Abstract
Tungsten has no known function in humans and is a relatively new contaminant, whereas molybdenum, its congener in the periodic table, is a nutritionally essential element. In addition to early studies on molybdosis in ruminants, their toxic effects in the form of tungstate and molybdate have been addressed primarily in rodents and are predominantly mediated by inducing oxidative stress in various tissues. The purpose of this study was to evaluate the differences between tungstate and molybdate in human liver (HepG2) and kidney (HEK293) cell lines in terms of retention in cells, effect on reactive oxygen species, and activities of xanthine oxidase and phosphatases. The cell lines were exposed to tungstate or molybdate (1 µM to 10 mM) for 24 h, lysed and analyzed for the above biochemical parameters. Despite the chemical similarity of the two anions, cell-specific differential effects were observed. At all concentrations, tungstate was retained more in HEK293 cells while molybdate was retained more in HepG2 cells. HepG2 cells were more sensitive to tungstate than molybdate, showing reduced viability at concentrations as low as 10 µM. Exposure to either anion resulted in the inhibition of protein tyrosine phosphatases at 1 mM and an increased production of reactive oxygen species (ROS) at 100 µM despite their inhibition of the ROS-producing molybdenum enzyme xanthine oxidase. In conclusion, the results indicate that excess of nutritionally essential molybdate or non-essential tungstate causes toxicity by affecting ROS- and phosphorylation-dependent signaling pathways and ensuing gene expression.
1. Introduction
Molybdenum and tungsten are in the same group of the periodic system of the elements as chromium and have essential functions in life, though tungsten only in archaebacteria and some other bacteria. Molybdenum and tungsten enzymes have common structural features and contain the pyranopterin cofactor, called molybdopterin/tungstopterin in the metal bound form (Tejada-Jiménez and Schwarz Citation2014; Cordas and Moura 2019; Kirk and Kc Citation2020). In humans, molybdenum is required for only four types of enzymes, sulfite oxidase, xanthine oxidase, aldehyde oxidase and mitochondrial aldoxime reducing enzyme. A 70 kg human has about five milligram molybdenum, which together with three milligram cobalt, are the lowest amounts of any essential trace element. With only 20 µg in the human body tungsten is orders of magnitude lower (Emsley Citation2011). Tungsten is being increasingly used in products such as mobile phones, computers, light bulbs, jewelry, ammunition, and implants (Idil and Donaldson Citation2018) The United States Department of Defense and the Environmental Protection Agency classified tungsten as an ‘emerging contaminant’ of concern (EPA Citation2008). In Fallon, Nevada, and other western US towns exposed to tungsten via drinking water, an association with a high incidence of childhood leukemia was noted (Rubin et al. Citation2007). Increased tungsten concentrations occur in drinking water in some areas as, for example, in the Mount Etna volcanic area where it exceeds the normal range in the urine of 27% of the inhabitants (Gianì et al. Citation2019). In a murine co-culture model of the pre/pro B-cell line BU-11 and the bone marrow stromal cell line BMS2, exposure to tungstate induces DNA damage and apoptosis of the developing lymphocytes (Guilbert et al. Citation2011). Similar findings were made with mice in vivo and the conclusion was essentially recapitulated (Kelly et al. Citation2013; Bolt et al. Citation2016).
The deleterious health effects of tungsten often originate through water and soil contamination (Datta et al. Citation2017; Zoroddu et al. Citation2018). In the soil, metallic tungsten is oxidized to soluble tungsten compounds that enter the food web and reach the drinking water supply. Vegetables can have levels of 0.2–16 mg/kg tungsten, leading to exposure through the diet (ATSDR 2005). Soluble tungsten compounds such as tungstate are readily absorbed in the intestine and distributed to tissues, in particular to bone and kidneys (Weber et al. Citation2008). While tungsten taken up through ingestion or inhalation is usually rapidly excreted, there is some retention in organs such as the liver and kidney and accumulation in the bone (Leggett Citation1997; Kelly et al. Citation2013). Administration of oral doses of 10 mg/kg over two weeks in rats resulted in significant accumulation in plasma (0.09 μg/g), intestine (0.64), liver (0.08), kidneys (1.18), femur (5.90) and uterus (0.15) (Weber et al. Citation2008).
Sodium tungstate induces oxidative stress in various tissues/organs. Intraperitoneal injection of 20 mg (41 mg/kg/d) was more toxic than oral gavage of 119 mg (238 mg/kg/d) given for two weeks (Sachdeva et al. Citation2013). Remarkably, the administration of antioxidants such as N-acetylcysteine and some flavonoids ameliorated the oxidative stress induced by sodium tungstate (Sachdeva and Flora Citation2014). N-acetylcysteine also reversed some of the oxidative stress-dependent neurotoxicity of tungstate (Sachdeva et al. Citation2015). Administration of tungstate in drinking water increased its concentrations in the blood and in tissues and elicited hematological changes, histological changes in the liver, kidneys and spleen, and oxidative stress-triggered apoptosis (Sachdeva et al. Citation2020). However, mechanistic insights into the health risk of tungsten remain elusive (Datta et al. Citation2017).
We followed up these observations made in rodents with investigations in cultured human liver and kidney cell lines. In particular, we compared tungstate and molybdate to arrive at an understanding of the molecular mechanisms of toxicity related to the formation of reactive oxygen species and effects on signal transduction pathways. Despite the chemical similarities of molybdate and tungstate, we found significant differences between the two anions in the cell lines in terms of retention in cells, effects on energy metabolism, and phosphatase activity.
2. Materials and methods
2.1. Materials
Molecular biology-grade Hepes, ethylenediaminetetraacetic acid (EDTA), tris(2-carboxyethyl) phosphine hydrochloride (TCEP), nitrilotriacetic acid (NTA), sodium tungstate, sodium molybdate, 2′,7′-dichloro-dihydro-fluorescein diacetate (DCFH-DA), xanthine oxidase and Triton X-100 were from Sigma-Aldrich; 6,8-difluoro-4-methylumbelliferyl phosphate (DiFMUP) and 6,8-difluoro-7-hydroxy-4-methylcoumarin (DiFMU) were from Invitrogen (Carlsbad, CA). Recombinant human PTP1B, residues 1–299, was from Millipore, and supplied in 50 mM Hepes, pH 7.2, 1 mM DTT, 1 mM EDTA, and 0.05% (v/v) NP-40. Cell culture medium (Dulbecco's Modified Eagle's medium, DMEM), fetal bovine serum (FBS) and antibiotics (penicillin and streptomycin) were from Gibco (Invitrogen). All other chemicals were analytical grade.
2.2. Cell culture
Human hepatoma HepG2 cells were cultured in DMEM, high glucose, GlutaMAXTM supplement, 10% (v/v) heat-inactivated FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Human embryonic kidney HEK293 cells were cultured in the same full medium. Both cells were maintained at 5% CO2 and 37 °C in a humidified atmosphere and passaged regularly by using trypsin/EDTA when confluence of 90% was reached.
2.3. Cell viability
The effects of tungstate or molybdate on cell metabolic activity was determined with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, which is an indicator of cell viability, proliferation, and cytotoxicity. Briefly, the cells were incubated in medium in a 96-well plate and then treated with tungstate or molybdate for 24 h at 37 °C. MTT solution was added to each well and the cells incubated at 37 °C for two hours. Absorbance readings were taken at 570 nm using a 96-well multiscanner (Synergy HT, BioTek, Winooski, VT).
2.4. Cellular extracts
HEK293 and HepG2 cells were washed with PBS twice and harvested by treatment with 0.25% (v/v) trypsin/EDTA solution. Medium was added and the cell suspension spun at 500×g for 5 min, 4 °C. Cells were then washed with phosphate-buffered saline (PBS), spun at 500×g for 5 min, 4 °C, resuspended in 1 mL PBS supplemented with 0.1% (v/v) Triton X-100, and kept on ice for up to 10 min. Cell lysates were harvested by centrifugation at 12,000×g for 30 min, 4 °C.
2.5. Protein quantification
Protein concentrations were determined spectrophotometrically (Synergy HT plate reader) using bicinchoninic acid (BCA) and bovine serum albumin as a standard.
2.6. Phosphatase assay
HepG2 and HEK293 cells were treated with different concentrations of tungstate and molybdate (10, 100, and 1000 µM) at 37 °C for one hour. After incubation, cells were washed with PBS, lysed as described under ‘cellular extracts’ and the supernatant used for the phosphatase assays. Aliquots in a total of 100 μL PBS were added into 96-well black optical bottom plates (Greiner Bio-One Ltd, Stonehouse, UK) containing 3 µM DiFMUP. The substrate DiFMUP generates DiFMU after the enzymatic removal of the phosphate group. DiFMU fluoresces at 460 nm when excited at 360 nm. Plates were read on a Synergy HT plate reader for 30 min, 25 °C. Phosphatase specific activities are expressed in nmoles/µg/min and calculated from the fluorescence readings of a standard line established with the product DiFMU.
2.7. Protein tyrosine phosphatase 1B (PTP1B) assay
Enzymatic activity of PTP1B was assayed fluorimetrically at 25 °C in a freshly prepared buffer containing 50 mM Hepes/Na+, pH 7.4, 0.1 mM TCEP, 1 mM NTA and 0.01% (v/v) Triton X-100 and with different concentrations of tungstate and molybdate. The enzyme was added to the buffer at a final concentration of 2.5 nM. The reaction was initiated by adding the fluorogenic phosphatase substrate (3 µM DiFMUP) and assayed as described under ‘Phosphatase assay’. PTP1B specific acitivity is expressed as nmoles/µg/min (Bellomo et al. Citation2014).
2.8. Xanthine oxidase (XO) assay
XO was assayed with a XO Assay Kit (Abcam; ab102522, Cambridge, UK). In this assay, XO oxidizes xanthine to uric acid and hydrogen peroxide (H2O2), which reacts stoichiometrically with the OxiRed Probe to generate a chromophore (at 570 nm) and fluorophore (at Ex/Em = 535/587 nm). Since the absorbance or fluorescence intensity is proportional to the concentration of XO, XO activity can be accurately measured. The kit detects 1-100 mU XO in a 100 µl reaction volume in the fluorescence assay (Synergy HT plate reader). XO activity is expressed as relative fluorescent units compared to the control (100%).
2.9. Inductively coupled plasma mass spectrometry (ICP-MS)
ICP-MS analysis of metals was performed on all components of the biochemical assays with a Perkin Elmer Life Science, model NexION 350 D with a Cetac ASX520 autosampler and Perkin Elmer’s Syngistix software. The instrument settings were in the standard mode: Argon flows – main: 18 L/min, auxiliary: 1.2 L/min, and nebulizer: 0.95 L/min; RF power 1600 W; liquid flow-rate: 0.25 ml/min; 184W and 95Mo isotopes were measured with a dwell time of 50 ms each; five replicates per reading (50 sweeps per replicate) with the mean of the replicates reported. Samples were prepared in 5% (v/v) HNO3-washed polypropylene tubes (Elkay, Basingstoke, UK). Cells were washed three times with PBS to remove any medium or extracellular metal ions before digestion with nitric acid/hydrogen peroxide (1:1, v/v). Ultrapure water was from an ELGA LabWater UK system maintained with strict control of the capacity of the cartridges.
2.10. Measurement of reactive oxygen species (ROS)
ROS was assayed with the fluorescent probe 2′,7′-dichloro-dihydro-fluorescein diacetate (DCFH-DA). The dye diffuses through the cell membrane and is hydrolyzed by intracellular esterases to non-fluorescent DCFH, which is oxidized to fluorescent dichlorofluorescein (DCF) in the presence of ROS. Tungstate or molybdate were added at different concentrations together with DCFH-DA at a final concentration of 5 µM and incubated at 37 °C for up to one hour. The fluorescence intensity was measured at different time intervals with Ex/Em = 485/530 nm (Synergy HT plate reader).
2.11. Data analysis and statistics
Data were analyzed with Microsoft Excel 2010. Data represent the mean percentage ± SD of three independent experiments. Statistical analysis was performed for each exposure condition compared with control (Dunnett test, *p < 0.01) using InStat (GraphPad Software).
3. Results
3.1. Tungstate or molybdate affect metabolic viability in a dose- and time-dependent way in HEK293 and HepG2 cell lines
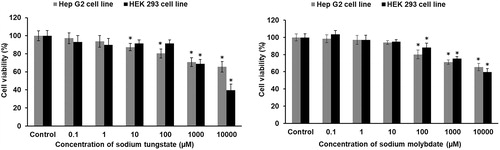
Human embryonic kidney (HEK293) and human hepatoma liver (HepG2) cells were treated with different concentrations of either tungstate or molybdate from 0.1 µM to 10 mM for 24 hours and viability determined by measuring mitochondrial dehydrogenase activity with the MTT assay. A dose-dependent decrease in cell viability was noted with both metal anions in the range of 1–10 mM (). No further decrease in cell viability was observed after 48 hours. Tungstate affected the two cell lines differently (, left panel), whereas the effect of molybdate on both cell lines was the same at concentrations >100 µM (, right panel). The liver cells were more sensitive to tungstate by two orders of magnitude in concentrations. For HepG2 cells, tungstate decreased viability at concentrations >10 µM (lower than molybdate) while for HEK293 cells a loss of viability became pronounced only at concentrations >1 mM (higher than molybdate).
Figure 1. Cytotoxicity of tungstate and molybdate in HEK293 and HepG2 cells. Cells were treated with concentrations ranging from 0.1 μM to 10 mM of either tungstate (left panel) or molybdate (right panel) for 24 hours and cytotoxicity assessed with the MTT assay. The percentage of cell viability was calculated with regard to the untreated control at 100% cell viability. Each treatment group was compared with the corresponding control. * p < 0.01, statistically significant difference (n = 3).
3.2. Tungsten or molybdenum concentrations in HEK293 and HepG2 cells after exposure to tungstate or molybdate
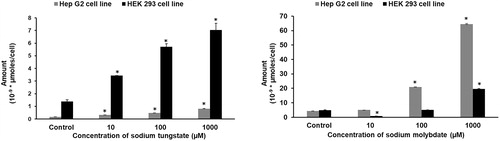
To determine how much tungstate or molybdate the cells retain, cellular tungsten and molybdenum concentrations were measured after continuous exposure for 24 hours (). Based on the results from the MTT assays, three concentrations (10 and 100 μM, and 1 mM) were chosen for the exposure. Retention of the anions was dose-dependent in both cell lines. Again, there is a significant difference: HEK293 cells retain more tungstate (, left panel) while HepG2 cells retain more molybdate (, right panel). The retention of molybdate in both cell lines is similar in magnitude but there is a 10-fold difference in the retention of tungstate.
Figure 2. Tungstate and molybdate uptake in HEK293 and HepG2 cells. Cells were treated with concentrations ranging from 10 μM to 1 mM of either tungstate (left panel) or molybdate (right panel) for 24 hours and the cellular metal concentrations determined by ICP-MS. * p < 0.01, statistically significant difference (n = 3).
3.3. Generation of reactive oxygen species (ROS) upon exposing HEK293 and HepG2 cells to tungstate or molybdate
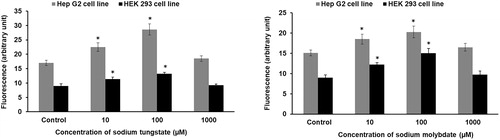
ROS production of the cell lines treated with tungstate or molybdate for one hour was assayed by fluorimetry with DCFH-DA. A significant increase in ROS levels was detected after exposure to 10 and 100 µM tungstate (, left panel) or molybdate (, right panel) in both cell lines. At the highest dose (1 mM), no further increase in ROS generation compared to control was observed.
Figure 3. Tungstate and molybdate generate reactive oxygen species in HEK293 and HepG2 cells. Cells were treated with concentrations ranging from 10 μM to 1 mM of either tungstate (left panel) or molybdate (right panel) for one hour and ROS determined with DCFH-DA. * p < 0.01, statistically significant difference (n = 3).
3.4. Effect of tungstate or molybdate on the activity of xanthine oxidase in HEK293 and HepG2 cells
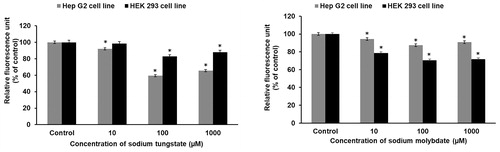
XO is a molybdenum enzyme and a significant source of cellular ROS. Exposure of the cell lines to tungstate (, left panel) or molybdate (, right panel) for one hour inhibited XO activity, indicating that the enzyme is not the source of the ROS observed upon exposure to either metal anion.
Figure 4. Tungstate and molybdate inhibit xanthine oxidase activity in HEK293 and HepG2 cells. Cells were treated with concentrations ranging from 10 μM to 1 mM of either tungstate (left panel) or molybdate (right panel) for one hour and xanthine oxidase assayed in cell lysates. The protein concentrations in the HepG2 and HEK293 lysates were 1.4 and 1.5 mg/ml, respectively. * p < 0.01, statistically significant difference (n = 3).
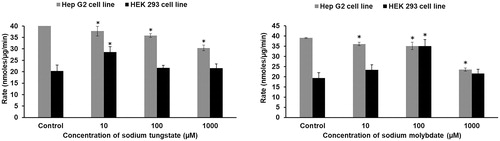
3.5. Effect of tungstate or molybdate on the activity of phosphatases in HEK293 and HepG2 cells
Phosphatase activity was analyzed to determine whether tungstate (, left panel) or molybdate (, right panel) affects cellular phosphorylation signaling pathways. Opposite effects were observed in the two cell lines. In HepG2 cells, tungstate or molybdate inhibited phosphatase activity in a dose-dependent way. In HEK293 cells, however, phosphatase activity increased at 10 µM tungstate or 100 µM molybdate.
Figure 5. Effect of tungstate and molybdate on phosphatase activity in HEK293 and HepG2 cells. Cells were treated with concentrations ranging from 10 μM to 1 mM of either tungstate (left panel) or molybdate (right panel) for one hour and phosphatase activity determined in cell lysates. The protein concentrations in HEK293 and HepG2 lysates were 2.3 and 2.1 mg/ml, respectively, in the phosphatase assays. * p < 0.01, statistically significant difference (n = 3).
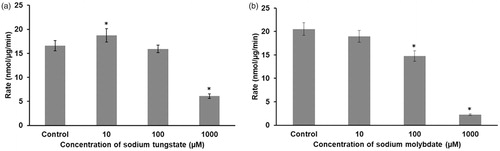
3.6. Effect of tungstate or molybdate on the activity of PTP-1B
Oxyanions such as vanadate, molybdate or tungstate are known protein tyrosine phosphatase inhibitors. Therefore, we examined whether incubation with tungstate or molybdate affects the activity of isolated human PTP-1B (). Both metal anions had similar inhibitory effects on the enzyme.
Figure 6. Tungstate or molybdate inhibit PTP-1B activity. The enzyme was incubated with concentrations ranging from 10 μM to 1 mM of either tungstate (a) or molybdate (b) and the enzymatic activity determined spectrofluorimetrically. The protein concentration was 2.5 nM and the substrate concentration was 3 µM. * p < 0.01, statistically significant difference (n = 3).
4. Discussion
Tungsten and molybdenum are used in several industrial and military applications throughout the world. While molybdenum is an essential micronutrient for humans, tungsten is non-essential. Tungsten toxicity has received attention lately because various anthropogenic activities expose humans to this otherwise very rare element in the air, in drinking water, and in the diet, for example, through produce that is grown on contaminated soil, or even through implants (Witten et al. Citation2012; Lemus and Venezia Citation2015; Bolt and Mann Citation2016; Idil and Donaldson Citation2018; Wasel and Freeman Citation2018; Furberg et al. Citation2019). A significant part of the exposure is through alloys with cobalt and nickel, posing additional issues of the toxicity of mixtures, and through nanoparticles (Chinde et al. Citation2017; Chinde and Grover, Citation2017; George et al. Citation2019; Božinović et al. Citation2020). Our investigations have been focused on the effects of tungstate and molybdate in the two human cell lines HEK293 (kidney) and HepG2 (liver). In this context, we selected a wide range of concentrations (1 to 10,000 µM) to reflect nutritional, supranutritional (pharmacologic) and toxic exposures. Tungsten exposure from dietary sources is expected to generate levels of ≤100 µM in the liver and kidneys (Johnson et al. Citation2010; Osterburg et al. Citation2010; Sachdeva et al. Citation2020). Concentrations as high as 68 µM and 119 µM in the liver and kidneys, respectively, were observed after administration of a single dose of 100 mg/kg (∼0.1 LD50) of tungstate to rats (McDonald et al. Citation2007). Concentrations between 3 and 13 µM in the liver and kidneys of rats were observed after a long-term exposure (120 days) to drinking water spiked with 50 to 200 ppm of sodium tungstate (Sachdeva et al. Citation2020). Thus, the observed effects at lower doses in our cell culture experiments are relevant to exposures in the diet, while the higher doses correspond to toxic exposures near the LD50 for tungsten.
Molybdate and tungstate are very similar structurally though there are some differences in their chemical reactivity (Hagen Citation2011). This molecular mimicry extends to other oxyanions of either metals such as chromate or nonmetals such as phosphate and sulfate (Bridges and Zalups Citation2010). In view of this similarity, it is remarkable that our investigations revealed differences in cellular retention and functions between the two anions and differential effects in the liver and kidney cell lines.
The 10-fold higher concentrations of tungstate in HEK293 cells in comparison to HepG2 cells () are in line with the published pharmacokinetic results of tungstate in rats. After administration of 10 mg/g tungstate to rats, 15-fold higher concentrations of tungstate in the kidneys (1.18 µg/g) were observed in comparison to the concentrations in the liver (0.08 µg/g) (Weber et al. Citation2008). Kidney cells seem to be adapted to this higher retention as they are much more resilient to the inhibitory effects of tungstate on energy metabolism (). There is a specific transporter for uptake of molybdate. The human molybdate transporter (gene product of MFSD5, major facilitator superfamily domain-containing protein 5) is expressed ubiquitously in human cells (https://ebi10.uniprot.org/uniprot/Q6N075#expression). When expressed in Saccharomyces cerevisiae, a Km value of 550 nM for molybdate was determined (Tejada-Jiménez et al. Citation2011; Tejada-Jiménez and Schwarz Citation2014). Its transport properties for tungstate are unknown. Hence, it is not clear how and whether the human molybdate transporter discriminates between the two anions.
Our results show differences in cellular retention of these anions, but the reason remains unknown. Employing a FRET-based molybdate sensor, oxalate-sensitive transporters were implicated in molybdate uptake in HEK293T cells with responses in the 0.3–30 µM range (Nakanishi et al. Citation2013). The molybdate concentrations in cells have been given as 0.5–10 µM, while serum concentrations are in the range 10-500 nM (Nakanishi et al. Citation2013). In the absence of specific exposure, the tungsten concentrations are about two orders of magnitude lower.
Cell viability after exposure to tungstate or molybdate was assessed with the MTT assay, which while often used for determining cytotoxicity actually measures mitochondrial metabolic activity and indicates proliferative capacity (Dunnick et al. Citation2014). Decreased viability in both cell lines at the higher doses of the oxyanions may be due to the formation of damaging ROS as detected with the DCFH-DA assay. ROS-induced damage results in cytotoxicity through mitochondrial damage and promotion of apoptosis (Jeng and Swanson Citation2006). The observations are in agreement with previous investigations of one of us (SS) and other investigations in duck renal tubular epithelial cells demonstrating the induction of oxidative stress by tungstate and molybdate in various organs such as the kidneys and the liver, overwhelming the cellular antioxidant defense (Sachdeva et al. Citation2013; Shi et al. Citation2017; Wang et al. Citation2020; Zhang et al. Citation2021).
The molecular pathways involved in the effects of increased cellular concentrations of molybdate or tungstate involve redox and phosphorylation signaling (Wu et al. Citation2019). We find that both anions inhibit XO, indicating that there is less hydrogen peroxide/superoxide formed (). Thus, the molybdoenzyme XO is not the source of the reactive species upon exposure. The XO-catalyzed reaction forms urate, which is a significant cellular antioxidant (Ames et al. Citation1981). This finding implies that a metabolic consequence of XO inhibition could be depriving cells of an important antioxidant. There is rather limited information about molybdenum toxicity in nonruminant species (Novotny Citation2011). In only three cases of environmental and occupational exposure, an increase and not a decrease of uric acid with gout-like arthritic symptoms has been observed. Our results from the MTT and DCFH-DA assays suggest that tungstate elicits mitochondrial dysfunction by inhibiting the mitochondrial respiratory chain, thus enhancing the production of ROS, with higher susceptibility to cell death in the kidney compared to the liver cell line. The effect of tungstate on the two cell lines is consistent with its effect in eliciting oxidative stress in isolated mitochondria (Cheraghi et al. Citation2019) and cell death in human cervical cancer (HeLa) cells (Rodriguez-Hernandez et al. Citation2013) and in human hepatoma cells (Johnson et al. Citation2010). In contrast to our results here, no significant effect of either tungstate or molybdate on growth inhibition of the PC-3 human prostate cancer cell line during a 3-day treatment was observed until a rather high concentration of either 1.4 mM tungstate or 4.0 mM molybdate was reached (Liu et al. Citation2012).
In assays of general phosphatase activities in cell lysates, we noticed effects of tungstate at concentrations as low as 10 µM, either activating (kidney cell line) or inhibiting (liver cell line) (). The same trend was observed with molybdate. Tungstate increased the activity of a cytosolic protein tyrosine kinase and cAMP levels in HepG2 cells, but the effect depends on the species and whether or not the cell line is derived from noncancerous or cancerous hepatocytes such as the HepG2 cell line (Johnson et al. Citation2010). Both tungstate and molybdate inhibit PTP-1B with estimated IC50 values of 210 µM (tungstate) and 200 µM (molybdate) (Singh and Maret Citation2017). We found no significant differences in the inhibition of PTP-1B by either molybdate or tungstate under the conditions of the assay (). Our findings demonstrate ‘off target’ effects of these metal anions, namely targets that do not employ the pyranopterin cofactor. Tungstate, molybdate and vanadate are tetra-oxo anions resembling phosphate and hence bind to phosphate-binding sites. A much stronger inhibition by tungstate (Ki = 1–25 µM) was noted for glucose-6 phosphatase (Foster et al. Citation1998). As of December 2020, the structures of 27 human proteins with bound tungstate have been determined (Research Collaboratory for Structural Bioinformatics (RCSB) protein data bank; www.rcsb.org). They include many different enzymes that use phosphate in their mechanism, such as phosphatases and phosphodiesterases. The enzymes are involved in numerous biochemical reactions that are crucial to cellular homeostasis, metabolism and phosphorylation signaling. Inhibition of these enzymes provides an explanation for the antidiabetic/antiobesity and antihypertensive pharmacological effects of these oxyanions (Fernández-Mariño et al. Citation2015). Such actions on phosphorylations are also the basis for their toxicological effects observed in pathways leading to gene expression, epigenetic modification, and for posing a carcinogenic potential (Murray et al. Citation2014, Citation2019; Wasel and Freeman Citation2018).
Like many essential elements at higher concentrations, molybdenum in the form of molybdate can be toxic as well (Zhuang et al. Citation2021). Historically, molybdosis was found in ruminants grazing on molybdenum-rich soils. The toxicity is due to a syndrome of secondary copper deficiency because molybdate is transformed into tetrathiomolybdate in the sulfur-rich environment of the ruminant digestive tract and tetrathiomolybdate binds copper strongly. In goats receiving an excessive amount of heptamolybdate in the drinking water, oxidative damage in kidney mitochondria was observed and linked to a secondary copper deficiency (Feng et al. Citation2020). Molybdenum seems to be less toxic to humans compared to ruminants, but there is evidence from human balance studies that relatively low amounts of dietary molybdenum affect copper metabolism, suggesting that diets rich in molybdenum can lead to copper deficiency (Deosthale and Gopalan Citation1974; Underwood Citation1977). Whether tungsten has a similar effect on copper metabolism is not known. A crystal structure of a copper chaperone binding to molybdate has been determined and suggests that molybdate interferes with copper trafficking proteins (Alvarez et al. Citation2010). If such a complex with copper chaperones forms in humans upon exposure to either molybdate or tungstate, it could tie up the copper chaperone for superoxide dismutase and not deliver copper to superoxide dismutase for its activity, resulting in oxidative stress.
5. Conclusions
Tungstate is considered an antagonist for nutritionally essential molybdate. Both metal anions become toxic to human cell lines at about the same concentrations and it is not clear whether there is biochemical discrimination between the two. Tungstate and molybdate are tetra-oxo anions which resemble phosphate and hence bind to phosphate binding sites. Accordingly, mechanisms of toxicity involve effects on phosphorylation signaling. In order to evaluate tungsten toxicity, it is important to consider the molybdenum status, and, in field experiments, what the exposure to molybdate or other oxyanions is at the same time, because effects may be additive.
| Abbreviations | ||
| PBS | = | phosphate-buffered saline |
| BCA | = | Bicinchoninic acid |
| PTP | = | Protein tyrosine phosphatase |
| XO | = | Xanthine oxidase |
| ROS | = | Reactive oxygen species |
| DCFH-DA | = | 2',7'-Dichloro-dihydro-fluorescein diacetate |
| DCF | = | 2',7'-Dichlorofluorescein |
| FBS | = | Fetal Bovine Serum |
| NTA | = | Nitrilotriacetic acid |
| DMEM | = | Dulbecco's Modified Eagle's medium |
| DiFMU | = | 6,8-difluoro-7-hydroxy-4-methylcoumarin |
| MTT | = | 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide |
Acknowledgements
The authors thank Andy Cakebread for metal analyses, Dr. Elisa Bellomo and Dr. Douglas Parsons for training Sherry Sachdeva with cell culture, Dr. Kshetrimayum Birla Singh, Dr. Sandra Carvalho and Prof. Christer Hogstrand for discussions. S.S. thanks the Director, Defence Research and Development Establishment, Gwalior, Madhya Pradesh, India and Dr. S.J.S. Flora for their support and encouragement. S.S. thanks the Department of Science and Technology, India and British Council UK for providing the financial support through a Newton-Bhabha fellowship. .
Disclosure statement
The authors have no conflict of interest.
Additional information
Funding
References
- Agency for Toxic Substances and Disease Registry (ATSDR) 2005. Toxicological profile for Tungsten. Atlanta (GA): U.S. Department of Health and Human Services, Public Health Service
- Alvarez HM, Xue Y, Robinson CD, Canalizo-Hernández MA, Marvin RG, Kelly RA, Mondragón A, Penner-Hahn JE, O'Halloran TV. 2010. Tetrathiomolybdate inhibits copper trafficking proteins through metal cluster formation. Science. 327(5963):331–334.
- Ames BN, Cathcart R, Schwiers E, Hochstein P. 1981. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 78(11):6858–6862.
- Bellomo E, Massarotti A, Hogstrand C, Maret W. 2014. Zinc ions modulate protein tyrosine phosphatase 1B activity. Metallomics. 6(7):1229–1239.
- Bolt AM, Grant MP, Wu TH, Flores MM, Plourde D, Kelly AD, Negro Silva LF, Lemaire M, Schlezinger JJ, Mwale F, Mann KK. 2016. Tungsten promotes sex-specific adipogenesis in the bone by altering differentiation of bone marrow-resident mesenchymal stromal cells. Toxicol Sci. 150(2):333–346.
- Bolt AM, Mann KK. 2016. Tungsten: an emerging toxicant, alone or in combination. Curr Environ Health Rep. 3(4):405–415.
- Božinović K, Nestić D, Centa UG, Ambriović-Ristov A, Dekanić A, de Bisschop L, Remškar M, Majhen D. 2020. In-vitro toxicity of molybdenum trioxide nanoparticles on human keratinocytes. Toxicology. 444:152564.
- Bridges CC, Zalups RK. 2010. Ionic and molecular mimicry and the transport of metal ions. In: Zalups RK, Koropatnick, J, editors, Cellular and molecular biology of metals, Boca Raton, FL: CRC Press, Taylor and Francis Group, LLC. p. 241–294.
- Cheraghi G, Hajiabedi E, Niaghi B, Nazari F, Naserzadeh P, Hosseini M-J. 2019. High doses of sodium tungstate can promote mitochondrial dysfunction and oxidative stress in isolated mitochondria. J Biochem Mol Toxicol. 33(4):e22266.
- Chinde S, Dumala N, Rahman MF, Kamal SSK, Kumari SI, Mahboob M, Grover P. 2017. Toxicological assessment of tungsten oxide nanoparticles in rats after acute oral exposure. Environ Sci Pollut Res Int. 24(15):13576–13593.
- Chinde S, Grover P. 2017. Toxicological assessment of nano and micron-sized tungsten oxide after 28days repeated oral administration to Wistar rats. Mutat Res. 819:1–13.
- Cordas CM, Moura JJ. 2019. Molybdenum and tungsten enzymes redox properties–A brief overview. Coord Chem Rev. 394:53–64.
- Datta S, Vero SE, Hettiarachchi GM, Johannesson K. 2017. Tungsten contamination of soils and sediments: current state of science. Curr Pollution Rep. 3(1):55–64.
- Deosthale Y, Gopalan C. 1974. The effect of molybdenum levels in sorghum (Sorghum vulgare Pers.) on uric acid and copper excretion in man. Br J Nutr. 31(3):351–355.
- Dunnick KM, Badding MA, Schwegler-Berry D, Patete JM, Koenigsmann C, Wong SS, Leonard SS. 2014. The effect of tungstate nanoparticles on reactive oxygen species and cytotoxicity in raw 264.7 mouse monocyte macrophage cells. J Toxicol Environ Health A. 77(20):1251–1268.
- Emsley J. 2011. The elements (A-Z). In Emsley J, Nature's building blocks: An A-Z guide to the elements. Oxford University Press, p. 571.
- EPA 2008. EPA, Emerging contaminant tungsten. Fact sheet, in 505-F-070-005. 2008 (https://nepis.epa.gov/Exe/tiff2png.cgi/P1000L3K.PNG?-r+75+-g+7+D%3A%5CZYFILES%5CINDEX%20DATA%5C06THRU10%5CTIFF%5C00000138%5CP1000L3K.TIF. ) accessed on 7 Oct 2020
- Feng J, Chen J, Xing C, Huang A, Zhuang Y, Yang F, Zhang C, Hu G, Mao Y, Cao H. 2020. Molybdenum induces mitochondrial oxidative damage in kidney of goats. Biol Trace Elem Res. 197(1):167–174.
- Fernández-Mariño AI, Cidad P, Zafra D, Nocito L, Domínguez J, Oliván-Viguera A, Köhler R, López-López JR, Pérez-García MT, Valverde MÁ, et al. 2015. Tungstate-targeting of BKαβ1 channels tunes ERK phosphorylation and cell proliferation in human vascular smooth muscle. PLoS One. 10(2):e0118148.
- Foster JD, Young SE, Brandt TD, Nordlie RC. 1998. Tungstate: a potent inhibitor of multifunctional glucose-6-phosphatase. Arch Biochem Biophys. 354(1):125–132.
- Furberg A, Arvidsson R, Molander S. 2019. Dissipation of tungsten and environmental release of nanoparticles from tire studs: A Swedish case study. J Clean Prod. 207:920–928.
- George I, Uboldi C, Bernard E, Sobrido MS, Dine S, Hagège A, Vrel D, Herlin N, Rose J, Orsière T, et al. 2019. Toxicological assessment of ITER-like tungsten nanoparticles using an in vitro 3D human airway epithelium model. Nanomaterials (Basel). 9(10):1374.
- Gianì F, Pandini G, Scalisi NM, Vigneri P, Fazzari C, Malandrino P, Russo M, Masucci R, Belfiore A, Pellegriti G, et al. 2019. Effect of low-dose tungsten on human thyroid stem/precursor cells and their progeny. Endocr Relat Cancer. 26(8):713–725.
- Guilbert C, Kelly ADR, Petruccelli LA, Lemaire M, Mann KK. 2011. Exposure to tungsten induces DNA damage and apoptosis in developing B lymphocytes. Leukemia. 25(12):1900–1904.
- Hagen WR. 2011. Cellular uptake of molybdenum and tungsten. Coord Chem Rev. 255(9-10):1117–1128.
- Idil AS, Donaldson N. 2018. The use of tungsten as a chronically implanted material. J Neural Eng. 15(2):021006.
- Jeng HA, Swanson J. 2006. Toxicity of metal oxide nanoparticles in mammalian cells. J Environ Sci Health A Tox Hazard Subst Environ Eng. 41(12):2699–2711.
- Johnson DR, Ang C-Y, Bednar AJ, Inouye LS. 2010. Tungsten effects on phosphate-dependent biochemical pathways are species and liver cell line dependent. Toxicol Sci. 116(2):523–532.
- Kelly ADR, Lemaire M, Young YK, Eustache JH, Guilbert C, Flores Molina M, Mann KK. 2013. In vivo tungsten exposure alters B-cell development and increases DNA damage in murine bone marrow. Toxicol Sci. 131(2):434–446.
- Kirk ML, Kc K. 2020. Molybdenum and tungsten cofactors and the reactions they catalyze. In: Sosa Torres M, Kroneck P, editors. Transition metals and sulfur – A strong relationship for life. Berlin: De Gruyter. p. 313–342.
- Leggett RW. 1997. A model of the distribution and retention of tungsten in the human body. Sci Total Environ. 206(2-3):147–165.
- Lemus R, Venezia CF. 2015. An update to the toxicological profile for water-soluble and sparingly soluble tungsten substances. Crit Rev Toxicol. 45(5):388–411.
- Liu TT, Liu YJ, Wang Q, Yang XG, Wang K. 2012. Reactive-oxygen-species-mediated Cdc25C degradation results in differential antiproliferative activities of vanadate, tungstate, and molybdate in the PC-3 human prostate cancer cell line. J Biol Inorg Chem. 17(2):311–320.
- McDonald JD, Weber WM, Marr R, Kracko D, Khain H, Arimoto R. 2007. Disposition and clearance of tungsten after single-dose oral and intravenous exposure in rodents. J Toxicol Environ Health A. 70(10):829–836.
- Murray FJ, Sullivan FM, Hubbard SA, Hoberman AM, Carey S. 2019. A two-generation reproductive toxicity study of sodium molybdate dihydrate administered in drinking water or diet to Sprague-Dawley rats. Reprod Toxicol. 84:75–92.
- Murray FJ, Sullivan FM, Tiwary AK, Carey S. 2014. 90-Day subchronic toxicity study of sodium molybdate dihydrate in rats. Regul Toxicol Pharmacol. 70(3):579–588.
- Nakanishi Y, Iida S, Ueoka-Nakanishi H, Niimi T, Tomioka R, Maeshima M. 2013. Exploring dynamics of molybdate in living animal cells by a genetically encoded FRET nanosensor. PLoS One. 8(3):e58175.
- Novotny JA. 2011. Molybdenum nutriture in humans. J Evid Based Complementary Altern Med. 16(3):164–168.
- Osterburg AR, Robinson CT, Schwemberger S, Mokashi V, Stockelman M, Babcock GF. 2010. Sodium tungstate (Na2WO4) exposure increases apoptosis in human peripheral blood lymphocytes. J Immunotoxicol. 7(3):174–182.
- Rodriguez-Hernandez CJ, Llorens-Agost M, Calbó J, Murguia JR, Guinovart J. 2013. Sodium tungstate modulates ATM function upon DNA damage. FEBS Lett. 587(10):1579–1586.
- Rubin CS, Holmes AK, Belson MG, Jones RL, Flanders WD, Kieszak SM, Osterloh J, Luber GE, Blount BC, Barr DB, et al. 2007. Investigating childhood leukemia in Churchill county, Nevada. Environ Health Perspect. 115(1):151–157.
- Sachdeva S, Chatterjee S, Flora S. 2020. Dose dependent changes in oxidative stress, hematological variables, tissue pathology, and apoptosis following chronic sodium tungstate exposure in rats. Med Drug Disc. 6:100045.
- Sachdeva S, Flora S. 2014. Efficacy of some antioxidants supplementation in reducing oxidative stress post sodium tungstate exposure in male Wistar rats. J Trace Elem Med Biol. 28(2):233–239.
- Sachdeva S, Kushwaha P, Flora S. 2013. Effects of sodium tungstate on oxidative stress enzymes in rats. Toxicol Mech Methods. 23(7):519–527.
- Sachdeva S, Pant SC, Kushwaha P, Bhargava R, Flora SJ. 2015. Sodium tungstate induced neurological alterations in rat brain regions and their response to antioxidants. Food Chem Toxicol. 82:64–71.
- Shi L, Cao H, Luo J, Liu P, Wang T, Hu G, Zhang C. 2017. Effects of molybdenum and cadmium on the oxidative damage and kidney apoptosis in duck. Ecotoxicol Environ Saf. 145:24–31.
- Singh KB, Maret W. 2017. The interactions of metal cations and oxyanions with protein tyrosine phosphatase 1B. Biometals. 30(4):517–527.
- Tejada-Jiménez M, Galván A, Fernández E. 2011. Algae and humans share a molybdate transporter. Proc Natl Acad Sci USA. 108:6420–6425.
- Tejada-Jiménez M, Schwarz G. 2014. Molybdenum and tungsten. In: Maret W, Wedd AG, editors. Binding, transport and storage of metal ions in biological cells. Royal Society of Chemistry, p. 223–259.
- Underwood E. 1977. Trace Elements in Human and Animal Nutrition, 4th ed. New York: Academic Press.
- Wang C, Nie G, Yang F, Chen J, Zhuang Y, Dai X, Liao Z, Yang Z, Cao H, Xing C, et al. 2020. Molybdenum and cadmium co-induce oxidative stress and apoptosis through mitochondria-mediated pathway in duck renal tubular epithelial cells. J Hazard Mater. 383:121157.
- Wasel O, Freeman JL. 2018. Comparative assessment of tungsten toxicity in the absence or presence of other metals. Toxics. 6(4):66.
- Weber WM, Marr R, Kracko D, Gao Z, McDonald JD, Ui Chearnaigh K. 2008. Disposition of tungsten in rodents after repeat oral and drinking water exposures. Toxicol Environm Chem. 90(3):445–455.
- Witten ML, Sheppard PR, Witten BL. 2012. Tungsten toxicity. Chem Biol Interact. 196(3):87–88.
- Wu TH, Bolt AM, Chou H, Plourde D, Jay ND, Guilbert C, Young YK, Kleinman CL, Mann KK. 2019. Tungsten blocks murine B lymphocyte differentiation and proliferation through downregulation of IL-7 receptor/Pax5 signaling. Toxicol Sci. 170(1):45–56.
- Zhang C, Lin T, Nie G, Hu R, Pi S, Wei Z, Wang C, Xing C, Hu G. 2021. Cadmium and molybdenum co-induce pyroptosis via ROS/PTEN/PI3K/AKT axis in duck renal tubular epithelial cells. Environ Pollut. 272:116403.
- Zhuang J, Nie G, Hu R, Wang C, Xing C, Li G, Hu G, Yang F, Zhang C. 2021. Inhibition of autophagy aggravates molybdenum-induced mitochondrial dysfunction by aggravating oxidative stress in duck renal tubular epithelial cells. Ecotoxicol Environ Saf. 209:111771.
- Zoroddu MA, Medici S, Peana M, Nurchi VM, Lachowicz JI, Laulicht-Glickc F, Costa M. 2018. Tungsten or wolfram: friend or foe? Curr Med Chem. 25(1):65–74.
