Abstract
Food allergy and other types of allergies are becoming epidemic in both the developed and developing countries. A large amount of information is available in literature that (xeno)estrogens can regulate the immune response in general, and the development of allergy in particular; however, the effect of (xeno)estrogens on food allergy is basically unknown. With increasing use of xenobiotics worldwide, chemicals with estrogenic activity have been accumulating in our environment. This review has summarized the current literature relating to the topic (xeno)estrogen regulation of food allergy. The effect of (xeno)estrogens on enterocytes, proteases for protein hydrolysis, dendritic cells and T-regulatory cells in the gastrointestinal tract has been discussed. Finally, considering the current confusion in literature regarding the effect of phytoestrogen genistein on the immune system, a brief discussion has been included for its effect on TH1-TH2 polarization, and possibly food allergy in its relation to windows of exposure. Sufficient evidences exist to support the notion that (xeno)estrogens can regulate food allergy, with the developmental periods more sensitive. Further clinical and animal studies are needed to determine the causal relationship between the exposure of (xeno)estrogens and incidence of food allergy, and the underlying mechanisms.
INTRODUCTION
Food allergy is becoming a major public health problem that affects 3–4% of adults and 6–8% of children in the United States, and is increasing in prevalence (CitationGizzarelli et al., 2006). This is demonstrated by the increase in emergency room visits due to food allergy, which have increased by a factor of 6 over a decade, as well as an increased incidence of anaphylaxis caused by food allergy (Citationde Blok et al., 2007). Food allergy often appears in early stages of the allergic reactions, and is a significant predictor for the development of asthma (CitationBener et al., 2005); however, asthma alone as a manifestation of a food allergy is rare and atypical (CitationOzol and Mete, 2008). The most common food allergies in adults are the following foods or food groups: cow's milk, eggs, fish, wheat, peanuts, soybean, crustacea (shrimp, crab and lobster), tree nuts (almond, hazel and walnut) and sesame seeds, and in children, the most common food allergens are present in milk, eggs, and peanuts (CitationLied, 2007).
Food allergies have several distinct clinical and immunologic entities (CitationVeres et al., 2003). These entities can be classified into those that are IgE mediated and those that are non-IgE mediated, a cell-mediated immunologic reaction involving both immune complex formation and complement deposition. IgE-mediated reactions are typically rapid in onset, mostly involve the skin, and may lead to anaphylaxis; whereas non-IgE-mediated disorders become evident hours to days after allergen ingestion, and mostly manifest in the gastrointestinal (GI) tract. IgE-mediated GI disorders include GI anaphylaxis and oral allergy syndrome. Non-IgE-mediated GI allergic disorders include food protein-induced enterocolitis, proctocolitis and enteropathy. Some GI diseases, such as eosinophilic esophagitis and gastroenteritis, involve both IgE and non-IgE mediated mechanisms (CitationChehade, 2007). In either case, various inflammatory cells and their mediators play a role in their immunopathogenesis. Activation of lymphocytes and the recruitment of eosinophils and mast cells are the key events of many of these diseases (CitationTakayama et al., 2007).
(Xeno)estrogen, Immune System and Allergy
Immune responses are sexually dimorphic in both the type and magnitude (CitationLamason et al., 2006). During their reproductive years, females have more vigorous cellular and humoral immune responses than males as evidenced by higher immunoglobulin concentrations and a greater ability to reject tumors and homografts. Estrogen appears to play a central role in mediating these effects. Estrogen is known to have biphasic effects: while estrogen at the levels of the menstrual cycle is stimulatory, it suppresses cell-mediated immune responses at higher concentrations, e.g., during pregnancy in which the levels of estrogen are 100 to 1000 times that of cycling females (CitationKovacs et al., 2002; CitationOhshima et al., 2007). There has been a significant increase in the prevalence of allergic diseases, including food allergies, over the past few decades. Furthermore, there are differences between men and women in the incidence of allergic diseases. Estrogen has been suggested to be involved in progression of diseases such as asthma, allergic rhinitis and dermatitis (CitationInadera, 2006).
During childhood more boys than girls are affected by asthma (CitationWjst and Dold, 1997) and atopic dermatitis (CitationBöhme et al., 2001), while this ratio is reversed at puberty when estrogens play a more important role in girls. Many epidemiological studies suggest that women are at increased risks of developing adult-onset asthma and also suffer from more severe asthma than men (CitationMelgert et al., 2007). With the onset of puberty the frequency of hospital admission for asthma is higher among females. A significant positive association between treatment with sex hormones and adult onset asthma in an epidemiological study of more than 100,000 nurses in the USA has also been reported (CitationTroisi et al., 1995).
Prenatal events appear to be crucial in programming the infant immune system. Allergic T-lymphocytes are typically primed in utero and subsequently reshaped during postnatal allergen exposure via immune deviation, leading to the eventual emergence of stable allergen-specific memory T-lymphocytes that are polarized toward either the TH1 (normal) or TH2 (atopic) phenotype (CitationHolt et al., 1999; CitationOhshima et al., 2007). Neonatal T-lymphocytes have a unique feature that they tend to develop a prolonged primary TH2 effector function while an impaired TH1 memory effector function (CitationRose et al., 2007), which may account for the presence of a high risk ‘window’ for allergic sensitization in early postnatal life.
Although no direct study has been performed to address the modulatory effect of perinatal estrogen treatment on the development of TH1/TH2 response and allergy in human later life, some implications may be obtained from the study of birth order. In a study involving over 11,000 men, CitationMatricardi et al. (1998) have shown that the prevalence of atopy is much higher among first-born men. The possible underlying mechanism is that there is a higher level of free estrogen in first pregnancy as result of a deficit in sex-steroid binding globulin production, which may predispose the first-born offspring toward the TH2-type immune response (CitationBernstein et al., 1986; CitationMartin, 2000).
An association of maternal use of oral contraceptive pills before birth with a higher risk of atopic diseases (asthma, allergic rhinitis and atopic eczema) in the offspring compared with children of mothers who had never taken hormonal pills has been reported (CitationChalubinski and Kowalski, 2006). Additionally, long-lasting immune modulatory effect of neonatal diethylstil-bestrol (DES, a synthetic estrogenic compound) treatment has been demonstrated in both human and animal studies (CitationKalland and Forsberg, 1978; CitationLuster et al., 1979; CitationWays et al., 1987). In mice, exposure to DES either in utero or in the first 5 days after birth diminished the delayed hypersensitivity responses (CitationKalland and Forsberg, 1978; CitationLuster et al., 1979). However, PHA-induced PBMC proliferation was enhanced in the in utero DES-exposed women (CitationWays et al., 1987). Studies with small number of DES daughters have also suggested functional alterations in natural killer cells and a hyper-responsive immune system. Autoimmune diseases occur more frequently (about a 2-fold increase) in women with prenatal DES exposure compared with controls. Although it is controversial (CitationBaird et al., 1996), asthma, arthritis, lupus and diabetes mellitus were reported more frequently among individuals with DES exposure (CitationVingerhoets et al., 1998).
Estrogen not only occurs as natural estrogens, but also as environmental estrogens (xenoestrogens). Environmental estrogens are present in plastics, detergents, diesel exhaust particles (DEP), pesticides and plants (phytoestrogens), such as DDT, polychlorinated biphenyls (PCBs), 4-nonylphenol, 4-octylphenol, 4-nitrophenol, genistein and many others. The concept of estrogenicity of an exogenous chemical is based on the property of these compounds to bind to, or modulate the function of the estrogen receptors (ERα or ERβ), and to act subsequently as transcription factors when binding directly to DNA through the estrogen response element. Although both ERα and ERβ have been described as nuclear receptors, they are also located in other organelles (e.g., the plasma membrane, cytosol, endoplasmic reticulum and mitochondria), and are able to bind estrogens to trigger rapid actions (CitationRopero, 2006). By interacting with ERs, the environmental estrogens, like endogenous estrogen, are capable of affecting cells of the immune system. In general, pesticide exposure causes a decrease in cell-mediated immunity and yet an increase in humoral immunity (CitationCrinnion, 2000).
These changes result in a decreased host resistance, and an increase in allergic diseases and certain cancers. The incidence of asthma has been found to be significantly associated with exposure to endocrine active environmental compounds such as carbamate insecticides, p, p-DDE and PCBs (CitationSenthilselvan et al., 1992; CitationReichrtova et al., 1999). Exposure to 4-tert-octylphenol, bisphenol A [2,2-(4,4-dihydroxy-diphenol) propane] and nonylphenol has also been reported to enhance interleukin (IL)-4 production in CD4+ T-lymphocytes and IgE production in antigen-primed mice (CitationLee et al., 2003, Citation2004). Allergic contact dermatitis from bisphenol A exposure has been reported widely (CitationMatthieu et al., 2003; CitationConnolly et al., 2006; CitationChu et al., 2006). Benzophenone, p-octylphenol, and tributyltin chloride can promote TH2 polarization (CitationKato et al., 2006), which may result in an increased development of atopic dermatitis-like lesions in DS-Nh mice after tributyltin exposure (CitationOhtaki et al., 2007). Additionally, there is evidence demonstrating that two estrogenic myco-toxins zearalenone and zearalenol can increase the expression of IL-5, a TH2 cytokine, in the EL-4 thymoma cells (CitationMarin et al., 1996).
(Xeno)estrogen and Food Allergy
The mucosal immune system is anatomically and functionally distinct from that found elsewhere in the body, e.g., the systemic immune responses. The mucosal immune system has developed specialized processes for antigen uptake, transport, processing and presentation, the production of distinctive immune effector cells and molecules, and a specific homing mechanism (CitationKraehenbuhl, 1998). For example, the duodenal intraepithelial lymphocytes from infants are difficult to activate in vitro but exhibit markers of activation when compared with peripheral blood lymphocytes (CitationPérez-Machado et al., 2004). GI-associated lymphoid tissue can be divided into loosely organized effector sites, which include the lamina propria (LP) and intraepithelial lymphocytes, and more organized structures, such as mesenteric lymph nodes (MLNs), Peyer's patches (PP), isolated lymphoid follicles, and cryptopatches (CP) (CitationNewberry and Lorenz, 2005). The PPs are secondary lymphoid tissues that are located along the wall of the small intestine, and they serve as the major sites for generation of immunity to intestinal antigens (CitationSato and Iwasaki, 2005).
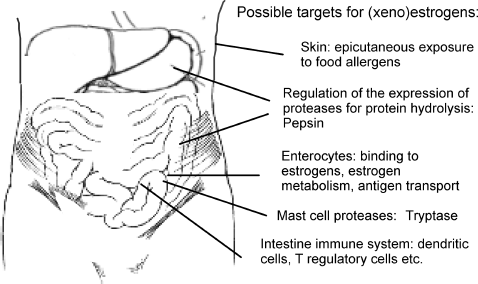
Fed antigens are absorbed from the intestinal lumen either via the villus epithelium and specialized M cells of the intestinal epithelium or the mucosal LP and processed by underlying antigen-presenting cells, and passed to the organized lymphoid tissues of the PP and MLNs to sensitize the naïve T- and B-lymphocytes (CitationGarside et al., 2004). It is believed that under normal circumstances, the gut immune system is relatively hyporesponsive to food antigens and commensal bacteria, and that this state of hyporesponsiveness (tolerance) is maintained by a number of important mechanisms including the integrity of gut epithelium and the presence of tolerogenic dendritic cells (DC) and T-regulatory (Treg) lymphocytes (CitationYang et al., 2007). Oral tolerance is a specific suppression of cellular and humoral immune responses to orally administered antigen upon subsequent immunization with the same antigen to prevent immune reactions to dietary antigens (CitationFinamore et al., 2003). Thus, any perturbation to the homeostasis between gut antigens and the gut immune system may lead to gut inflammation and food allergy ().
Significant actions for female sex steroids and xenoestrogens in the GI tract have been identified in various experimental models and clinical settings (CitationKawano et al., 2004). DES treatment inhibited Trichinella spiralis expulsion and tissue reactions in the small intestine of adult mice by altering the immune responses that mediate expulsion of adult worms from the gut (CitationLuebke et al., 1984). Estrogen-treated mice exhibited higher levels of Candida albicans colonization when compared to control mice; this was most evident in the small intestine and reproductive tract, which may be related to suppressed delayed-type hypersensitivity (DTH) responses (CitationHamad et al., 2002). Early studies in mice have shown that stimulation of the mononuclear phagocyte system by estrogen can prevent the oral ovalbumin (OVA)-induced systemic tolerance (CitationMowat and Parrot, 1983). After exposure of mice to DEP, the airborne particulates reached not only the lung but also the gut. Marked deposits of DEP in the mouse intestinal tissue following exposure suggest that DEP may act as a mucosal adjuvant in the gut and play a role in food allergy (CitationKadkhoda et al., 2004). Indeed, there is evidence that exposure to DEP can block induction of oral tolerance in mice (CitationYoshino et al., 1998, Citation2002; CitationYoshino and Sagai, 1999).
Additionally, benzo(a)pyrene has also been shown to enhance systemic TH1 and TH2 immune responses by acting as a mucosal adjuvant in the gut and may play a role in food allergy induction (CitationKadkhoda et al., 2004). In humans, food allergy prevalence is higher in boys before puberty, while this sex ratio is reversed after puberty (CitationDunnGalvin et al., 2006). The data from a Norwegian national register of severe allergic reactions to food showed a strong dominance of reactions by females (60%) over males (40%); however, this sex difference was not apparent at 18 months of age (CitationLovik et al., 2003, Citation2004).
Nonetheless, there remains much to learn about the relationships between food allergies and (xeno)estrogen exposure. Why there are not clearer gender differences in the prevalence of food allergy if sex hormones and xenoestrogens are indeed playing a big role? The answer to this question is not known; however, it might be related to the time of exposure. The fetus, neonate, and child are often more sensitive than adults to environmental toxicants, especially estrogenic compounds (CitationAldridge et al., 2003). T-Lymphocytes in the cord blood of babies born to atopic mothers respond to aeroallergens as well as food allergens to which the mother was exposed during her pregnancy (CitationBecklake and Kauffmann, 1999).
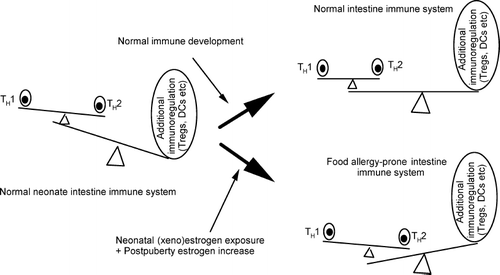
Neonates normally display a TH2 cytokine bias in the gut (CitationScott et al., 2002), and at the same time, it seems that neonates of many species have more—and more potent—Tregs than adults, which may help explain why young animals show greater susceptibility to infections (CitationHartigan-O'Connor et al., 2007; CitationRouse and Sehrawat, 2007). If the neonatal immune system is not able to down-regulate the pre-existing TH2 dominance effectively, an allergic phenotype may develop (CitationCalder et al., 2006). It is possible that exposure to estrogen-like substances during the developmental stages may have contributed to the dramatic increase in the food allergy worldwide by shifting the immune development toward a TH2-biased state with less suppressor cell activity, which can be further exacerbated by estrogen exposure after puberty (). Bisphenol A is recognized as a xenoestrogen due to its binding activity to estrogen receptors, and that it plays either estrogenic or anti-estrogenic roles in vitro. CitationOhshima et al. (2007) have shown that developmental exposure to bisphenol A interrupted the development of oral tolerance to food allergens in mice.
(Xeno)estrogen and Enterocytes
Enterocytes, the columnar epithelial cells in GI, are the major absorptive cells of the intestine, and they are born at the bottom of the crypts, pass through childhood migrating up the walls of the crypts, then settle down briefly to enjoy an absorptive adulthood on the villi. The differentiation of enterocytes is a dynamic process during which reinforcement of cell-cell adhesion favors migration along the crypt-to-villus axis (CitationSchreider et al., 2002). The rapidity of enterocyte migration and maturation may provide a protective mechanism against carcinogenic toxins (CitationLin et al., 1999). The expression of ERs by epithelial cells of each segment of the rat intestine (duodenum, jejunum, ileum, and colon) has been reported (CitationThomas et al., 1993; CitationGejima et al., 2007).
In piglets, CitationChen et al. (2005) have shown that the mRNA expression of both ERα and ERβ can be detected in the small intestine. In addition, the GI mucosa has a high capacity for the metabolism of 17β -estradiol (E2) (CitationMichnovicz and Rosenberg, 1992; CitationRuoff and Dziuk, 1994; CitationEnglish et al., 2000). Estrogen treatment can increase the expression of ERα in abomasum and ERβ in rectum (CitationPfaffl et al., 2003). These observations are important because these ER positive cells are the first to interact with the environmental estrogens uptaken orally. The phytoestrogen genistein (GEN), at the level present in soy infant formula, is bioactive in the small intestine of piglets, and results in reduced enterocyte proliferation and migration (CitationChen et al., 2005); however, it has no effect in adult mice (CitationBooth et al., 1999).
Recent epidemiological studies suggest that combined estrogen and progestogen hormone replacement therapy reduces the incidence of colorectal cancer (CRC) in post-menopausal women, and the protective effect against CRC seems to be through ERs (CitationKonstantinopoulos et al., 2003; CitationWada-Hiraike et al., 2005). ERβ is the predominant ER expressed in colonic tissues. An ERβ -selective agonist has been reported to be an effective treatment in animal models of inflammatory bowel disease. These beneficial effects of estrogen treatment may be due to its modulatory effect on the immune responses (CitationHarnish et al., 2004). However, the effect of estrogen on enterocytes and its contribution to the food allergic response in intestine is not known.
Normally, adult absorptive cells do not absorb macromolecules from the lumen, although neonatal absorptive cells are capable of endocytosing macromolecules from maternal milk, including immunoglobulins. Thus, the intestinal epithelium constitutes an efficient barrier to the penetration of massive amounts of food antigens. Additionally, the intestinal epithelium also regulates the antigenic information transmitted to the mucosal immune system. Transcytosis is a membrane traffic pathway of polarized cells in which ligand is internalized at one cell surface, sorted away from lysosomally directed ligands in the early sorting endosomes, and transported across the cell without entry into lysosomes (CitationBurgess and Stanley, 1994). All proteins are absorbed across mucosal epithelia by transcellular transport and/or through interstitial spaces among the epithelial cells but not at equal levels (CitationMatsuda et al., 2006).
However, the intestinal epithelial barrier function is abnormal in individuals with food allergies (CitationTu et al., 2005). In the first stage of food allergic response, food allergens are transferred intact across the epithelium into the LP mucosa of the digestive tract to initiate the interaction between food antigens and the mucosal immune system (CitationHeyman and Desjeux, 2000; CitationFujita et al., 2007). The transepithelial antigen transport in sensitized rodents can be enhanced by binding of the antigen to IgE-CD23 (Fcε RII) on the apical surface of epithelial cells, which is result from the secondary effect of an abnormal immunological response (CitationDesjeux and Heyman, 1994; CitationBevilacqua et al., 2004; CitationTu and Perdue, 2006).
Ethinylestradiol can increase the transcytosis of asialofetuin and low-density lipoprotein (LDL) through mechanisms that alter the intracellular trafficking of the asialoglyco-protein receptor (CitationBurgess and Stanley, 1994). In addition, estrogen causes intracellular diversion of LDL from the endocytic pathway, which would normally result in degradation in lysosomes, into a transcellular pathway resulting in the discharge of undegraded apo-lipoprotein B into the bile (CitationBurgess and Stanley, 1997). Whether estrogen affects the food allergen transport across the intestinal epithelial barrier similarly is currently unknown.
(Xeno)estrogen and Proteases
The allergenicity of food proteins is dependent on their stability during digestion, e.g., resistance to digestive enzymes. One possibility that (xeno)estrogens affect the antigenicity of food allergens is by affecting the protein hydrolysis. Both partially and extensively hydrolyzed infant formulas have demonstrated a potential in protecting from allergic diseases such as atopic eczema and food allergy (CitationBeyer, 2007; Citationvon Berg, 2007). Reduction in allergenicity and induction of oral tolerance of wheat gliadin by deamidation has been reported (CitationKumagai et al., 2007). Pepsin plays an important role in the digestion of peanut protein allergens, which is inhibited at high pH (CitationKopper et al., 2004). The stomach in infants shows a low amount of secretary pepsin (CitationYoshino et al., 2004). Importantly, gastrin-stimulated secretory rate of gastric H+ and pepsin were markedly inhibited in ovari-ectomized rat after 7 days of estradiol treatment (80 μ g/kg/day) in comparison to ovariectomized untreated rat and sham control (CitationPiyachaturawat et al., 1983). In addition, tributyrin also significantly (20–50%) inhibited both fetal and postnatal aminopeptidase N (ApN) activity in the small intestine and colon (CitationKushak and Winter, 1999).
Tryptase, an abundant mast cell protease that is specifically released in large quantities from activated mast cells, can regulate mast cell activation and control allergic reaction by cleaving IgE to prevent it from binding to both allergens and Fcε Rs (Rauter et al., 2008). The expression of tryptase in duodenal mucosa was decreased in children with food allergy (CitationAugustin et al., 2001). Interestingly, estrogen can negatively regulate the uterine secretion of implantation serine proteinase (ISP) 1 and 2 at the posttranscriptional level. The ISP1 gene may encode the embryo-derived enzyme strypsin, while the ISP2 gene encodes a related tryptase, which is expressed in endometrial glands (CitationO'Sullivan et al., 2004).
(Xeno)estrogen and Dendritic Cells (DC) in GI Tract
Dendritic cells are recognized for their ability to internalize antigens at an extremely low concentration and for their highly efficient presentation of these antigens in the context of the major histocompatibility complex (MHC) Class II molecules to naive T-lymphocytes. The DC are believed to play an essential role in regulating the balance between immunogenic and tolerogenic responses to mucosal antigens by controlling T-lymphocyte differentiation and activation via cytokine co-stimulatory and co-inhibitory signals (CitationNeurath et al., 2002). The intestine and its lymphoid organs contain large number of DC (including a number of unique subsets), and expansion of DC in mice using the cytokine flt3 ligand (flt3L) or enhancement of their survival with the TNFR family member RANK-ligand (up-regulate IL-10 in mucosal DC and IL-12 in splenic DC) markedly increased the induction of oral tolerance in these mice (CitationBilsborough and Viney, 2004; CitationFleeton et al., 2004).
The incoming antigens are sampled by the DC that resides just under the subepithelial dome region. This local sampling of antigens by DC in the PP is critical for the induction of adaptive mucosal immunity (CitationSato and Iwasaki, 2005). In contrast to splenic DC that induces CD4+ T-lymphocyte proliferation, it is possible that intestinal DC induce minimal T-lymphocyte proliferation or even suppress CD4+ T-lymphocyte proliferation. The phenotype of intestinal DC is not fixed, and can be modulated by adjuvants or inflammatory cytokines and, possibly, (xeno)estrogens. There is evidence that neonatal DCs are intrinsically biased against Th-1 immune responses (CitationLangrish et al., 2002). However, other reports suggest that neonatal DCs are fully competent in their innate immune functions (CitationSun et al., 2003), and their MHC class I antigen processing and presentation pathway is also functional (CitationGold et al., 2007).
The endocrine system regulates the maturation of different DC subtypes. The expression of ERα and ERβ by DC at all stages of differentiation has been reported (CitationSalem et al., 2000; CitationSapino et al., 2003; CitationMao et al., 2005). The ER antagonists ICI 182,780 and tamoxifen can block the differentiation of the E2-responsive DC population (CitationPaharkova-Vatchkova et al., 2004). E2 can enhance DC antigen-presenting function in vivo in the absence of inflammation and thus promote autoimmunity (CitationPaharkova-Vatchkova et al., 2004), possibly by enhancing the T-lymphocyte stimulatory capacity of mature DC since estrogen-exposed DC express higher levels of MHC class II and co-stimulatory molecules (CitationPettersson et al., 2004). Other potentiating effects of estrogen include: E2 enhances the secretion of IL-6 in immature DC (iDC); E2 modulates the production of the DC-derived chemokines IL-8, MCP-1, MDC, and TARC; E2 provides a signal essential for migration of mature DC toward CCL19/MIP3b (CitationBengtsson et al., 2004).
The endocytosis is an important property of immature DC. During the process of the maturation, DC appear high stimulatory capacity and low endocytosis, and E2 decreases the endocytosis of DC (CitationYang et al., 2006). On the other hand, estrogen treatment did not alter the function of mouse plasmacytoid DC that were derived from estrogen-resistant myeloid progenitors (CitationHarman et al., 2006). In experimental autoimmune encephalomyelitis, systemic E2 treatment led to decreased numbers of DC migrating into the central nervous system at disease onset, and decreased DC production of tumor necrosis factor (TNF)-α, IL-12, and interferon (IFN)-γ (CitationLiu et al., 2002). Taken together, a consistent pattern of estrogen effects on DCs does not emerge from studies reported so far, possibly due to the heterogeneity. Nonetheless, it suggests that the function of DC, including those residing in the GI, can be affected by estrogen.
(Xeno)estrogen and Tregs in GI Tract
The gut is home to a large number of Tregs, and several types of Tregs have been induced after a repeated feeding of a low dose of antigen. These include TGF-β -producing TH3 cells (CitationPérez-Machado et al., 2003), IL-10-producing Tr1 cells (CitationBattaglia et al., 2004), and CD4+ CD25+ Foxp3+ T-lymphocytes (CitationSun et al., 2007), Natural killer T (NKT) cells (CitationRocha-Campos et al., 2006), CD8+ T suppressor lymphocytes and TCR γ δ T-lymphocytes (CitationMengel et al., 1995; CitationKe et al., 1997). Large amount of evidence suggests for cross talk between these Tregs (CitationChen et al., 2003; CitationChung et al., 2005; CitationBattaglia et al., 2006; CitationLy et al., 2006; CitationDiPaolo et al., 2007; CitationWu et al., 2007). Furthermore, as mentioned above, the immune system of neonates is empowered with a tremendous regulatory potential, which can be developed independently of thymus (CitationDujardin et al., 2004).
A subset of well-characterized Tregs is the CD4+ CD25+ Foxp3+ T-lymphocytes. Based on their origin, two types of CD4+ Tregs have been described, e.g., naturally occurring Tregs (nTregs) that originate and mature in the thymus, and inducible (or adaptive) Tregs (iTregs). The iTregs develop extra-thymically from conventional CD4+ T-lymphocytes that are activated under conditions of impaired co-stimulatory signaling or are induced by suppressive cytokines and drugs (CitationDe Luca et al., 2007). Both experimental animal and human studies have convincingly demonstrated that CD4+ CD25+ Tregs are crucial in the regulation of food allergy (CitationKarlsson et al., 2004; CitationHerberth et al., 2006; CitationTorgerson et al., 2007; Citationvan Wijk et al., 2007; CitationYang et al., 2007).
Oral exposure to protein induces activation of functional CD4+ CD25+ T-lymphocytes in the gut-draining lymph nodes in mouse models (CitationTorgerson et al., 2007). The studies from Citationvan Wijk et al. (2007) have suggested that the induction of oral tolerance to peanut extract (PE) is impaired in CD4+ CD25+ T-lymphocyte-depleted animals; in sensitized mice, the depletion of CD4+ CD25+ T-lymphocytes results in increased allergic responses to PE.
These studies suggest that CD4+ CD25+ T-lymphocytes play an important role in both controlling tolerance induction and regulating the intensity of allergic responses to peanut. Patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome, or X-linked autoimmunity-allergic dysregulation syndrome lack CD4+ CD25+ Tregs because of a Foxp3 mutation, and they often develop food allergic reactions and increase specific IgE antibody levels accompanied by severe eczema (CitationHerberth et al., 2006). Children, who have outgrown their cow's milk allergy and developed tolerance, have a higher percentage of CD4+ CD25+ T-lymphocytes with regulatory function in the peripheral blood than allergic children (CitationKarlsson et al., 2004; CitationTorgerson et al., 2007).
Although some evidences suggest that estrogen treatment in adult animals at levels equal or higher than that found during pregnancy expands the compartment of CD4+ CD25+ Tregs (CitationPolanczyk et al., 2004, Citation2006; CitationTai et al., 2008), the role estrogen at lower levels on CD4+ CD25+ Tregs is not known, and not even a mention of its developmental effect in mucosal tissue. This is important considering the biphasic effect of estrogen on the immune response, as discussed earlier. However, it should be noted that prenatal exposure to bisphenol A has been shown to augment both TH1 and TH2 responses at least partially by decreasing CD4+ CD25+ Tregs in adult mice (CitationOhshima et al., 2007). Similarly, developmental exposure to genistein also decreased CD4+ CD25+ T-lymphocytes in adult female mice (discussed next).
NKT cells are increasingly recognized as important immunoregulatory cells. They are defined as expressing both natural killer 1 (a member of the natural killer cell receptor family, NKR-P1, CD161) and CD3 surface markers (CitationGrose et al., 2007). CD1d represents a restriction element for invariant NKT (iNKT) cells. The role of CD1d in the regulation of intestinal immune responses was first suggested by experiments showing that a mixed lymphocyte reaction between intestinal epithelial cells and CD8+ T-lymphocytes was inhibited by a mAb against CD1.
Large amounts of CD1d positive cells have been found in the LP of patients with cow's milk hypersensitivity both at the time of GI manifestations and during asymptomatic periods when compared to healthy individuals who did not show any CD1d expression in the duodenum. The localization of CD1d positive cells corresponded to areas where B-lymphocytes, plasma cells, and DC were present (CitationUlanova et al., 2000). The iNKT cells are also detected in the duodenum of healthy individuals using α -galactosylceramide loaded CD1d tetramers; however, they are deficient in subjects with coeliac disease (CitationGrose et al., 2007).
iNKT cells can function to skew adaptive immunity toward TH2 responses (CitationMeyer et al., 2007). CD4+ iNKT cells are thought to be the major source of TH2 cytokines (CitationSakuishi et al., 2007); IL-4 and IL-13 secreted by iNKT cells has been highlighted in the pathogenesis of asthma (CitationAkbari et al., 2003; CitationMeyer et al., 2006). However, negative regulation of TH2-responses by the activated NKT has also been reported (CitationIwamura and Nakayama, 2007). Neonatal NKT cells differ from their adult counterparts: they express markers of activation, such as CD25; they are polyclonal; and they do not produce cytokines in response to primary stimulation (CitationD'Andrea et al., 2000). Limited information is currently available for estrogen's effect on iNKT cells. Gourdy et al. (2005) have shown that adult estrogen treatment increased IFNγ production by peripheral iNKT cells. However, the effect of (xeno)estrogen exposure during various period of life on the mucosal NKT cells is unknown.
Other Routes of (Xeno)estrogen Exposure
We have presented above possible targets of (xeno)estrogen in modulating food allergy as a result of oral/dietary exposure to food proteins. However, there is increasing evidence that sensitization to food allergens can be acquired via other routes. It became evident that some children had allergic reactions following their apparent first ingestion of peanuts. IgE-mediated food allergy has co-morbid conditions, such as atopic dermatitis and asthma. Impaired function of the epidermal skin barrier is characteristic of atopic dermatitis, and sensitization to allergens through the skin is a possibility.
A cohort study has shown that the use of peanut-containing creams was more common in children who subsequently became allergic to peanut than in control infants, suggesting that some sensitization to peanut may have occurred through the skin (CitationLack et al., 2003). In animal models, CitationStrid et al. (2005) have shown that epicutaneous exposure to peanut protein prevents oral tolerance induction. In addition, exposure to antigens from the mother's skin is one of the key events that promote maturation of the infant's gut and gut-associated immune systems (CitationCalder et al., 2006). There is likely to be occupational and residential exposure to (xeno)estrogens, and thus, examination of interaction between food allergen and (xeno)estrogens following dermal exposure will not only shed light on the effect of routes of exposure on food allergy induction but also the role of (xeno)estrogens in this complex disease.
Genistein, Windows of Exposure, TH1-TH2 Polarization and Food Allergy
Genistein, a major isoflavone in most soy products, is consumed in supraphysiologic levels as nutritional supplements and pharmaceuticals (CitationLi et al., 2003). GEN has a 2-phenyl-naphthalene-type chemical structure sharing several features in common with that of E2, including a pair of hydroxyl groups separated by a similar distance, and has been demonstrated to interact with estrogen receptors in vivo (CitationMartin et al., 1978). GEN exhibits weak estrogenic activity on the order of 10-2 to 10-3 compared to that of E2 (CitationMiksicek, 1994), but it is present in the body in concentrations much higher than those of endogenous estrogens (CitationAdlercreutz et al., 1993). GEN has higher affinity to ERβ than to ERα. Infants fed soy milk formulas have plasma isoflavone levels that are orders of magnitude higher than those of infants fed human or cows' milk (CitationSetchell et al., 1997). Despite the hypothesized beneficial effects of GEN (e.g., decreased incidences of some hormone-related cancers), there are concerns about the potential long-term effects of this compound on human health, especially that of infants and young children.
A retrospective multiple controlled cohort study has indicated that there was an increase in the use of allergy drugs in young adults (with statistically significant changes in females) who had been fed soy formula during infancy as compared to those who were fed cow milk formula from the age of less than 9 days old when both groups were healthy-term infants whose mothers elected not to breastfeed (CitationStrom et al., 2001).
Furthermore, soy frequently causes food allergy especially in childhood (CitationChristensen et al., 2004). In a study conducted in Sweden, consumption of soy was thought to be responsible for several fatal food anaphylaxis in children and young adults either by itself or by synergizing with peanut (CitationFoucard et al., 1999). Phytoestrogens have also been detected in amniotic fluid (CitationDoerge et al., 2001), suggesting that in utero exposure also occurs. Furthermore, the metabolic and/or excretion rates of GEN are different between mother and fetus, and once GEN is transferred to the fetus, it tends to stay in the fetus longer than in the mother (CitationTodaka et al., 2005).
As reviewed above, estrogen can decrease cell-mediated immunity in mice (CitationHolmdahl and Jansson 1988; CitationSalem et al., 2000) and in women (CitationHoek et al., 1995). It has been suggested that the mechanism of the suppression is through ERs on the target cells. Because of the similarities of GEN to estrogen, and its affinity to ERs, studies have been done to see if GEN has immunosuppressive effects. CitationYellayi et al. (2002) have shown that subcutaneous injections of GEN not only suppressed humoral immunity but also decreased thymic weight and produced thymic atrophy; however, the dose of GEN was high and route of exposure was physiologically irrelevant (CitationCooke et al., 2006). Similar shortcomings existed in studies by CitationVerdrengh et al. (2003), who have shown that subcutaneous injection of GEN-suppressed DTH reactions.
We have investigated the effects of developmental GEN exposure on serum total IgE production, following dermal exposure to the allergen trimellitic anhydride (TMA) in B6C3F1 mice using a physiologically relevant exposure route, e.g., oral exposure. Increased serum total IgE production while decreased IgG2a and IgG2b production was observed in the in utero GEN-exposed adult female B6C3F1 mice (CitationGuo et al., 2005). Furthermore, increased IgE production in these mice was associated with a decrease in the percentage of CD4+ CD25+ T-lymphocytes, and increases in the production of cytokines (IL-2 and IL-4), expression of B7.2 by B-lymphocytes and activity of eosinophil peroxidases. In our study with younger female mice, e.g., at postnatal day (PND) 38, in utero exposure to GEN did not affect the total serum IgE production in response to TMA, suggesting that sexual maturation was necessary to manifest an enhanced IgE production (CitationGuo et al., 2005; data not shown).
The exact mechanism underlying the developmental basis of the adult disease is currently unclear. In contrast, an enhancement in TH1 immune response was observed in our studies with GEN exposure in adult B6C3F1 mice. Our studies have demonstrated that exposure of adult female B6C3F1 mice to GEN by gavage at doses of 2–20 mg/kg increased the activities of cytotoxic T-lymphocytes (CTLs) in both ex vivo (CitationGuo et al., 2001) and in vivo systems (CitationGuo et al., 2007). Moreover, increased activities of CTLs correlated with an increase in the production of IFNγ and activation of signal transducers and activators of transcription (STAT) 1 and STAT4.
These results are confirmed indirectly by CitationKogiso et al. (2006) who showed that OVA-specific TH2-antibody IgG1 production was decreased following GEN exposure in adult mice. The fact that GEN exposure in adults enhances cell-mediated immunity agrees with epidemiological studies that there is a negative correlation between GEN intake and allergic reactions. There are reports that GEN exposure in adult may provide some protection against asthma (CitationSmith et al., 2004) and reduce the incidence of chronic respiratory symptoms (CitationButler et al., 2004; CitationMiyake et al., 2005). Therefore, exposure to GEN may modulate TH1/TH2 balance depending on the window of exposure (in utero versus adult exposure).
CONCLUSION
There are sufficient evidences to make us believe (xeno)estrogens can regulate the development of food allergy. These (xeno)estrogens may exert their effects on the enterocytes, proteases involved in protein digestion and allergy regulation, intestinal DC and Tregs through binding to ERs directly or through interfering the binding of endogenous estrogens to the ERs. Early developmental periods may be particularly sensitive. More research (both clinical and animal studies) using specific immunomodulatory compounds (e.g., GEN) interfering with immunoregulatory mechanisms (e.g., the TH1/TH2 balance) may help us obtain information that we are currently lacking, including gender and sex differences on food allergy.
Supported in part from ES012286 and NIEHS contract NO1-ES-05454.
REFERENCES
- H. Adlercreutz, H. Markkanen, and S. Watanabe. (1993). Plasma concentrations of phyto-oestrogens in Japanese men. Lancet 342:1209–1210.
- O. Akbari, P. Stock, E. Meyer, M. Kronenberg, S. Sidobre, T. Nakayama, M. Taniguchi, M.J. Grusby, R.H. DeKruyff, and D.T. Umetsu. (2003). Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nature Med. 9:582–588.
- J.E. Aldridge, J.A. Gibbons, M.M. Flaherty, M.L. Kreider, J.A. Romano, and E.D. Levin. (2003). Heterogeneity of toxicant response: sources of human variability. Toxicol. Sci. 76:3–20.
- M. Augustin, T.J. Karttunen, and J. Kokkonen. (2001). TIA1 and mast cell tryptase in food allergy of children: increase of intraepithelial lymphocytes expressing TIA1 associates with allergy. J. Pediatr. Gastroenterol. Nutr. 32:11–18.
- D.D. Baird, A.J. Wilcox, and A.L. Herbst. (1996). Self-reported allergy, infection, and autoimmune diseases among men and women exposed in utero to diethylstilbestrol. J. Clin. Epidemiol. 49:263–266.
- M. Battaglia, C. Gianfrani, S. Gregori, and M.G. Roncarolo. (2004). IL-10-producing T-regulatory Type 1 cells and oral tolerance. Ann. N. Y. Acad. Sci. 1029:142–153.
- M. Battaglia, A. Stabilini, E. Draghici, B. Migliavacca, S. Gregori, E. Bonifacio, and M.G. Roncarolo. (2006). Induction of tolerance in Type 1 diabetes via both CD4+ CD25+ T-regulatory cells and T-regulatory Type 1 cells. Diabetes 55:1571–1580.
- M.R. Becklake, and F. Kauffmann. (1999). Gender differences in airway behavior over the human life span. Thorax 54:1119–1138.
- A. Bener, I.A. Janahi, and A. Sabbah. (2005). Genetics and environmental risk factors associated with asthma in schoolchildren. Allerg. Immunol. (Paris) 37:163–168.
- A.K. Bengtsson, E.J. Ryan, D. Giordano, D.M. Magaletti, and E.A. Clark. (2004). 17β -estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood 104:1404–1410.
- L. Bernstein, R.H. Depue, R.K. Ross, H.L. Judd, M.C. Pike, and B.E. Henderson. (1986). Higher maternal levels of free estradiol in first compared to second pregnancy: Early gestational differences. J. Natl. Cancer Inst. 76:1035–1039.
- C. Bevilacqua, G. Montagnac, A. Benmerah, C. Candalh, N. Brousse, N. Cerf-Bensussan, M.H. Perdue, and M. Heyman. (2004). Food allergens are protected from degradation during CD23-mediated transepithelial transport. Int. Arch. Allergy Immunol. 135:108–116.
- K. Beyer. (2007). Hypoallergenicity: A principle for the treatment of food allergy. Nestle Nutr. Workshop Ser. Pediatr. Program 59:37–43.
- J. Bilsborough, and J.L. Viney. (2004). In vivo enhancement of dendritic cell function. Ann. N. Y. Acad. Sci. 1029:83–87.
- M. Böhme, A. Svensson, I. Kull, S.L. Nordvall, and C.F. Wahlgren. (2001). Clinical features of atopic dermatitis at two years of age: A prospective, population-based case-control study. Acta Derm. Venereol. 81:193–197.
- C. Booth, D.F. Hargreaves, J.A. O'Shea, and C.S. Potten. (1999). In vivo administration of genistein has no effect on small intestinal epithelial proliferation and apoptosis, but a modest effect on clonogen survival. Cancer Lett. 144:169–175.
- J.W. Burgess, and K.K. Stanley. (1994). 17α -Ethinylestradiol increases transcytosis of asialoglycoproteins in rat liver. J. Biol. Chem. 269:3482–3488.
- J.W. Burgess, and K.K. Stanley. (1997). Estrogen-stimulated transcytosis of desialylated ligands and α 2 macroglobulin in rat liver. Biochim. Biophys. Acta 1359:48–58.
- L.M. Butler, W.P. Koh, H.P. Lee, M.C. Yu, and S.J. London. (2004). Dietary fiber and reduced cough with phlegm: A cohort study in Singapore. Am. J. Respir. Crit. Care Med. 170:279–287.
- P.C. Calder, S. Krauss-Etschmann, E.C. de Jong, C. Dupont, J.S. Frick, H. Frokiaer, J. Heinrich, H. Garn, S. Koletzko, G. Lack, G. Mattelio, H. Renz, P.T. Sangild, J. Schrezenmeir, T.M. Stulnig, T. Thymann, A.E. Wold, and B. Koletzko. (2006). Early nutrition and immunity-progress and perspectives. Br. J. Nutr. 96:774–790.
- M. Chalubinski, and M.L. Kowalski. (2006). Endocrine disrupters–potential modulators of the immune system and allergic response. Allergy 61:1326–1335.
- M. Chehade. (2007). IgE and non-IgE-mediated food allergy: Treatment in 2007. Curr. Opin. Allergy Clin. Immunol. 7:264–268.
- A.C. Chen, M.A. Berhow, K.A. Tappenden, and S.M. Donovan. (2005). Genistein inhibits intestinal cell proliferation in piglets. Pediatr. Res. 57:192–200.
- W. Chen, W. Jin, N. Hardegen, K.J. Lei, L. Li, N. Marinos, G. McGrady, and S.M. Wahl. (2003). Conversion of peripheral CD4+ CD25-naive T-cells to CD4+ CD25+ regulatory T-cells by TGFβ induction of transcription factor Foxp3. J. Exp. Med. 198:1875–1886.
- H.R. Christensen, S. Brix, and H. Frøkiaer. (2004). Immune response in mice to ingested soya protein: Antibody production, oral tolerance and maternal transfer. Br. J. Nutr. 91:725–732.
- C.Y. Chu, A. Pontén, C.C. Sun, and S.H. Jee. (2006). Concomitant contact allergy to the resins, reactive diluents and hardener of a Bisphenol A/F-based epoxy resin in subway construction workers. Contact Dermatitis 54:131–139.
- Y. Chung, S.H. Lee, D.H. Kim, and C.Y. Kang. (2005). Complementary role of CD4+ CD25+ regulatory T-cells and TGFβ in oral tolerance. J. Leukocyte Biol. 77:906–913.
- M. Connolly, L. Shaw, I. Hutchinson, A.J. Ireland, M.G. Dunnill, and J.E. Sansom. (2006). Allergic contact dermatitis from Bisphenol-A-glycidyldimethacrylate during application of orthodontic fixed appliance. Contact Dermatitis 55:367–368.
- P.S. Cooke, V. Selvaraj, and S. Yellayi. (2006). Genistein, estrogen receptors, and the acquired immune response. J. Nutr. 136:704–708.
- W.J. Crinnion. (2000). Environmental medicine. Part 4: pesticides-biologically persistent and ubiquitous toxins. Altern. Med. Rev. 5:432–447.
- A. D'Andrea, D. Goux, C. De Lalla, Y. Koezuka, D. Montagna, A. Moretta, P. Dellabona, G. Casorati, and S. Abrignani. (2000). Neonatal invariant Vα 24+ NKT lymphocytes are activated memory cells. Eur. J. Immunol. 30:1544–1550.
- B.M. de Blok, B.J. Vlieg-Boerstra, J.N. Oude Elberink, E.J. Duiverman, A. DunnGalvin, J.O. Hourihane, J.R. Cornelisse-Vermaat, L. Frewer, C. Mills, and A.E. Dubois. (2007). A framework for measuring the social impact of food allergy across Europe: A EuroPrevall state of the art paper. Allergy 62:733–737.
- A. De Luca, C. Montagnoli, T. Zelante, P. Bonifazi, S. Bozza, S. Moretti, C. D'Angelo, C. Vacca, L. Boon, F. Bistoni, P. Puccetti, F. Fallarino, and L. Romani. (2007). Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of Rorc. J. Immunol. 179:5999–6008.
- J.F. Desjeux, and M. Heyman. (1994). Milk proteins, cytokines, and intestinal epithelial functions in children. Acta Paediatr. Jpn. 36:592–596.
- R.J. DiPaolo, C. Brinster, T.S. Davidson, J. Andersson, D. Glass, and E.M. Shevach. (2007). Autoantigen-specific TGFβ -induced Foxp3 D'Andrea regulatory T-cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T-cells. J. Immunol. 179:4685–4693.
- D.R. Doerge, M.I. Churchwell, H.C. Chang, R.R. Newbold, and K.B. Delclos. (2001). Placental transfer of the soy isoflavone genistein following dietary and gavage administration to Sprague Dawley rats. Reprod Toxicol. 15:105–110.
- H.C. Dujardin, O. Burlen-Defranoux, L. Boucontet, P. Vieira, A. Cumano, and A. Bandeira. (2004). Regulatory potential and control of Foxp3 expression in newborn CD4+ T-cells. Proc. Natl. Acad. Sci. USA 101:14473–14478.
- A. DunnGalvin, J.O. Hourihane, L. Frewer, R.C. Knibb, J.N. Oude Elberink, and I. Klinge. (2006). Incorporating a gender dimension in food allergy research: A review. Allergy 61:1336–1343. 2006
- M.A. English, S.V. Hughes, K.F. Kane, M.J. Langman, P.M. Stewart, and M. Hewison. (2000). Estrogen inactivation in the colon: Analysis of the expression and regulation of 17β -hydroxysteroid dehydrogenase isozymes in normal colon and colonic cancer. Br. J. Cancer 83:550–558.
- A. Finamore, M. Roselli, N. Merendino, F. Nobili, F. Vignolini, and E. Mengheri. (2003). Zinc deficiency suppresses the development of oral tolerance in rats. J. Nutr. 133:191–198.
- M. Fleeton, N. Contractor, F. Leon, J. He, D. Wetzel, T. Dermody, A. Iwasaki, and B. Kelsall. (2004). Involvement of dendritic cell subsets in the induction of oral tolerance and immunity. Ann. N. Y. Acad. Sci. 1029:60–65.
- T. Foucard, and I. Malmheden Yman. (1999). A study on severe food reactions in Sweden—is soy protein an underestimated cause of food anaphylaxis?. Allergy 54:261–265.
- M. Fujita, R. Baba, M. Shimamoto, Y. Sakuma, and S. Fujimoto. (2007). Molecular morphology of the digestive tract; Macromolecules and food allergens are transferred intact across the intestinal absorptive cells during the neonatal-suckling period. Med. Mol. Morphol. 40:1–7.
- P. Garside, O. Millington, and K.M. Smith. (2004). The anatomy of mucosal immune responses. Ann. N. Y. Acad. Sci. 1029:9–15.
- K. Gejima, H. Kawaguchi, M. Souda, H. Kawashima, T. Komokata, N. Hamada, Y. Umekita, R. Sakata, and H. Yoshida. (2007). Expression of estrogen receptor-α protein in the rat digestive tract. In Vivo 21:487–492.
- F. Gizzarelli, S. Corinti, B. Barletta, P. Iacovacci, B. Brunetto, C. Butteroni, C. Afferni, R. Onori, M. Miraglia, G. Panzini, G. Di Felice, and R. Tinghino. (2006). Evaluation of allergenicity of genetically modified soybean protein extract in a murine model of oral allergen-specific sensitization. Clin. Exp. Allergy 36:238–248.
- M.C. Gold, T.L. Robinson, M.S. Cook, L.K. Byrd, and H.D. Ehlinger. (2007). Human neonatal dendritic cells are competent in MHC Class I antigen processing and presentation. PLoS ONE 2 (9):e957. doi:10.1371/journal.pone.0000957
- R.H. Grose, A.G. Cummins, and F.M. Thompson. (2007). Deficiency of invariant natural killer T-cells in coeliac disease. Gut 56:790–795.
- T.L. Guo, W. Auttachoat, and R.P. Chi. (2005). Genistein enhancement of respiratory allergen trimellitic anhydride-induced IgE production by adult B6C3F1 mice following in utero and post-natal exposure. Toxicol Sci. 87:399–408.
- T.L. Guo, R.P. Chi, D.M. Hernandez, W. Auttachoat, and J.F. Zheng. (2007). Decreased 7,12-dimethylbenz[a]anthracene-induced carcinogenesis coincides with the induction of anti-tumor immunities in adult female B6C3F1 mice pretreated with genistein. Carcinogenesis 28:2560–2566.
- T.L. Guo, J.A. McCay, L.X. Zhang, R.D. Brown, L. You, N.A. Karrow, D.R. Germolec, and K.L. WhiteJr.. (2001). Genistein modulates immune responses and increases host resistance to B16F10 tumor in adult female B6C3F1 mice. J. Nutr. 131:3251–3258.
- M. Hamad, K.H. Abu-Elteen, and M. Ghaleb. (2002). Persistent colonization and transient suppression of DTH responses in an estrogen-dependent vaginal candidosis murine model. New Microbiol. 25:65–73.
- B.C. Harman, J.P. Miller, N. Nikbakht, R. Gerstein, and D. Allman. (2006). Mouse plasmacytoid dendritic cells derive exclusively from estrogen-resistant myeloid progenitors. Blood 108:878–885.
- D.C. Harnish, L.M. Albert, Y. Leathurby, A.M. Eckert, A. Ciarletta, M. Kasaian, and J.C. KeithJr.. (2004). Beneficial effects of estrogen treatment in the HLA-B27 transgenic rat model of inflammatory bowel disease. Am. J. Physiol. 286:G118–125.
- D.J. Hartigan-O'Connor, K. Abel, and J.M. McCune. (2007). Suppression of SIV-specific CD4+ T-cells by infant but not adult macaque regulatory T-cells: Implications for SIV disease progression. J. Exp. Med. 204:2679–2692.
- G. Herberth, C. Daegelmann, A. Weber, S. Röder, T. Giese, U. Krämer, R.P. Schins, H. Behrendt, M. Borte, I. Lehmann, and the LISAplus Study Group. (2006). Association of neuropeptides with TH1/TH2 balance and allergic sensitization in children. Clin. Exp. Allergy 36:1408–1416.
- M. Heyman, and J.F. Desjeux. (2000). Cytokine-induced alteration of the epithelial barrier to food antigens in disease. Ann. N. Y. Acad. Sci. 915:304–311.
- A. Hoek, Y. van Kasteren, M. de Haan-Meulman, H. Hooijkaas, J. Schoemaker, and H.A. Drexhage. (1995). Analysis of peripheral blood lymphocyte subsets, NK cells, and delayed-type hypersensitivity skin tests in patients with premature ovarian failure. Am. J. Reprod. Immunol. Microbiol. 33:495–502.
- R. Holmdahl, and L. Jansson. (1988). Estrogen-induced suppression of collagen arthritis. III. Adult thymectomy does not affect the course of arthritis or the estrogen-mediated suppression of T-cell immunity. Brain Behav. Immun. 2:123–132.
- P.G. Holt, C. Macaubas, P.A. Stumbles, and P.D. Sly. (1999). The role of allergy in the development of asthma. Nature 402:B12–17.
- H. Inadera. (2006). The immune system as a target for environmental chemicals: Xenoestrogens and other compounds. Toxicol. Lett. 164:191–206.
- C. Iwamura, and T. Nakayama. (2007). Role of α -galactosylceramide-activated Vα 14 natural killer T-cells in the regulation of allergic diseases. Allergol. Int. 56:1–6.
- K. Kadkhoda, Z. Pourpak, A. Akbar Pourfathallah, and A. Kazemnejad. (2004). The ex vivo study of synergistic effects of polycyclic aromatic hydrocarbon, benzo(a)pyrene with ovalbumin on systemic immune responses by oral route. Toxicology 199:261–265.
- T. Kalland, and J.G. Forsberg. (1978). Delayed hypersensitivity response to oxazolone in neonatally-estrogenized mice. Cancer Lett. 4:141–146.
- M.R. Karlsson, J. Rugtveit, and P. Brandtzaeg. (2004). Allergen-responsive CD4+ CD25+ regulatory T-cells in children who have outgrown cow's milk allergy. J. Exp. Med. 199:1679–1688.
- T. Kato, S. Tada-Oikawa, K. Takahashi, K. Saito, L. Wang, A. Nishio, R. Hakamada-Taguchi, S. Kawanishi, and K. Kuribayashi. (2006). Endocrine disruptors that deplete glutathione levels in APC promote TH2 polarization in mice leading to the exacerbation of airway inflammation. Eur. J. Immunol. 36:1199–1209.
- N. Kawano, T. Koji, Y. Hishikawa, K. Murase, I. Murata, and S. Kohno. (2004). Identification and localization of estrogen receptor alpha- and beta-positive cells in adult male and female mouse intestine at various estrogen levels. Histochem. Cell Biol. 121:399–405.
- Y. Ke, K. Pearce, J.P. Lake, H.K. Ziegler, and J.A. Kapp. (1997). γ δ T-Lymphocytes regulate the induction and maintenance of oral tolerance. J. Immunol. 158:3610–3618.
- M. Kogiso, T. Sakai, K. Mitsuya, T. Komatsu, and S. Yamamoto. (2006). Genistein suppresses antigen-specific immune responses through competition with 17β -estradiol for estrogen receptors in ovalbumin-immunized BALB/c mice. Nutrition 22:802–809.
- P.A. Konstantinopoulos, A. Kominea, G. Vandoros, G.P. Sykiotis, P. Andricopoulos, I. Varakis, G. Sotiropoulou-Bonikou, and A.G. Papavassiliou. (2003). Oestrogen receptor-β (ERβ) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumor's dedifferentiation. Eur. J. Cancer 39:1251–1258.
- R.A. Kopper, N.J. Odum, M. Sen, R.M. Helm, J.S. Stanley, and A.W. Burks. (2004). Peanut protein allergens: Gastric digestion is carried out exclusively by pepsin. J. Allergy Clin. Immunol. 114:614–618.
- E.J. Kovacs, K.A. Messingham, and M.S. Gregory. (2002). Estrogen regulation of immune responses after injury. Mol. Cell. Endocrinol. 193:129–135.
- J.P. Kraehenbuhl. (1998). The gut-associated lymphoid tissue: A major site of HIV replication and CD4 cell loss. Trends Microbiol. 6:419–420.
- H. Kumagai, A. Suda, H. Sakurai, H. Kumagai, S. Arai, N. Inomata, and Z. Ikezawa. (2007). Improvement of digestibility, reduction in allergenicity, and induction of oral tolerance of wheat gliadin by deamidation. Biosci. Biotechnol. Biochem. 71:977–985.
- R.I. Kushak, and H.S. Winter. (1999). Regulation of intestinal peptidases by nutrients in human fetuses and children. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 124:191–198.
- G. Lack, D. Fox, K. Northstone, J. Golding, and Avon Longitudinal Study of Parents and Children Study Team. (2003). Factors associated with the development of peanut allergy in childhood. New Engl. J. Med. 348:977–985.
- R. Lamason, P. Zhao, R. Rawat, A. Davis, J.C. Hall, J.J. Chae, R. Agarwal, P. Cohen, A. Rosen, E.P. Hoffman, and K Nagaraju. (2006). Sexual dimorphism in immune response genes as a function of puberty. BMC Immunol. 22:7:2.
- C.L. Langrish, J.C. Buddle, A.J. Thrasher, and D. Goldblatt. (2002). Neonatal dendritic cells are intrinsically biased against TH1 immune responses. Clin. Exp. Immunol. 128:118–123.
- M.H. Lee, S.W. Chung, B.Y. Kang, J. Park, C.H. Lee, S.Y. Hwang, and T.S. Kim. (2003). Enhanced interleukin-4 production in CD4+ T-cells and elevated immunoglobulin E levels in antigen-primed mice by bisphenol A and nonylphenol, endocrine disruptors: Involvement of nuclear factor-AT and Ca2 +. Immunology 109:76–86.
- M.H. Lee, E. Kim, and T.S. Kim. (2004). Exposure to 4-tert-octylphenol, an environmentally persistent alkylphenol, enhances interleukin-4 production in T-cells via NF-AT activation. Toxicol. Appl. Pharmacol. 197:19–28.
- S. Li, S.D. Hursting, B.J. Davis, J.A. McLachlan, and J.C. Barrett. (2003). Environmental exposure, DNA methylation, and gene regulation: Lessons from diethylstilbesterol-induced cancers. Ann. N. Y. Acad. Sci. 983:161–169.
- G.A. Lied. (2007). Gastrointestinal food hypersensitivity: Symptoms, diagnosis and provocation tests. Turk. J. Gastroenterol. 18:5–13.
- J.H. Lin, M. Chiba, and T.A. Baillie. (1999). Is the role of the small intestine in first-pass metabolism overemphasized?. Pharmacol. Rev. 51:135–158.
- H.Y. Liu, A.C. Buenafe, A. Matejuk, A. Ito, A. Zamora, J. Dwyer, A.A. Vandenbark, and H. Offner. (2002). Estrogen inhibition of EAE involves effects on dendritic cell function. J. Neurosci. Res. 70:238–248.
- M. Lovik, E. Namork, C. Faeste, and E. Egaas. (2004). The Norwegian national reporting system and register of severe allergic reactions to food. Norsk. Epidemiol. 14:155–160.
- M. Lovik, H.G. Wilker, B.A. Stensby, R. Kjelkevik, A.K. Sommer, and B. Mangschou. The Norwegian national reporting system and register of severe allergic reactions to foodClinical Immunology and Allergy in MedicineG. Marone. JGC Publishers, Napoli, (2003), 461–466.
- R.W. Luebke, M.I. Luster, J.H. Dean, and H.T. Hayes. (1984). Altered host resistance to Trichinella spiralis infection following subchronic exposure to diethylstilbestrol. Int. J. Immunopharmacol. 6:609–617.
- M.I. Luster, R.E. Faith, J.A. McLachlan, and G.C. Clark. (1979). Effect of in utero exposure to diethylstilbestrol on the immune response in mice. Toxicol. Appl. Pharmacol 47:279–285.
- D. Ly, Q.S. Mi, S. Hussain, and T.L. Delovitch. (2006). Protection from Type 1 diabetes by invariant NK T-cells requires the activity of CD4+ CD25+ regulatory T-cells. J. Immunol. 177:3695–3704.
- A. Mao, V. Paharkova-Vatchkova, J. Hardy, M.M. Miller, and S. Kovats. (2005). Estrogen selectively promotes the differentiation of dendritic cells with characteristics of Langerhans cells. J. Immunol. 175:5146–5151.
- M.L. Marin, J. Murtha, W. Dong, and J.J. Pestka. (1996). Effects of mycotoxins on cytokine production and proliferation in EL-4 thymoma cells. J. Toxicol. Environ. Health 48:379–396.
- J.T. Martin. (2000). Sexual dimorphism in immune function: The role of prenatal exposure to androgens and estrogens. Eur. J. Pharmacol 405:251–261.
- P.M. Martin, K.B. Horwitz, D.S. Ryan, and W.L. McGuire. (1978). Phytoestrogen interaction with estrogen receptors in human breast cancer cells. Endocrinology 103:1860–1867.
- P.M. Matricardi, F. Franzinelli, A. Franco, G. Caprio, F. Murru, D. Cioffi, L. Ferrigno, A. Palermo, N. Ciccarelli, and F. Rosmini. (1998). Sibship size, birth order, and atopy in 11,371 Italian young men. J. Allergy Clin. Immunol. 101:439–444.
- T. Matsuda, T. Matsubara, and S. Hino. (2006). Immunogenic and allergenic potentials of natural and recombinant innocuous proteins. J. Biosci. Bioeng. 101:203–211.
- L. Matthieu, A.F. Godoi, J. Lambert, and R. Van Grieken. (2003). Occupational allergic contact dermatitis from bisphenol A in vinyl gloves. Contact Dermatitis 49:281–283.
- B.N. Melgert, A. Ray, M.N. Hylkema, W. Timens, and D.S. Postma. (2007). Are there reasons why adult asthma is more common in females?. Curr. Allergy Asthma Rep. 7:143–150.
- J. Mengel, F. Cardillo, L.S. Aroeira, O. Williams, M. Russo, and N.M. Vaz. (1995). Anti-γ δ T-cell antibody blocks the induction and maintenance of oral tolerance to ovalbumin in mice. Immunol. Lett. 48:97–102.
- E.H. Meyer, R.H. DeKruyff, and D.T. Umetsu. (2007). iNKT cells in allergic disease. Curr. Topics Microbiol. Immunol. 314:269–291.
- E.H. Meyer, S. Goya, O. Akbari, G.J. Berry, P.B. Savage, M. Kronenberg, T. Nakayama, R.H. DeKruyff, and D.T. Umetsu. (2006). Glycolipid activation of invariant T-cell receptor+ NK T-cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T-cells. Proc. Natl. Acad. Sci. USA 103:2782–2787.
- J.J. Michnovicz, and D.W. Rosenberg. (1992). Oxidative metabolism of estrogens in rat intestinal mitochondria. Biochem. Pharmacol. 43:1847–1852.
- R.J. Miksicek. (1994). Interaction of naturally occurring nonsteroidal estrogens with expressed recombinant human estrogen receptor. J. Steroid Biochem. Mol. Biol. 49:153–160.
- Y. Miyake, S. Sasaki, Y. Ohya, S. Miyamoto, I. Matsunaga, T. Yoshida, Y. Hirota, and H. Oda. (2005). Soy, isoflavones, and prevalence of allergic rhinitis in Japanese women: The Osaka Maternal and Child Health Study. J. Allergy Clin. Immunol. 115:1176–1183.
- A.M. Mowat, and D.M. Parrot. (1983). Immunological responses to fed protein antigens in mice. IV. Effects of stimulating the reticuloendothelial system on oral tolerance and intestinal immunity to ovalbumin. Immunology 50:547–554.
- M.F. Neurath, S. Finotto, and L.H. Glimcher. (2002). The role of TH1/TH2 polarization in mucosal immunity. Nat. Med. 8:567–573.
- R.D. Newberry, and R.G. Lorenz. (2005). Organizing a mucosal defense. Immunol. Rev. 206:6–21.
- Y. Ohshima, A. Yamada, S. Tokuriki, M. Yasutomi, N. Omata, and M. Mayumi. (2007). Transmaternal exposure to Bisphenol a modulates the development of oral tolerance. Pediatr. Res. 62:60–64. 2007
- K. Ohtaki, M. Aihara, H. Takahashi, H. Fujita, K. Takahashi, T. Funabashi, T. Hirasawa, and Z. Ikezawa. (2007). Effects of tributyltin on the emotional behavior of C57BL/6 mice and the development of atopic dermatitis-like lesions in DS-Nh mice. J. Dermatol. Sci. 47:209–216.
- C.M. O'Sullivan, J.L. Ungarian, K. Singh, S. Liu, J. Hance, and D.E. Rancourt. (2004). Uterine secretion of ISP1 & 2 tryptases is regulated by progesterone and estrogen during pregnancy and the endometrial cycle. Mol. Reprod. Dev. 69:252–259.
- D. Ozol, and E. Mete. (2008). Asthma and food allergy. Curr. Opin. Pulm. Med. 14:9–12.
- V. Paharkova-Vatchkova, R. Maldonado, and S. Kovats. (2004). Estrogen preferentially promotes the differentiation of CD11c+CD11b(intermediate) dendritic cells from bone marrow precursors. J. Immunol. 172:1426–1436.
- M.A. Pérez-Machado, P. Ashwood, M.A. Thomson, F. Latcham, R. Sim, J.A. Walker-Smith, and S.H. Murch. (2003). Reduced transforming growth factor-β 1-producing T-cells in the duodenal mucosa of children with food allergy. Eur. J. Immunol. 33:2307–2315.
- M.A. Pérez-Machado, P. Ashwood, F. Torrente, C. Salvestrini, R. Sim, M.A. Thomson, J.A. Walker-Smith, and S.H. Murch. (2004). Spontaneous TH1 cytokine production by intra-epithelial but not circulating T-cells in infants with or without food allergies. Allergy 59:346–353.
- A. Pettersson, C. Ciumas, V. Chirsky, H. Link, Y.M. Huang, and B.G. Xiao. (2004). Dendritic cells exposed to estrogen in vitro exhibit therapeutic effects in ongoing experimental allergic encephalomyelitis. J. Neuroimmunol. 156:58–65.
- M.W. Pfaffl, I.G. Lange, and H.H. Meyer. (2003). The gastrointestinal tract as target of steroid hormone action: Quantification of steroid receptor mRNA expression (AR, ERα, ERβ, and PR) in 10 bovine gastrointestinal tract compartments by kinetic RT-PCR. J. Steroid Biochem. Mol. Biol. 84:159–166.
- P. Piyachaturawat, P. Sretarugsa, L. Limlomwongse, and S. Sahaphong. (1983). Effect of estradiol treatment on the secretion and structure of gastric H+ and pepsin-secreting cells in the rat. Res. Commun. Chem. Pathol. Pharmacol. 42:81–93.
- M.J. Polanczyk, B.D. Carson, S. Subramanian, M. Afentoulis, A.A. Vandenbark, S.F. Ziegler, and H. Offner. (2004). Cutting edge: Estrogen drives expansion of the CD4+ CD25+ regulatory T-cell compartment. J. Immunol. 173:2227–2230.
- M.J. Polanczyk, C. Hopke, A.A. Vandenbark, and H. Offner. (2006). Estrogen-mediated immunomodulation involves reduced activation of effector T-cells, potentiation of Treg cells, and enhanced expression of the PD-1 co-stimulatory pathway. J. Neurosci. Res. 84:370–378.
- I. Rauter, M.T. Krauth, K. Westritschnig, F. Horak, S. Flicker, A. Gieras, A. Repa, N. Balic, S. Spitzauer, J. Huss-Marp, K. Brockow, U. Darsow, H. Behrendt, J. Ring, F. Kricek, P. Valent, and R. Valenta. (2007). Mast cell-derived proteases control allergic inflammation through cleavage of IgE. J. Allergy Clin. Immunol. 2007:Sep 27 [Epub ahead of print]
- E. Reichrtová, P. Ciznár, V. Prachar, L. Palkovicová, and M. Veningerová. (1999). Cord serum immunoglobulin E related to the environmental contamination of human placentas with organochlorine compounds. Environ. Health Perspect. 107:895–899.
- A.C. Rocha-Campos, R. Melki, R. Zhu, N. Deruytter, D. Damotte, M. Dy, A. Herbelin, and H.J. Garchon. (2006). Genetic and functional analysis of the Nkt1 locus using congenic NOD mice: Improved Vα 14-NKT cell performance but failure to protect against Type 1 diabetes. Diabetes 55:1163–1170.
- A.B. Ropero, P. Alonso-Magdalena, C. Ripoll, E. Fuentes, and A Nadal. (2006). Rapid endocrine disruption: Environmental estrogen actions triggered outside the nucleus. J. Steroid Biochem. Mol. Biol. 102:163–169.
- S. Rose, M. Lichtenheld, M.R. Foote, and B. Adkins. (2007). Murine neonatal CD4+ cells are poised for rapid TH2 effector-like function. J. Immunol. 178:2667–2678.
- B.T. Rouse, and S. Sehrawat. (2007). Regulatory T-cells and infectious disease. Immune Network 7:167–172.
- W.L. Ruoff, and P.J. Dziuk. (1994). Absorption and metabolism of estrogens from the stomach and duodenum of pigs. Domest. Anim. Endocrinol. 11:197–208.
- K. Sakuishi, S. Oki, M. Araki, S.A. Porcelli, S. Miyake, and T. Yamamura. (2007). Invariant NKT cells biased for IL-5 production act as crucial regulators of inflammation. J. Immunol. 179:3452–3462.
- M.L. Salem, G. Matsuzaki, K. Kishihara, G.A. Madkour, and K. Nomoto. (2000). β -Estradiol suppresses T-cell-mediated DTH through suppression of antigen-presenting cell function and TH1 induction. Int. Arch. Allergy Immunol. 121:161–169.
- A. Sapino, P. Cassoni, E. Ferrero, M. Bongiovanni, L. Righi, N. Fortunati, P. Crafa, R. Chiarle, and G. Bussolati. (2003). Estrogen receptor-α is a novel marker expressed by follicular dendritic cells in lymph nodes and tumor-associated lymphoid infiltrates. Am. J. Pathol. 163:1313–1320.
- A. Sato, and A. Iwasaki. (2005). Peyer's patch dendritic cells as regulators of mucosal adaptive immunity. Cell. Mol. Life Sci. 62:133–1338.
- C. Schreider, G. Peignon, S. Thenet, J. Chambaz, and M. Pinçon-Raymond. (2002). Integrin-mediated functional polarization of Caco-2 cells through E-cadherin-actin complexes. J. Cell Sci. 115:543–552.
- F.W. Scott, P. Rowsell, G.S. Wang, K. Burghardt, H. Kolb, and S. Flohé. (2002). Oral exposure to diabetes-promoting food or immunomodulators in neonates alters gut cytokines and diabetes. Diabetes 51:73–78.
- A. Senthilselvan, H.H. McDuffie, and J.A. Dosman. (1992). Association of asthma with use of pesticides. Results of a cross-sectional survey of farmers. Am. Rev. Respir. Dis. 146:884–887.
- K.D. Setchell, L. Zimmer-Nechemias, J. Cai, and J.E. Heubi. (1997). Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet 350:23–27.
- L.J. Smith, J.T. Holbrook, R. Wise, M. Blumenthal, A.J. Dozor, J. Mastronarde, and L. Williams. (2004). Dietary intake of soy genistein is associated with lung function in patients with asthma. J. Asthma 41:833–843.
- J. Strid, J. Hourihane, I. Kimber, R. Callard, and S. Strobel. (2005). Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clin. Exp. Allergy 35:757–766.
- B.L. Strom, R. Schinnar, E.E. Ziegler, K.T. Barnhart, M.D. Sammel, G.A. Maconesm, V.A. Stallings, J.M. Drulis, S.E. Nelson, and S.A. Hanson. (2001). Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA 15:807–814.
- C.M. Sun, L. Fiette, M. Tanguy, C. Leclerc, and R. Lo-Man. (2003). Ontogeny and innate properties of neonatal dendritic cells. Blood 102:585–591.
- C.M. Sun, J.A. Hall, R.B. Blank, N. Bouladoux, M. Oukka, J.R. Mora, and Y. Belkaid. (2007). Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 Treg cells via retinoic acid. J. Exp. Med. 204:1775–1785.
- P. Tai, J. Wang, H. Jin, X. Song, J. Yan, Y. Kang, L. Zhao, X. An, X. Du, X. Chen, S. Wang, G. Xia, and B. Wang. (2008). Induction of regulatory T-cells by physiological level estrogen. J. Cell. Physiol. 214:456–464.
- N. Takayama, O. Igarashi, M.N. Kweon, and H. Kiyono. (2007). Regulatory role of Peyer's patches for the inhibition of OVA-induced allergic diarrhea. Clin. Immunol. 123:199–208.
- M.L. Thomas, X. Xu, A.M. Norfleet, and C.S. Watson. (1993). The presence of functional estrogen receptors in intestinal epithelial cells. Endocrinology 132:426–430.
- E. Todaka, K. Sakurai, H. Fukata, H. Miyagawa, M. Uzuki, M. Omori, H. Osada, Y. Ikezuki, O. Tsutsumi, T. Iguchi, and C. Mori. (2005). Fetal exposure to phytoestrogens–the difference in phytoestrogen status between mother and fetus. Environ. Res. 99:195–203.
- T.R. Torgerson, A. Linane, N. Moes, S. Anover, V. Mateo, F. Rieux-Laucat, O. Hermine, S. Vijay, E. Gambineri, N. Cerf-Bensussan, A. Fischer, H.D. Ochs, O. Goulet, and F.M. Ruemmele. (2007). Severe food allergy as a variant of IPEX syndrome caused by a deletion in a non-coding region of the FOXP3 gene. Gastroenterology 132:1705–1717.
- R.J. Troisi, F.E. Speizer, W.C. Willett, D. Trichopoulos, and B. Rosner. (1995). Menopause, post-menopausal estrogen preparations, and the risk of adult-onset asthma. A prospective cohort study. Am. J. Respir. Crit. Care Med. 152:1183–1188.
- Y. Tu, and M.H. Perdue. (2006). CD23-mediated transport of IgE/immune complexes across human intestinal epithelium: Role of p38 MAPK. Am. J. Physiol. 291:G532–538.
- Y. Tu, S. Salim, J. Bourgeois, V. Di Leo, E.J. Irvine, J.K. Marshall, and M.H. Perdue. (2005). CD23-mediated IgE transport across human intestinal epithelium: Inhibition by blocking sites of translation or binding. Gastroenterology 129:928–940.
- M. Ulanova, M. Torebring, S.A. Porcelli, U. Bengtsson, J. Magnusson, O. Magnusson, X.P. Lin, L.A. Hanson, and E. Telemo. (2000). Expression of CD1d in the duodenum of patients with cow's milk hypersensitivity. Scand. J. Immunol. 52:609–617.
- F. van Wijk, E.J. Wehrens, S. Nierkens, L. Boon, A. Kasran, R. Pieters, and L.M. Knippels. (2007). CD4+ CD25+ T-cells regulate the intensity of hypersensitivity responses to peanut, but are not decisive in the induction of oral sensitization. Clin. Exp. Allergy 37:572–581.
- M. Verdrengh, I.M. Jonsson, R. Holmdahl, and A. Tarkowski. (2003). Genistein as an anti-inflammatory agent. Inflamm. Res. 52:341–346.
- G. Veres, M. Westerholm-Ormio, J. Kokkonen, A. Arato, and E. Savilahti. (2003). Cytokines and adhesion molecules in duodenal mucosa of children with delayed-type food allergy. J. Pediatr. Gastroenterol. Nutr. 37:27–34.
- A.J. Vingerhoets, J. Assies, K. Goodkin, G.L. Van Heck, and M.H. Bekker. (1998). Prenatal diethylstilbestrol exposure and self-reported immune-related diseases. Eur. J. Obstet. Gynecol. Reprod. Biol. 77:205–209.
- A. von Berg. (2007). The concept of hypoallergenicity for atopy prevention. Nestle Nutr. Workshop Ser. Pediatr. Program 59:49–57.
- O. Wada-Hiraike, O. Imamov, H. Hiraike, K. Hultenby, T. Schwend, Y. Omoto, M. Warner, and J.A. Gustafsson. (2006). Role of estrogen receptor beta in colonic epithelium. Proc. Natl. Acad. Sci. USA 103:2959–2964.
- S.C. Ways, J.F. Mortola, N.J. Zvaifler, R.J. Weiss, and S.S. Yen. (1987). Alterations in immune responsiveness in women exposed to diethylstilbestrol in utero. Fertil. Steril. 48:193–197.
- M. Wjst, and S. Dold. (1997). Is asthma an endocrine disease?. Pediatr. Allergy Immunol. 8:200–204.
- L. Wu, and L. van Kaer. (2007). Role of NKT cells in the digestive system. II. NKT cells and diabetes. Am. J. Physiol. Gastrointest. Liver Physiol. 293:G919–922.
- L. Yang, Y. Hu, and Y. Hou. (2006). Effects of 17β -estradiol on the maturation, nuclear factor κ B p65 and functions of murine spleen CD11c-positive dendritic cells. Mol. Immunol. 43:357–366.
- P.C. Yang, Z. Xing, C.M. Berin, J.D. Soderholm, B.S. Feng, L. Wu, and C. Yeh. (2007). TIM-4 expressed by mucosal dendritic cells plays a critical role in food antigen-specific TH2 differentiation and intestinal allergy. Gastroenterology 2007:Aug 2 [Epub ahead of print]
- S. Yellayi, A. Naaz, M.A. Szewczykowski, T. Sato, J.A. Woods, J. Chang, M. Segre, C.D. Allred, W.G. Helferich, and P.S. Cooke. (2002). The phytoestrogen genistein induces thymic and immune changes: A human health concern?. Proc. Natl. Acad. Sci. USA 99:7616–7621.
- S. Yoshino, H. Hayashi, S. Taneda, H. Takano, M. Sagai, and Y. Mori. (2002). Effect of diesel exhaust particle extracts on induction of oral tolerance in mice. Toxicol. Sci. 66:293–297.
- S. Yoshino, and M. Sagai. (1999). Induction of systemic TH1 and TH2 immune responses by oral administration of soluble antigen and diesel exhaust particles. Cell. Immunol. 192:72–78.
- K. Yoshino, K. Sakai, Y. Mizuha, A. Shimizuike, and S. Yamamoto. (2004). Peptic digestibility of raw and heat-coagulated hen's egg white proteins at acidic pH range. Int. J. Food Sci. Nutr. 55:635–640.
- S. Yoshino, M. Ohsawa, and M. Sagai. (1998). Diesel exhaust particles block induction of oral tolerance in mice. J. Pharmacol. Exp. Ther. 287:679–683.