring. A detailed overview of the TRIHYDE setup as well as the gas sample production procedure is described in Ref. [Citation3].
Fig. 1. Sketch of the TRIHYDE facility for the production of reference gas samples containing tritiated molecules. For inline gas analysis, the loop includes a BGA, a BIXS, and a LARA.
The SoS measurement was performed using a binary gas analyzer (BGA244) based on resonance frequencies in a temperature-stabilized cavity (40°C). The device was integrated with VCR connections. All wetted materials, mainly electropolished 304 stainless steel, were tritium compatible. The transducers were made from a 25-μm-thick polymid (Kapton) foil, which showed no degradation of performance, i.e., drifts or leaks, after tritium exposure to about 1 g during the measurement campaigns. The stated measurement accuracy is on the order of 0.5 m/s and is mainly defined by the pressure sensor (Honeywell STP0030) and temperature sensor (BGA244 internal). The SoS was measured with a 4.4-Hz sampling rate, and the measured velocity was internally normalized to normal temperature and pressure (NTP) conditions (20°C and 1013.25 hPa).
II.B. Procedure
For all mixtures, the initial fill gases were in ortho-para-equilibrium and filled to a combined pressure of both mixing volumes to 500 hPa at room temperature. Prior to circulating the gas volumes contained in the mixing vessels, the remaining parts of the mixing loop were evacuated to hPa. For all mixtures, the initial gas purities were measured using mass spectrometry and Raman spectroscopy. While circulating in the loop, the constituents homogenized, and due to the beta-enhanced self-equilibration process, the heteronuclear isotopologues (HT, DT, and HD) started to form. This first-order process continued until the molar fractions in chemical equilibrium of the respective isotopologues were formed. See Refs. [Citation3,Citation4] for more details on the equilibration process.
For calculating the final molar fractions, the initial fill pressures of the mixing vessels, the measured initial gas purities, and the chemical equilibrium constant from Ref. [Citation9] were used. The SoS was measured after chemical equilibrium was reached and no composition change was detectable (SoS variation %/h). In both campaigns, i.e., the preparation of the hydrogen-tritium and deuterium-tritium mixtures, the mixtures with equimolar factions (50:50), for which the molar faction uncertainty is highest, were performed twice to test for repeatability.
III. RESULTS
The SoS for a single isotopologue can be derived from the first law of thermodynamics assuming adiabatic interaction between individual molecules shown in EquationEq. (1(1)
(1) ), using the adiabatic index
, the universal gas constant
, the absolute temperature
, and the molecular weight
[Citation10]:
This was done for all six hydrogen isotopologues using the measured temperature during the preparation procedure.
For a gas mixture of components , the total molar mass
is divided into a sum of the molar mass fractions of each constituent, i.e.,
, where
represents the molar fraction as a number between zero and one, and
is the molar mass of each isotopologue. In addition, the adiabatic index
for each constituent needs to be considered in a similar way using
. However, assuming an ideal gas, the adiabatic coefficients for the hydrogen isotopologues are identical, and one may use a single
-value. In order to obtain the effective SoS of such a mixture,[Citation11] one therefore needs to apply EquationEq. (2)
(2)
(2) :
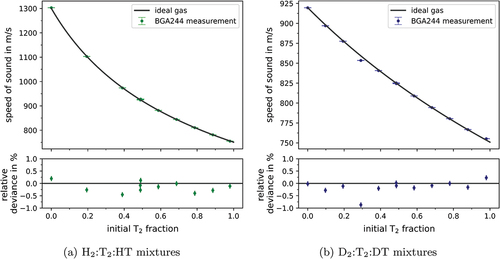
In , the ideal gas values based on EquationEq. (2)(2)
(2) are compared to the measured velocities. For both campaigns, the measured values agree very well with the expected velocities, with deviations on the order of 0.1% over the whole concentration range. In conjunction with the LARA measurements presented in Ref. [Citation3], this agreement independently shows the high accuracy, i.e., precision and trueness, of the produced reference samples. The observed minor deviations can have multiple causes: (1) uncertainties in the initial gas purity, (2) drift in the pressure transducers, and (3) operator error during the sample preparation. The latter is the most plausible cause for the biggest deviation in of around 30% initial
fraction, where likely some part of the loop volume was not sufficiently evacuated. As a consequence, the “tainted” sample was disregarded and not used in further analyses.
Due to the location of the BGA on the feed side of the circulation pump, it was not possible to perform measurements with the circulation pump running. Although the BGA244 specifications state an operating flow rate from 0 up to 5000 standard cubic centimeters, it is likely that, due to the proximity, pump-associated pressure fluctuations interfered with the resonance measurements inside the cavity. As a consequence, only static measurements have been performed, but the placement of the BGA will be improved in the future.
In addition, one can use the measured SoS of a mixture to derive the individual SoS in a single isotopologue. This measurement is straightforward for a single homonuclear isotopologue; however, the tritiated heteronuclear isotopologues HT and DT cannot be individually isolated due to the immediately commencing decomposition into the respective chemical equilibrium components. As a consequence, to our knowledge, no experimental data have been published yet, although theoretical efforts are ongoing.[Citation14] Using EquationEq. (2)(2)
(2) with the molar fractions given by the sample preparation and leaving the individual SoS as a free parameter in a multivariant fit, we obtain the results stated in . In general, the derived experimental velocities agree with the theoretical values for an ideal gas. The large uncertainties, based on a bootstrap estimate, as described in Ref. [Citation15], are dominated by the small number of data points for the multivariant fit. However, this analysis should be seen as a proof of principle and will improve with additional measurements.
TABLE I Derived SoS for Pure Tritiated Isotopologues at NTP Conditions*
IV. CONCLUSION AND OUTLOOK
It was shown that SoS measurements are an easy-to-implement and accurate quality flag to validate reference gas samples containing tritiated molecules. The agreement between manometrically derived and measured SoSs of the gas mixtures also highlights the accuracy and repeatability achievable with the TRIHYDE facility. The production of well-defined tritiated gas samples in chemical equilibrium also enabled first SoS measurements of tritiated isotopologues, which are in agreement with the theoretical data, but the associated uncertainty is currently limited by statistics. In general, the presented methodology can be expanded to include other gas species of interest, e.g., helium and argon, as well as the ortho-para ratio.[Citation16] Therefore SoS measurement is a promising tool to cover a wide range of tritium-containing mixtures present in typical tritium processing applications.[Citation17–19]
Acknowledgments
We acknowledge the support of the Helmholtz Association, the German Ministry for Education and Research BMBF (05A17VK2), and the Deutsche Forschungsgemeinschaft DFG (Graduate School GSC 1085 - KSETA) in Germany.
Disclosure Statement
No potential conflict of interest was reported by the authors.
References
- P. FICHET et al., “Review of the Different Techniques to Analyse Tritium,” D2.1, TRANSAT Research and Innovation (RIA) (2018).
- The KATRIN COLLABORATION and M. AKER et al., “The Design, Construction, and Commissioning of the KATRIN Experiment,” J. Instrum., 16, 8, T08015 (2021); https://doi.org/10.1088/1748-0221/16/08/T08015.
- S. NIEMES et al., “Accurate Reference Gas Mixtures Containing Tritiated Molecules: Their Production and Raman-Based Analysis,” Sensors, 21, 18, 6170 (2021); https://doi.org/10.3390/s21186170.
- T. UDA, K. OKUNO, and Y. NARUSE, “Hydrogen Isotope Exchange Reaction Rates in Tritium, Hydrogen and Deuterium Mixed Gases,” Radiochim. Acta, 56, 209 (1992); https://doi.org/10.1016/j.fusengdes.2015.05.056.
- M. DOUBEK et al., “Speed-of-Sound Based Sensors for Environmental Monitoring,” Proc. 2016 IEEE SENSORS, p. 1, Orlando, Florida, October 30–November 03, 2016 (2016); https://doi.org/10.1109/ICSENS.2016.7808873.
- M. SCHLÖSSER et al., “Raman Spectroscopy at the Tritium Laboratory Karlsruhe,” Fusion Sci. Technol., 67, 3, 555 (2015); https://doi.org/10.13182/FST14-T78.
- F. PRIESTER et al., “A New Compact Easy-to-Use Raman System for All Hydrogen Isotopologues,” Sensors, 22, 10, 3952 (2022); https://doi.org/10.3390/s22103952.
- M. RÖLLIG et al., “Development of a Compact Tritium Activity Monitor and First Tritium Measurements,” Fusion Eng. Des., 100, 177 (2015); https://doi.org/10.1016/j.fusengdes.2015.05.056.
- W. M. JONES, “Thermodynamic Functions for Tritium and Tritium Hydride. The Equilibrium of Tritium and Hydrogen with Tritium Hydride. The Dissociation of Tritium and Tritium Hydride,” J. Chem. Phys., 16, 11, 1077 (1948); https://doi.org/10.1063/1.1746727.
- G. HALLEWELL et al., “A Sonar-Based Technique for the Ratiometric Determination of Binary Gas Mixtures,” Nucl. Instrum. Methods Phys. Res., Sect. A, 264, 2–3, 219 (1988); https://doi.org/10.1016/0168-9002(88)90912-6.
- M. SUCHENEK and T. BOROWSKI, “Measuring Sound Speed in Gas Mixtures Using a Photoacoustic Generator,” Int. J. Thermophys., 39, 11 (2018); https://doi.org/10.1007/s10765-017-2335-2.
- P. J. MOHR, D. B. NEWELL, and B. N. TAYLOR, “CODATA Recommended Values of the Fundamental Physical Constants: 2014,” Rev. Mod. Phys., 88, 3, 035009 (2016); https://doi.org/10.1103/RevModPhys.88.035009.
- A. M. H. van der VEEN et al., “Interpretation and Use of Standard Atomic Weights (IUPAC Technical Report),” Pure Appl. Chem., 93, 5, 629 (2021); https://doi.org/10.1515/pac-2017-1002.
- X. MA et al., “Speed of Sound in Hydrogen Isotopes Derived from the Experimental PVT Data and an Improved Quantum Law of Corresponding State,” Sci. Rep., 10, 975 (2020); https://doi.org/10.1038/s41598-020-58011-9.
- M. SCHLÖSSER et al., “Accurate Calibration of the Laser Raman System for the Karlsruhe Tritium Neutrino Experiment,” J. Mol. Struct., 1044, 61 (2013); https://doi.org/10.1016/j.molstruc.2012.11.022.
- S. EISENHUT et al., “Cryostat for the Provision of Liquid Hydrogen with a Variable Ortho-Para Ratio for a Low-Dimensional Cold Neutron Moderator,” EPJ Web Conf., 231, 04001 (2020); https://doi.org/10.1051/epjconf/202023104001.
- M. STURM et al., “Kilogram Scale Throughput Performance of the KATRIN Tritium Handling System,” Fusion Eng. Des., 170, 112507 (2021); https://doi.org/10.1016/j.fusengdes.2021.112507.
- C. C. KLEPPER and JET CONTRIBUTORS et al., “Extending Helium Partial Pressure Measurement Technology to JET DTE2 and ITER,” Rev. Sci. Instrum., 87, 11, 11D442 (2016); https://doi.org/10.1063/1.4963713.
- J. WILSON et al., “The ITER Tokamak Exhaust Processing System Design and Substantiation,” Fusion Sci. Technol., 75, 8, 794 (2019); https://doi.org/10.1080/15361055.2019.1642089.