Abstract
Multi-walled carbon nanotubes (MWCNTs) have industrial applications in the nanotechnology field. The physico-chemical properties of MWCNTs vary greatly depending on MWCNT manufacture and application. It has been pointed out that their needle shape and high durability are important factors that determine the biopersistence of fibers and can lead to inhalation toxicity or cytotoxicity. In this study, we prepared six suspensions of MWCNTs differing in diameter and length, and performed in vitro cell-based assays for 24 h using NR8383 rat alveolar macrophages. Rigid, needle-shaped MWCNTs with a large diameter (>50 µm) penetrated the cytoplasm and decreased cell survival without generating intracellular reactive oxygen species (ROS), significantly up-regulated many genes involved in inflammatory responses, response to oxidative stress and apoptosis, and extracellular matrix degradation. Bent MWCNTs with a small diameter (<20 µm) were phagocytosed in vacuole-like cellular compartments and decreased cell survival along with intracellular ROS generation. Straight, thin MWCNTs with a small diameter (<20 µm) caused a slight intracellular ROS generation but no decrease in cell viability. Some straight, long, and thin MWCNTs were found in the mitochondria and near the nuclei; however, no mutagenesis was observed. The in vitro cell-based assays showed high cytotoxicity of MWCNTs with a large diameter (>50 µm), moderate and low cytotoxicity of MWCNTs with a small diameter (<20 µm). These results suggested that the diameter of MWCNTs considerably contributes to their cytotoxicity.
1. Introduction
Multi-walled carbon nanotubes (MWCNTs) have industrial applications in the nanotechnology field. However, because of their needle shape and high durability, MWCNTs can cause inhalation toxicity. Kasai et al. (Citation2016) reported that MWNT-7, a rigid, needle-shaped MWCNT, lead to increased incidence of bronchioloalveolar carcinoma in male and female rats, although there is no development of pleural mesothelioma in both sexes. In 2014, the World Health Organization and the International Agency for Research on Cancer classified MWNT-7 into group 2B (‘Possibly carcinogenic to humans’) and, because of a lack of carcinogenicity data, other MWCNTs, except MWNT-7, into group 3 (‘Not classifiable as to its carcinogenicity to humans’; Grosse et al. Citation2014). MWNT-7 show cytotoxicity and the ability to pierce the mesothelial cell membrane in vitro and subsequent inflammogenicity and mesotheliomagenicity in vivo (Nagai et al. Citation2011). However, the carcinogenic mechanism is still unclear, and further studies are required to fully elucidate the effects of MWNT-7 and other types of MWCNTs on carcinogenicity.
The respiratory system is an important route of exposure to nanomaterials. Inhalation toxicity of MWCNTs is likely an exposure route for putative events in manufacturing workers. An in vivo intratracheal instillation test with 1.0 mg/kg MWNT-7 showed no evidence of chronic inflammation, such as angiogenesis or fibrosis, in rat lungs (Kobayashi et al. Citation2010). These results suggested that MWNT-7 are processed and cleared by alveolar macrophages (Kobayashi et al. Citation2010). Fujita et al. (Citation2016) reported that MWNT-7 instillation in doses of 0.15 or 1.5 mg/kg markedly impacted the alveoli immediately, with pulmonary inflammation reducing in a time-dependent manner following MWNT-7 instillation. MWNT-7 instillation induced more pleural inflammation compared with single-walled carbon nanotubes (SWCNTs). MWNT-7 translocation in mediastinal lymph nodes was observed, suggesting that after pleural penetration, MWNT-7 undergo lymphatic drainage to mediastinal lymph nodes (Fujita et al. Citation2016). Rydman et al. (Citation2014) showed that mice exposed to MWNT-7 for 4 h/day for four consecutive days developed a novel innate immunity-mediated, allergic-like airway inflammation-like reaction. However, exposure to flexible, tangled MWCNTs did not cause morphologically evident lung inflammation in mice (Rydman et al. Citation2014). Some studies have also conducted inhalation toxicity tests for MWCNTs other than MWNT-7. Pulmonary inflammation was induced following a single intratracheal instillation with MWCNTs in rats, as evidenced by a transient neutrophil response in low-dose groups (3.3 mg/kg), and a small granulomatous lesion and persistent neutrophil infiltration in high-dose groups (0.66 mg/kg; Morimoto et al. Citation2012). However, few in vivo animal studies have compared the inhalation toxicity of MWNT-7 with other MWCNTs. Based on the results of MWNT-7 inhalation toxicity, data are still lacking to generalize the inhalation toxicity of all MWCNTs.
The physico-chemical properties of MWCNTs, such as diameter, length, and rigidity/flexibility, greatly vary depending on MWCNT manufacture and application. We previously proposed that the characterization of the physico-chemical properties of nanomaterials in a cell culture medium is essential for evaluating their effects on cellular processes (Horie and Fujita Citation2011; Horie et al. Citation2012). The pulmonary toxicity of SWCNTs is closely associated with the bundle size (Fujita et al. Citation2013). Poulsen et al. (Citation2015) analyzed the pulmonary response to intratracheal exposure of short, thin, curled MWCNTs or long, thick MWCNTs and found remarkably similar effects on the transcriptome, especially in key processes, inflammation, and acute-phase response. However, they found notable differences between the two types of MWCNTs in the expression of several fibrosis-associated genes (Poulsen et al. Citation2015). Gaté et al. (Citation2019) examined the lung toxicity of ‘long and thick’ and ‘short and thin’ MWCNTs in rats by intratracheal instillation or inhalation exposure. Xu et al. (Citation2014) reported that compared with small MWCNTs (L = 3 μm; D = 15 nm), large, needle-shaped MWCNTs (L = 8 μm; D = 150 nm) have a higher risk of causing asbestos-like pleural lesions related to mesothelioma development. These reports suggested that physico-chemical properties of the MWCNTs are closely related to biological effects.
In this study, we assessed cytotoxicity of MWCNTs with different lengths and fiber diameters by examining cell viability, intracellular reactive oxygen species (ROS) levels, pro-inflammatory cytokine expression, intracellular MWCNT uptake, and comprehensive gene expression profiling in NR8383 rat alveolar macrophges. Alveolar macrophages play an important role in defending the body against foreign particles. We used NR8383 cells to study phagocytosis and cytotoxicity of CNTs and exfoliated graphene (Fujita et al. Citation2015, Citation2018). The results suggested that NR8383 cells were useful to evaluate acute in vivo toxic assessment following SWCNT exposure. In this study, NR8383 cells were used to examine the cytotoxicity of MWCNT to evaluate inhalation toxicity of MWCNTs.
2. Materials and methods
2.1. Test materials and their preparation
Bulk MWNT-7 and Nikkiso-MWCNTs were obtained from Mitsui and Co., Ltd. (Tokyo, Japan) and Nikkiso Co., Ltd. (Tokyo, Japan), respectively. Bulk FloTube 9000 CNano-MWCNTs and NC7000™ Nanocyl-MWCNTs were purchased from Marubeni Information Systems (Tokyo, Japan) and Nanocyl SA (Chiba, Japan), respectively. All MWCNTs were separately dispersed into a 10 mg/mL bovine serum albumin (BSA; NACALAI TESQUE, INC., Kyoto, Japan) solution dissolved in UltraPure™ DNase/RNase-Free distilled water (Thermo Fisher Scientific, Waltham, MA, USA) using a Branson 1510 J-DTH ultrasonic bath (Branson Ultrasonics, Emerson Japan, Ltd., Atsugi, Japan). The MWCNTs were centrifuged, and the resulting supernatant was filtered through a cell strainer with a 40-μm nylon mesh (Nippon Becton Dickinson Company, Ltd., Tokyo, Japan) to remove large carbon nanotube (CNT) agglomerates. The filtrates were adjusted at a concentration of 1.0 mg/mL and used as MWCNT stock suspensions: MW1 (MWNT-7), MW2 (Nikkiso-MWCNTs), MW3 (CNano-MWCNTs), and MW4 (Nanocyl-MWCNTs). Bulk Taiyo Nippon Sanso-MWCNTs were obtained from Taiyo Nippon Sanso Corporation (Tokyo, Japan) and two types of suspensions with different MWCNT lengths were prepared. Bulk MWCNTs were stirred for 1 h in 10 mg/mL BSA and dispersed using an ultrasonic homogenizer for 2 h. The solution was redispersed using a NanoVater™ L-AD200 wet atomization device (YOSHIDA KIKAI CO., LTD., Nagoya, Japan), centrifuged, and the resulting supernatant was filtered through a cell strainer with a 100-μm nylon mesh (Nippon Becton Dickinson Company, Ltd.). Filtrates adjusted at a concentration of 0.5 mg/mL were used as MWCNT stock suspension MW5. Then, bulk MWCNTs was dispersed into 10 mg/mL BSA using a Branson Sonifier® 250 ultrasonic homogenizer (Branson Ultrasonics, Emerson Japan, Ltd.) for 0.5 h were centrifuged, and the resulting supernatant was filtered through a cell strainer with a 100-μm nylon mesh (Nippon Becton Dickinson Company, Ltd.). After centrifugation of the filtrate, the collected precipitate was redispersed using a Branson 1510J-DTH ultrasonic bath (Branson Ultrasonics, Emerson Japan, Ltd.), and the resulting suspension was filtered through a cell strainer with a 100-μm nylon mesh (Nippon Becton Dickinson Company, Ltd.). Filtrates adjusted at a concentration of 0.5 mg/mL were used as long length MWCNT stock suspension MW6. MW5 and MW6 were adjustable at a concentration of 0.5 mg/mL; suspensions at a concentration of 1.0 mg/mL or more could not technically be prepared.
The concentration of MWCNTs in the six stock suspensions (MW1–4: 1.0 mg/mL; MW5 and 6: 0.5 mg/mL) was determined by measuring their ultraviolet (UV)-visible absorption spectra using a UV-2550 spectrophotometer (Shimadzu Corporation, Kyoto, Japan) at wavelengths of 600–800 nm (Fujita et al. Citation2017). The stock suspensions were adjusted to a 10- to 1000-fold dilution series to the required concentration in cell culture medium for use in in vitro cell-based assays and then used for a battery of genotoxicity tests. The zeta potentials of MWCNTs dispersed in the stock suspensions were measured using a Zetasizer Nano zeta potential analyzer (Malvern Panalytical Ltd., Malvern, UK). Hitachi H-7000 and H-7100 transmission electron microscopy (TEM) systems at 75 kV and 100 kV (Hitachi, Ltd., Tokyo, Japan), respectively, were used to observe MWCNTs in the stock suspensions. The lengths and fiber diameters of the MWCNTs were measured from ∼1000 or 200 MWCNTs bundles using a JEOL JEM-1010 TEM (Jeol Ltd., Tokyo, Japan) at 100 kV. A Limulus amebocyte lysate test (Associates of Cape Cod, Inc., East Falmouth,MA, USA; data not shown) was conducted to find endotoxins in the six MWCNT stock suspensions, but none was detected.
2.2. Cell culture and exposure to MWCNTs
The six stock suspensions of MWCNTs were diluted 10-fold in Ham’s F-12K (Kaighn’s) culture medium (Life Technologies Japan Ltd., Tokyo, Japan) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS; Hana-Nesco Bio, Tokyo, Japan), 100 units/mL of penicillin, and 100 μg/mL of streptomycin (F-12K + FBS medium). These working solutions were used for in vitro cell-based assays, as described later. The concentrations (MW1–4: 1–100 µg/mL; MW5 and 6: 0.5–50 µg/mL) were set to 10, 100 and 1000-fold dilution of the maximum concentration of the stock suspensions. NR8383 rat alveolar macrophage cells (CRL-2192™) obtained from the American Type Culture Collection (Manassas, VA, US) were cultured in 75 cm2 cell culture flasks in F-12K + FBS medium in a humidified atmosphere of 95% air and 5% CO2 at 37 °C. After centrifugation, F-12K + FBS medium was removed and replaced with the working solutions; the cell density at the time of replacement was ∼3 × 105 cells/mL. The NR8383 cells were seeded into 6-well or 96-well plates (Nippon Becton Dickinson Company, Ltd.) and incubated for 24 h in a humidified atmosphere of 95% air and 5% CO2 at 37 °C for in vitro cell-based assays.
2.3. Cell survival assay
Cell survival with MWCNTs was examined by the 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt (WST-1)-based assay (Takara Bio Inc., Kusatsu, Japan). After treating NR8383 cells with the working solutions (MW1–4: 1–100 µg/mL; MW5 and 6: 0.5–50 µg/mL) in quadruplicate, the supernatant was removed. WST-1, diluted to 1:10 (v/v) with F-12K + FBS medium, was added to each well. According to the manufacturer’s instructions, the reaction with WST-1 reagent to the cells was performed for 1 h at 37 °C. Sample absorbance at a wavelength of 450 nm was measured using a Model 680 microplate reader (Bio-RadLaboratories, Inc., California,CA, USA), with absorbance at a wavelength of 750 nm as reference. Data were normalized to the absorbance values of control cells. The measured values were calculated with or without MWCNT in the absence of cells. TEM observation validated that no WST-1 crystals can be found within or attached to MWCNTs (data not shown). We convinced that there was no interference of MWCNTs absorption with the colorant absorption at selected wavelengths. To confirm the test system, 100-μM mitomycin C and 100-μM pyocyanin (Sigma-Aldrich, Tokyo, Japan) were used as positive controls (data not shown).
2.4. Intracellular ROS assay
Intracellular ROS were detected using 2′,7′-dichlorodihydrofluorescin diacetate (DCFH-DA; Sigma-Aldrich) dissolved in dimethyl sulfoxide. After treating NR8383 cells with the working solutions (MW1–4: 1–100 µg/mL; MW5 and 6: 0.5–50 µg/mL) for 24 h in quadruplicate, the supernatant was removed; fresh F-12K without FBS medium containing 10 μM DCFH-DA solution was added to each well, and the samples were incubated for 30 min at 37 °C. The cells were then washed once with Dulbecco’s phosphate-buffered saline (DPBS) and resuspended in DPBS. The samples were excited with a 488 nm argon laser in a Guava flow cytometer with guavaSoft™ (Merck Millipore, Burlington, MA, USA). Data were collected from 1000-gated events. To confirm the test system, 100-μM pyocyanin was used as a positive control (data not shown).
2.5. Pro-inflammatory cytokine assay
After treating NR8383 cells with the working solutions (MW1–4: 1–100 µg/mL; MW5 and 6: 0.5–50 µg/mL) for 24 h in quadruplicate, pro-inflammatory cytokines interleukin (IL)-1α, IL-1β, IL-18, macrophage inflammatory protein-1 alpha (MIP-1α), interferon-gamma (INF-γ), macrophage chemoattractant protein-1 (MCP-1), and tumor necrosis factor alpha (TNF-α) in cell suspensions were measured using a MILLIPLEX® MAP Rat Cytokine/Chemokine Magnetic Bead Panel and a Luminex 200 System (Merck Millipore).
2.6. TEM observations of cell morphology
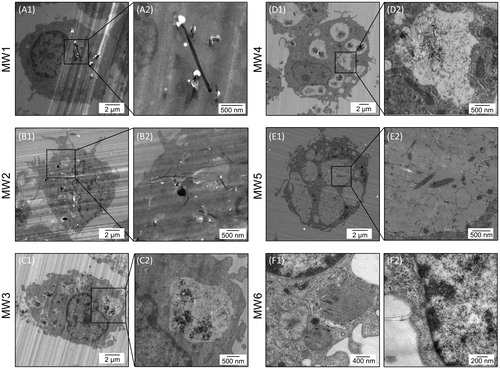
After treating NR8383 cells with the working solutions (MW1–4: 100 µg/mL; MW5 and 6: 50 µg/mL) for 24 h, the cells were fixed sequentially in 1.2% (v/v) glutaraldehyde for 1 h at 20 °C, fixed in 1% osmium oxide solution for 1 h at 4 °C, dehydrated in ethanol, and embedded in a commercially available epoxy resin (TAAB Laboratories Equipment Ltd., Aldermaston, UK). Samples were transferred to fresh resin in capsules and polymerized for >48 h at 60 °C. Hitachi H-7000 and H-7100 TEM systems at 75 kV (Hitachi, Ltd.) were used to observe the intracellular distribution of MWCNTs and morphological changes in NR8383 cells.
2.7. RNA extraction and DNA microarray experiments
Due to suitable for assessment of gene expression, the concentration of MWCNTs in the working solutions for the DNA microarray experiment was set to the maximum concentration at which cell survival was observed. NR8383 cells incubated with the working solutions (n = 4; MW1–4: 100 µg/mL; MW5 and 6: 50 µg/mL) for 24 h were collected, and total RNA was extracted using RNeasy Mini Kit (QIAGEN K.K., Tokyo, Japan) according to the manufacturer’s instructions. The RNA was quantified using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific), and sample qualities were monitored using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Next, cyanine-3-labeled complementary RNA (cRNA) was prepared from RNA using a Low Input Quick Amp Labeling kit (Agilent Technologies) according to the manufacturer’s instructions, followed by RNeasy column purification (QIAGEN K.K.). Then, each labeled cRNA probe was used separately for hybridization to a 4 × 44 K G4131F Whole Rat Genome Microarray (Agilent Technologies) for 17 h at 65 °C. Hybridized microarray slides were washed according to the manufacturer’s instructions and scanned using a G2565BA DNA Microarray Scanner (Agilent Technologies) at a resolution of 5 μm. Finally, the scanned images were analyzed numerically using Agilent Feature Extraction ver. 12.0.3.1 (Agilent Technologies).
2.8. Microarray data analysis
Normalized data were analyzed using GeneSpring GX ver. 14.8 (Agilent Technologies). Log-fold-changes (FCs) represent the ratio of the normalized intensity of MWCNT-exposed samples to the normalized intensity of control samples. Genes with log FC values > 1 were considered up-regulated, while those with log FC values < −1 were considered down-regulated.
Gene expression profiling of NR8383 cells exposed to 100 μg/mL of MW1, MW2, MW3, and MW4 or 50 μg/mL of MW5 and MW6 for 24 h was performed by using DNA microarrays in order to compare each comprehensive biological response. We focused on expressed genes involved in inflammatory responses, response to oxidative stress and apoptosis, and extracellular matrix (ECM) degradation by exposure of MWCNTs in MW1–MW6 for 24 h. The gene expression profiles obtained for each experimental group were uploaded to the Gene Expression Omnibus database (accession no. GSE137008; http://www.ncbi.nlm.nih.gov/projects/geo/).
2.9. Bacterial reverse mutation tests
Bacterial reverse mutation tests were conducted according to the Organization for Economic Co-operation and Development (OECD) Guideline for Testing of Chemicals 471 ‘Bacterial Reverse Mutation Test’ (OECD Citation1997). Histidine-requiring ring Salmonella typhimurium strains TA98, TA100, TA1535, and TA1537, as well as tryptophan-Escherichia coli mutant WP2uvrA, were cultured in nutrient broth at 37 °C with shaking. AF-2; [2-(2-furyl)-3-(5-nitro-2-furyl) acrylamide], NaN3; [sodium azide], 9-AA; [9-aminoacridine hydrochloride], and 2-AA; [2-aminoanthraceme] were used for positive controls. The tests were performed after pre-incubation in the presence or absence of metabolic activation using a rat liver S9 fraction pretreated with enzyme-inducing agents, phenobarbital and 5,6-benzoflavone, and mixed with cofactor-I (Oriental Yeast Co. Ltd., Tokyo, Japan) to a final concentration of 10% (v/v) S9; 10 mg/mL of BSA was used as the vehicle control.
In a preliminary cytotoxicity test, to determine the appropriate concentration range, all strains were tested at concentrations of 1.56, 3.13, 6.25, 12.5, 25, and 50 µg of MWCNTs/plate. Based on the preliminary results, the main test was conducted at concentrations of 1.56, 3.13, 6.25, 12.5, 25, and 50 μg of MWCNTs/plate. In both tests, duplicate plates were used for each concentration. The results were judged positive if a twofold or larger increase was observed in the number of revertant colonies in the MW6-treated groups compared with the vehicle control, with a dose–response relationship.
2.10. In vitro mammalian chromosomal aberration tests
In vitro mammalian chromosomal aberration tests were conducted according to the OECD Guideline for Testing of Chemicals 473 ‘In vitro Mammalian Chromosomal Aberration Test’ (OECD Citation2016). The Chinese hamster lung fibroblast cell line CHL/IU was cultured in Eagle’s minimum essential medium (Life Technologies Japan Ltd.) containing 10% heat-inactivated newborn calf serum (Life Technologies Japan Ltd.). CHL/IU cells were incubated in a 5% CO2 atmosphere at 37 °C, and 10 mg/mL of BSA was used as the vehicle control.
A preliminary test was performed for cell growth inhibition at concentrations of 1.56, 3.13, 6.25, 12.5, 25, and 50 μg/mL of MWCNTs. Based on the results of the preliminary test, 12.5, 25, and 50 μg/mL were selected as concentrations of MWCNTs for the main study. Mitomycin C (Kyowa Kirin Co., Ltd., Tokyo, Japan) and cyclophosphamide (SHIONOGI & CO., LTD., Osaka, Japan) were used as positive controls. Duplicate plates were used for each concentration. The test was performed in the presence and absence of metabolic activation with the S9 mix. The experiments included short-term exposure (6 h, with and without metabolic activation) and continuous exposure (24 h, without metabolic activation). In both experiments, colcemid at a final concentration of 0.2 μg/mL (Life Technologies Japan Ltd.) was added 2 h before cell harvesting. Chromosomal preparations were air-dried and stained with 1.2% (v/v) Giemsa solution (Merck, KGaA, Germany) for 15 min at room temperature. Finally, 150 metaphases/slide (300 metaphases/dose) were examined for structural and numerical aberrations.
2.11. Mammalian erythrocyte micronucleus tests
Mammalian erythrocyte micronucleus tests were conducted according to the OECD Guideline for Testing of Chemicals 474 ‘Mammalian Erythrocyte Micronucleus Test’ (OECD Citation2014). Briefly, 9-week-old Crlj:CD1 (ICR) mice were purchased from Charles River Laboratories Japan (Yokohama, Japan) and divided into six experimental groups (n = 5 per group). The mice were housed in metal cages with 52 ± 10% humidity at a controlled temperature of 23 °C and fed a chow diet ad libitum. Since a preliminary test showed no clear gender differences in toxicity (data not shown), male mice were used. Three groups were intratracheally instilled with 0.125, 0.25, and 0.5 mg/kg of MWCNT stock suspensions daily for two consecutive days, corresponding to total doses of 0.25, 0.5, and 1.0 mg/kg, respectively. The fourth group was untreated, while the remaining two groups were treated with 1 mL/kg of BSA as a vehicle control and cyclophosphamide (CP: 50 mg/kg) as a positive control, respectively. The femurs were excised, and the bone marrow was flushed into test tubes. The percentage of micronucleated bone marrow polychromatic erythrocytes (MNPCEs) was calculated based on observations of 2000 polychromatic erythrocytes (PCEs) per mouse. The percentage of PCEs among all erythrocytes was determined by counting 500 erythrocytes per mouse.
All procedures and animal handling were approved by the Institutional Animal Care and Use Committee of the National Institute of Advanced Industrial Science and Technology, and the BioSafety Research Center Inc., Iwata, Japan.
2.12. Statistical analysis
Differences between the control and experimental groups were evaluated using the unequal variance Welch’s t-test, which is suitable regardless of whether the two analyzed groups have similar or dissimilar variance. All statistical analyses were conducted with a significance level of α = 0.05 (p < 0.05). Numerical values were represented as the mean ± standard deviation or mean ± standard error. The significance of differences between treated samples and untreated controls was determined by analysis of variance (ANOVA) using Dunnett’s or Steel’s tests for multiple comparisons. The significance of differences in the MNPCE frequency between the vehicle control and other groups was determined using the Kastenbaum–Bowman test. The percentage of PCEs among the total erythrocyte population was analyzed using Dunnett’s test. All statistical analyses were conducted with significance levels of α = 0.01 or 0.05 (p < 0.01 or p < 0.05).
3. Results and discussion
3.1. Characterization of MWCNTs
Characteristics of bulk MWCNTs and MWCNTs dispersed in stock suspension and in F-12K + FBS medium are shown in . shows TEM images of MWCNTs in stock suspensions MW1–MW6. MWCNTs in MW1–MW5 had a distribution of approximately equal length (0.37–0.67 μm). MWCNTs in MW6 were dispersed relatively with a long length comparatively. Consequently, MWCNTs were grouped into (i) rigid, needle-shaped MWCNTs (MW1 and MW2), (ii) bent MWCNTs (MW3 and MW4), and (iii) straight, long, thin MWCNTs (MW5 and MW6). CNTs usually form large aggregates in cell culture media containing large amounts of salts and proteins (Horie et al. Citation2012). In this study, MWCNTs with different physico-chemical properties in all stock suspensions were visually confirmed to be unaggregated after preparation (data not shown). In addition, the negative zeta potentials of MW1–MW5 revealed MWCNTs to be dispersed in the stock suspensions. The zeta potentials of MW6 were deemed inadequate on the basis of the MWCNT length. Some chemicals such as Pluronic F-127 or polyoxyethylene sorbitan monooleate (Tween80) are useful for CNT dispersants. However, induction of intracellular ROS level was observed in cells exposed to CNT dispersion including each reagent except BSA (Horie et al. Citation2013). Therefore, we believed that the method of sonication and centrifugation using BSA performed in this study can be sufficiently dispersed in cell culture medium even for MWCNTs differing in diameter and length, and MWCNTs dispersed in stock suspension could be used for in vitro cell-based assay and a battery of genotoxicity tests.
Figure 1. TEM images of MWCNTs dispersed in stock suspensions MW1–MW6 for in vitro cell-based assays and genotoxicity tests. TEM: transmission electron microscopy; MWCNT: multi-walled carbon nanotube.
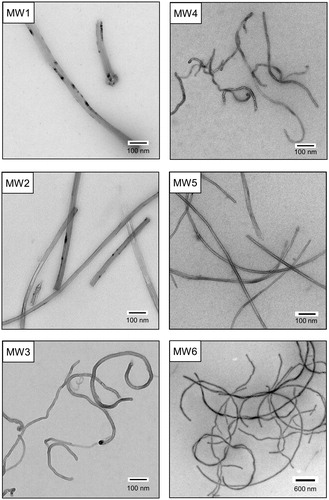
Table 1. Characteristics of bulk MWCNTs and MWCNTs dispersed in stock suspensions.
3.2. Cell survival, intracellular ROS, and pro-inflammatory cytokines
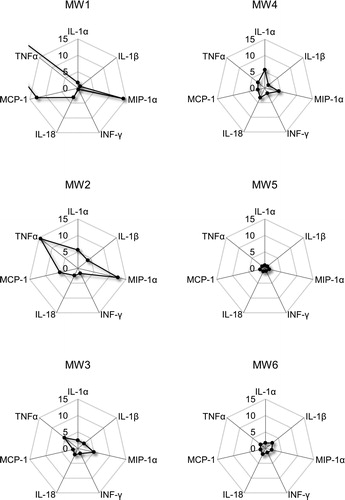
Cell viability significantly decreased after 24 h exposure to 100 µg/mL of MW1–MW3. However, there was no significant change in cell viability in 100 or 50 µg/mL of MW4–MW6 (). There were no significant changes in intracellular ROS generation after exposure to 100 μg/mL of MW1 and MW2, while intracellular ROS generation significantly increased after exposure to 100 or 50 μg/mL of MW3–MW6 (). These results show that MW1 and MW2 decrease cell survival without generating intracellular ROS. MW3 decrease cell survival along with intracellular ROS generation. MW4, MW5 and MW6 geneaused a slight intracellular ROS generation but no decrease in cell viability. The reason why ROS was not generated by MW1 and MW2 may be related to the cell uptake of MWCNT. This will be discussed from the results of the TEM observation as described below. Furthermore, the levels of pro-inflammatory cytokines IL-1α, IL-1β, MIP-1α, INF-γ, IL-18, MCP-1, and TNF-α were higher in NR8383 cells treated with MW1–MW4 and MW6 compared with control cells (). The levels of pro-inflammatory cytokines in NR8383 cells treated with MW1 and MW2 were especially high. Overall, these results suggest that MWCNTs with a large diameter (>50 µm) are strongly cytotoxic in NR8383. In addition, the results in cells exposed to MW5 or MW6 present that the length of the linear thin MWCNT has no significant cellular effect.
Figure 2. Effects of MWCNTs on NR8383 cell survival. After exposure to MWCNTs dispersed in cell culture medium for 24 h, NR8383 cell survival was measured using the WST-1 assay. The viability of MWCNTs-treated cells in the WST-1 assay are expressed as a percentage of untreated control cells. Values are mean ± SE of four individual experiments. *p < 0.05; **p < 0.01 (vs. control cells, Dunnett, and ANOVA). MWCNT: multi-walled carbon nanotube; WST-1: 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt; SE: standard error; ANOVA: analysis of variance.
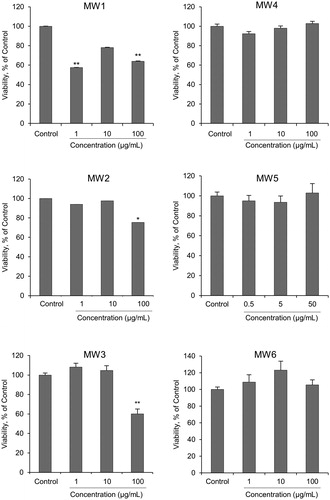
Figure 3. Intracellular ROS level in NR8383 cells exposed to MWCNTs dispersed in cell culture medium for 24 h. Intracellular ROS levels were measured using the DCFH assay and flow cytometry. DCFH fluorescence in NR8383 cells is expressed as a ratio compared to untreated control cells. Values are mean ± SE of four individual experiments. **p < 0.01 (vs. control cells, Steel, and Kruskal–Wallis). ROS: reactive oxygen species; MWCNT: multi-walled carbon nanotube; DCFH: 2′,7′-dichlorodihydrofluorescin; SE: standard error.
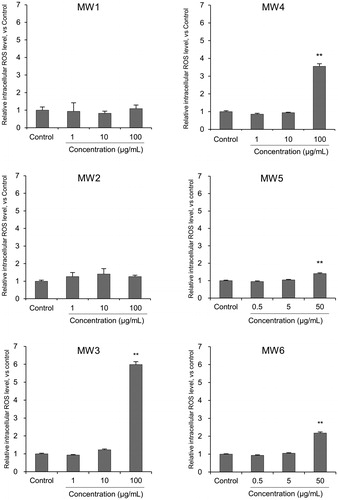
Figure 4. Cytokine levels in NR8383 cells exposed to MWCNTs for 24 h. Values are expressed as ratios compared to untreated control cells. The ratio of TNF-α in MW1 to the control was 47.5. MWCNT: multi-walled carbon nanotube; TNF-α: tumor necrosis factor alpha.
A recent literature reports on the relationship between MWCNT diameter and cytotoxicity. The study demonstrated that straight MWCNTs (maximum length = ∼4.0 µm; median diameter = ∼67 nm) were strongly toxic, while highly bent MWCNTs (maximum length = 1.3, 0.8, or 0.4 µm; median diameter = ∼11 nm) induced no measurable cytotoxicity in HL-60 cells (Westphal et al. Citation2019). These results correspond to those of our study on the cytotoxicity of MWCNTs with different diameters. On the other hand, another study showed that there was no difference in the activation of and cytotoxicity to murine macrophages. All types of MWCNT (thin: diameter = ∼50 nm; thick: diameter = ∼150 nm; tangled diameter = ∼ 2–20 nm) affected murine macrophages (RAW264.7) similarly in terms of cytotoxic effects (Nagai et al. Citation2011). Cell type must be considered to generalize the relationship between MWCNT diameter and cytotoxicity.
3.3. Cellular morphology and MWCNT uptake
TEM revealed several rigid, needle-shaped MWCNTs in the cytoplasm in NR8383 cells exposed to MW1 (A1 and A2) and MW2 (B1 and B2), maintaining their physical structure. On the other hand, numerous bent MWCNT aggregates were phagocytosed in the vacuole-like compartments of NR8383 cells exposed to MW3 (C1 and C2) and MW4 (D1 and D2). Many straight, thin MWCNTs were localized in the cytoplasm, vacuole-like compartments, and other organelles of NR8383 cells exposed to MW5 (E1 and E2). No MWCNTs were observed within the nucleus in any of the samples. These results suggested that bent or straight, thin MWCNTs were translocated to the phagosome, maintaining their physical structure. Furthermore, straight, long, thin MWCNTs were observed in the cytoplasm, vacuole-like compartments, and mitochondria and near the nuclei of NR8383 cells exposed to MW6 (F1 and F2; ). TEM observation demonstrated that differences in MWCNT uptake by NR8383 cells affect cell survival, intracellular ROS generation, and pro-inflammatory cytokine induction. In our previous study, relatively large carbon-based nanomaterials, exfoliated graphene (= ∼ 10 nm) were incorporated into cells and decreased cell survival without generating intracellular ROS in NR8383 (Fujita et al. Citation2018). NR8383 cells phagocytized SWCNTs (diameter = ∼ 3 nm) and generated intracellular ROS in a concentration-dependent manner (Fujita et al. Citation2015). Taken together, our findings suggest that rigid, needle-shaped MWCNTs with a large diameter (>50 µm) that are not phagocytosed may cause cell survival but not generate intracellular ROS in macrophages. Meanwhile, bent MWCNTs with a small diameter (<20 µm) that are phagocytosed in the vacuole-like compartments of cells may cause slight cell survival but generate intracellular ROS.
3.4. Gene expression profiling
The expression patterns of up- or down-regulated genes evaluated using heat map images were clustered into two major groups (): MW1 and MW2, and MW3–MW6. The gene expression profile for MW6 was closer to that for MW4 compared with MW5. shows representative up- or down-regulated genes. The result suggests that rigid, needle-shaped MWCNTs with a large diameter (MW1 and MW2) markedly elicit cellular responses meanwhile straight, thin MWCNTs (MW5) do not induce strong cellular responses. The expression of Arg1 and Nos2 was significantly up-regulated in all experimental groups. Interestingly, long, linear SWCNTs and exfoliated graphene up-regulated Arg1 and Nos2 in NR8383 cells (Fujita et al. Citation2015, Citation2018). These genes might be useful as biomarkers for exposure to nanomaterials such as MWCNTs in alveolar macrophages. Many significantly up-regulated genes (Arg1, Ccl2, Ccl3, Ccl4, Ccl7, Ccl9, Cxcl1, Cxcl2, Cxcl3, Il1rn, Itgam, Spp1, Nos2, Hmox1, Serpine1, Sod2, Lcn2, Mmp12, Ctsk, and Col15a1) were overlapped in MW1 and MW2 compared with MW3–MW6. In MW5, the exposure concentration and level were lower compared with MW1–MW4. No representative genes were involved in the response to oxidative stress and apoptosis, and ECM degradation was markedly up-regulated by MW5. These results were similar to those of a study on gene expression with short, linear SWCNTs in NR8383 cells (Fujita et al. Citation2015). Like SWCNTs, the cytotoxicity of straight, thin MWCNTs is closely related to their length.
Figure 6. Effects of MWCNTs on gene expression in NR8383 cells. The heat map generated from comprehensive DNA microarray data reflects differential expression of genes in NR8383 cells exposed to MW1–MW6 for 24 h. MWCNT: multi-walled carbon nanotube.
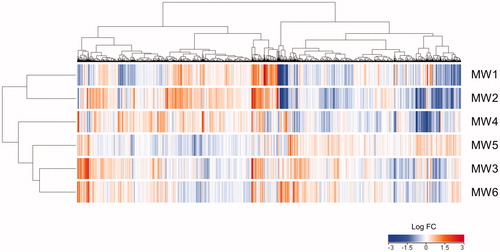
Table 2. Fold changes of selected expressed genes involved in inflammatory responses, response to oxidative stress and apoptosis, and extracellular matrix (ECM) degradation up-regulated in NR8383 cells by MWCNT exposure.
3.5. Genotoxicity tests
Some straight, long, thin MWCNTs were observed near the nuclei of NR8383 cells exposed to MW6 (). These results of intracellular MWCNT uptake showed that thin MWCNTs directly act on DNA. We therefore evaluated mutagenesis of MWCNTs in MW6 by using a battery of genotoxicity tests. In both − S9 and + S9 treatments of bacterial reverse mutation tests, twofold or greater increases in reverse mutation colonies were not observed compared with those of the vehicle control group. This confirmed the reproducibility of test results. Positive controls (MMC and CP) indicated clear mutation-inducing effects on respective test strains (Supplementary Figure 1).
Preliminary cytotoxicity tests for the in vitro mammalian chromosomal aberration assay demonstrated that no growth inhibition at concentrations of 1.56, 3.13, 6.25, 12.5, 25, and 50 µg/mL of MWCNTs following short-term (6 h) exposure with or without the S9 mix or continuous (24 h) exposure without the S9 mix. In the main test, MWCNTs did not increase the number of structural and numerical chromosomal aberrations at any concentration (12.5, 25, or 50 µg/mL) following short-term exposure with or without the S9 mix or continuous exposure without the S9 mix (Supplementary Figure 2). MMC and CP resulted in a marked increase in structural chromosomal aberrations, confirming that the test was performed appropriately.
In mammalian erythrocyte micronucleus tests, we found no statistically significant increase in the percentage of MNPCEs at any concentration of MWCNTs dispersed in MW6 compared with the negative control. In addition, we observed no statistically significant reduction in the PCE ratio. The MNPCE percentage in the negative control was within the standard value derived from background data, whereas the MNPCE percentage in the positive control significantly increased compared with the negative control (Supplementary Figure 3).
The same MWCNTs used in this study (MWNT-7, Nikkiso) were reported by Asakura et al. (Citation2010) and Ema et al. (Citation2012) to be negative for genotoxicity. Many other types of MWCNTs have also been reported to be negative for genotoxicity by individual genotoxicity tests (Szendi and Varga Citation2008; Di Sotto et al. Citation2009; Wirnitzer et al. Citation2009; Kim et al. Citation2011). For most nanomaterials, it is not clear whether they directly interact with DNA. We also do not know whether indirect effects, such as inflammation-mediated oxidative stress, might infer a threshold for the genotoxicity of some nanomaterials (Landsiedel et al. Citation2009). Excessive and persistent intracellular ROS generation from inflammatory cells is considered the hallmark of the secondary genotoxicity of nonfibrous and fibrous particles (Schins Citation2002). Therefore, the only practical approach is to use a battery of genotoxicity tests covering a wide range of mechanisms, as in this study. The results of a battery of genotoxicity tests concluded that MW6 exhibit no genotoxicity.
4. Conclusions
The physico-chemical properties of MWCNTs vary greatly depending on MWCNT manufacture and application. Therefore, it is difficult to generalize the degree of MWCNT cytotoxicity. The toxicity of CNTs depends on properties of the CNTs such as their structure, length and aspects ratios, surface area, degree of aggregation, extent of oxidation, bound functional group(s), method of manufacturing, concentration, and dose (Kayat et al. Citation2011). Another factor contributing to the toxicity of inhaled nanomaterials is the impairment of alveolar clearance by macrophages (Ma-Hock et al. Citation2013). Intracellular distribution is important to understand the mechanisms of the toxic effects of nanomaterials (Guo et al. Citation2011).
This study shows that cytotoxicity increased in the order of MW1, MW2 > MW3, MW4 > MW6 > MW5. In other words, rigid, needle-shaped MWCNT with a large diameter (>50 µm), which is an obstacle to cellular uptake, has the greater cytotoxicity, and MWCNT with a small diameter (<20 µm) to be taken up is lower. These results suggest that the diameter of MWCNTs is a crucial parameter for their cytotoxicity, and consequently, contribute inhalation toxicity via alveolar clearance.
In vivo animal studies are necessary to elucidate inhalation toxicity of individual MWCNTs, although there are concerns about the inhalation effects of MWCNTs and for reasons of high cost and animal welfare. In vitro models cultured at air–liquid interface of different levels of the lung or a three-dimensional tissue models of the human airway have been advancing (Upadhyay and Palmberg Citation2018; Jackson et al. Citation2018). As these technologies progress, the results of in vitro cell-based assays are expected to be used for stratifying the inhalation toxicity of MWCNTs.
Supplemental Material
Download PDF (36.2 KB)Supplemental Material
Download PDF (28.6 KB)Supplemental Material
Download PDF (152.5 KB)Acknowledgments
The authors thank Ms. Emiko Kobayashi for her help with the TEM analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Asakura M, Sasaki T, Sugiyama T, Takaya M, Koda S, Nagano K, Arito H, Fukushima S. 2010. Genotoxicity and cytotoxicity of multi-wall carbon nanotubes in cultured Chinese hamster lung cells in comparison with chrysotile A fibers. Jrnl of Occup Health. 52:155–166.
- Di Sotto A, Chiaretti M, Carru GA, Bellucci S, Mazzanti G. 2009. Multi-walled carbon nanotubes: Lack of mutagenic activity in the bacterial reverse mutation assay. Toxicol Lett. 184:192–197.
- Ema M, Imamura T, Suzuki H, Kobayashi N, Naya M, Nakanishi J. 2012. Evaluation of genotoxicity of multi-walled carbon nanotubes in a battery of in vitro and in vivo assays. Regul Toxicol Pharmacol. 63:188–195.
- Fujita K, Endoh S, Maru J, Okazaki T, Tajima N, Shinohara N, Uchino K, Obara S, Morimoto Y, Izumi H. 2017. General procedures for safety tests on carbon nanomaterials. https://en.aist-riss.jp/assessment/6226/
- Fujita K, Fukuda M, Endoh S, Kato H, Maru J, Nakamura A, Uchino K, Shinohara N, Obara S, Nagano R, et al. 2013. Physical properties of single-wall carbon nanotubes in cell culture and their dispersal due to alveolar epithelial cell response. Toxicol Mech Methods. 23:598–609.
- Fujita K, Fukuda M, Endoh S, Maru J, Kato H, Nakamura A, Shinohara N, Uchino K, Honda K. 2016. Pulmonary and pleural inflammation after intratracheal instillation of short single-walled and multi-walled carbon nanotubes. Toxicol Lett. 257:23–37.
- Fujita K, Fukuda M, Endoh S, Maru J, Kato H, Nakamura A, Shinohara N, Uchino K, Honda K. 2015. Size effects of single-walled carbon nanotubes on in vivo and in vitro pulmonary toxicity. Inhal Toxicol. 27:207–223.
- Fujita K, Take S, Tani R, Maru J, Obara S, Endoh S. 2018. Assessment of cytotoxicity and mutagenicity of exfoliated graphene. Toxicol In Vitro. 52:195–202.
- Gaté L, Knudsen KB, Seidel C, Berthing T, Chézeau L, Jacobsen NR, Valentino S, Wallin H, Bau S, Wolff H, et al. 2019. Pulmonary toxicity of two different multi-walled carbon nanotubes in rat: Comparison between intratracheal instillation and inhalation exposure. Toxicol Appl Pharmacol. 375:17–31.
- Grosse Y, Loomis D, Guyton KZ, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Scoccianti C, Mattock H, et al. 2014. Carcinogenicity of fluoro-edenite, silicon carbide fibres and whiskers, and carbon nanotubes. Lancet Oncol. 15:1427–1428.,
- Guo YY, Zhang J, Zheng YF, Yang J, Zhu XQ. 2011. Cytotoxic and genotoxic effects of multi-wall carbon nanotubes on human umbilical vein endothelial cells in vitro. Mutat Res. 721:184–191.
- Horie M, Fujita K. 2011. Chapter four. Toxicity of metal oxides nanoparticles. In: James CF, editor. Advances in molecular toxicology, vol. 5. Oxford: Elsevier; p. 145–178.
- Horie M, Kato H, Fujita K, Endoh S, Iwahashi H. 2012. In vitro evaluation of cellular response induced by manufactured nanoparticles. Chem Res Toxicol. 25:605–619.
- Horie M, Stowe M, Tabei M, Kato H, Nakamura A, Endoh S, Morimoto Y, Fujita K. 2013. Dispersant affects the cellular influences of single-wall carbon nanotube: the role of CNT as carrier of dispersants. Toxicol Mech Methods. 23:315–322.
- Jackson GR Jr, Maione AG, Klausner M, Hayden PJ. 2018. Prevalidation of an acute inhalation toxicity test using the EpiAirway in vitro human airway model. Appl In Vitro Toxicol. 4:149–158.
- Kasai T, Umeda Y, Ohnishi M, Mine T, Kondo H, Takeuchi T, Matsumoto M, Fukushima S. 2016. Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats. Part Fibre Toxicol. 13:53.
- Kayat J, Gajbhiye V, Tekade RK, Jain NK. 2011. Pulmonary toxicity of carbon nanotubes: a systematic report. Nanomedicine. 7:40–49.
- Kim JS, Lee K, Lee YH, Cho HS, Kim KH, Choi KH, Lee SH, Song KS, Kang CS, Yu IJ. 2011. Aspect ratio has no effect on genotoxicity of multi-wall carbon nanotubes. Arch Toxicol. 85:775–786.
- Kobayashi N, Naya M, Ema M, Endoh S, Maru J, Mizuno K, Nakanishi J. 2010. Biological response and morphological assessment of individually dispersed multi-wall carbon nanotubes in the lung after intratracheal instillation in rats. Toxicology. 276:143–153.
- Landsiedel R, Kapp MD, Schulz M, Wiench K, Oesch F. 2009. Genotoxicity investigations on nanomaterials: Methods, preparation and characterization of test material, potential artifacts and limitations—many questions, some answers. Mutat Res. 681:241–258.
- Ma-Hock L, Strauss V, Treumann S, Küttler K, Wohlleben W, Hofmann T, Gröters S, Wiench K, van Ravenzwaay B, Landsiedel R. 2013. Comparative inhalation toxicity of multi-wall carbon nanotubes, graphene, graphite nanoplatelets and low surface carbon black. Part Fibre Toxicol. 10:23.
- Morimoto Y, Hirohashi M, Ogami A, Oyabu T, Myojo T, Todoroki M, Yamamoto M, Hashiba M, Mizuguchi Y, Lee BW, et al. 2012. Pulmonary toxicity of well-dispersed multi-wall carbon nanotubes following inhalation and intratracheal instillation. Nanotoxicology. 6:587–599.,
- Nagai H, Okazaki Y, Chew SH, Misawa N, Yamashita Y, Akatsuka S, Ishihara T, Yamashita K, Yoshikawa Y, Yasui H, et al. 2011. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc Natl Acad Sci U S A. 6:1330–1338.
- OECD. 1997. Test No. 471: Bacterial Reverse Mutation Test. OECD Publishing.
- OECD. 2014., Test No. 474: Mammalian Erythrocyte Micronucleus Test. OECD Publishing.
- OECD. 2016. Test No. 473: In vitro Mammalian Chromosomal Aberration Test. OECD Publishing.
- Poulsen SS, Saber AT, Williams A, Andersen O, Købler C, Atluri R, Pozzebon ME, Mucelli SP, Simion M, Rickerby D, et al. 2015. MWCNTs of different physicochemical properties cause similar inflammatory responses, but differences in transcriptional and histological markers of fibrosis in mouse lungs. Toxicol Appl Pharmacol. 284:16–32.,
- Rydman EM, Ilves M, Koivisto AJ, Kinaret PAS, Fortino V, Savinko TS, Lehto MT, Pulkkinen V, Vippola M, Hämeri KJ, et al. 2014. Inhalation of rod-like carbon nanotubes causes unconventional allergic airway inflammation. Part Fibre Toxicol. 11:48.
- Schins RP. 2002. Mechanisms of genotoxicity of particles and fibers. Inhal Toxicol. 14:57–78.
- Szendi K, Varga C. 2008. Lack of genotoxicity of carbon nanotubes in a pilot study. Anticancer Res. 28:349–352.
- Upadhyay S, Palmberg L. 2018. Air-liquid interface: relevant in vitro models for investigating air pollutant-induced pulmonary toxicity. Toxicol Sci. 164:21–30.
- Westphal GA, Rosenkranz N, Brik A, Weber D, Föhring I, Monsé C, Kaiser N, Hellack B, Mattenklott M, Brüning T, et al. 2019. Multi-walled carbon nanotubes induce stronger migration of inflammatory cells in vitro than asbestos or granular particles but a similar pattern of inflammatory mediators. Toxicol In Vitro. 58:215–223.
- Wirnitzer U, Herbold B, Voetz M, Ragot J. 2009. Studies on the in vitro genotoxicity of baytubes®, agglomerates of engineered multi-walled carbon-nanotubes (MWCNT). Toxicol Lett. 186:160–165.
- Xu J, Alexander DB, Futakuchi M, Numano T, Fukamachi K, Suzui M, Omori T, Kanno J, Hirose A, Tsuda H. 2014. Size- and shape-dependent pleural translocation, deposition, fibrogenesis, and mesothelial proliferation by multiwalled carbon nanotubes. Cancer Sci. 105:763–769.