Abstract
The carbamate pyridostigmine bromide (PB) is the only fielded pharmacological prophylaxis for military use against nerve agents. Previous studies have shown differences in the PB-pretreatment efficacy for various nerve agents and in the influence of post-exposure treatment with common antidotes. In the present study, the aim was to evaluate the possibility of using an ex vivo rat precision-cut lung slice model to determine the impact of PB pretreatment on VX-induced bronchoconstriction. In addition, the efficacy of post-exposure treatment with atropine sulfate following PB-prophylaxis was investigated.
Bronchoconstriction was induced by electric-field stimulation and was significantly aggravated by 10 µM PB. Airway recovery was decreased by both 1 and 10 µM PB. Evaluation of acetylcholineesterese inhibition by PB showed that the lower concentration met the clinical criteria of residual enzyme activity while the higher concentration completely inhibited the activity. Exposure to VX with or without pretreatment demonstrated similar contractions. However, VX-incubation following pretreatment caused decreased airway relaxation compared to pretreatment alone. Atropine treatment following PB- and VX-exposure significantly decreased the maximum airway contraction and increased the relaxation.
In conclusion, no beneficial effect of PB-prophylaxis on VX-induced contractions was observed. The atropine efficacy to relax airways was significant demonstrating the importance of efficient post-exposure therapeutics to protect against the life-threatening respiratory contractions.
Introduction
Medical prophylaxis is measures used to prevent illness and can include vaccines, antibiotics and other drugs. The carbamate pyridostigmine bromide (PB) is the only approved pharmacological prophylaxis for military use by the US Food and Drug Administration to reduce lethality following nerve agent poisoning (US Food and Drug Administration, Citation2003). PB acts through reversible binding to AChE and thereby prevents the nerve agent from irreversible bind to the enzyme (Layish et al. Citation2005). The anticipated result is the elimination of the nerve agent from the human body and PB subsequently leaves the enzyme spontaneously, restoring the normal AChE function. Attributable to the mechanism of action of PB, the drug is frequently used for the treatment of other diseases, for example, stimulation of cholinergic signaling, and thereby improving muscle strength in patients with myasthenia gravis (Lorenzoni et al. Citation2020).
PB is a peripherally acting substance due to the polar chemical properties under normal physiological conditions, which poorly can pass the blood-brain barrier (BBB), although some studies have demonstrated that PB can enter the central nervous system due to BBB-leakage triggered by, e.g. stress (Beck et al. Citation2001; Myhrer & Aas, Citation2016; Macht et al. Citation2020). Pretreatment with PB alone will not protect against poisoning and rapid administration of medical countermeasures is required following nerve agent exposure (US Food and Drug Administration, Citation2003). The standard treatment for nerve agent poisoning consists of the antimuscarinic drug atropine and an oxime AChE-reactivator, commonly obidoxime or pralidoxime (2-PAM) (Amend et al. Citation2020; Thiermann & Worek, Citation2022). As an alternative to PB, the non-polar carbamate physiostigmine (PHY) has been evaluated for pretreatment against nerve agent intoxication (Miller et al. Citation1993; Wetherell et al. Citation2002). To avoid poor drug bioavailability by oral administration, the administration of PHY by transdermal patches or utilizing liposomes for extended release has also been investigated (Park et al. Citation2018; Nam et al. Citation2020). However, the neuroactive properties of PHY may cause adverse effects that would result in impaired soldier performance. For that reason, co-administration of PHY with a low-dose anticholinergic drug has been suggested and has demonstrated less undesired effects during prophylactic use (Von Bredow et al. Citation1991; Bonhage et al. Citation2009). Other centrally or peripherally acting anticholinesterase drugs for prophylactic use have also demonstrated reduced lethality after exposure to several organophosphorus nerve agents and pesticides (Aracava et al. Citation2009; Lenina et al. Citation2020).
During the Gulf War in the early 1990s, PB was used among U.S. and British soldiers for protection against potential Iraqi nerve agent attacks. Among soldiers taking PB, the most common adverse effects reported were minor intestinal and urinary symptoms (Keeler et al. Citation1991). After the war, about one-third of the veterans experienced chronic multi-symptomatic Gulf War Illness, and the extensive use of PB was suggested to have contributed to the disease (Joyce & Holton, Citation2020; Carpenter et al. Citation2021). However, no consensus on the exact cause has been identified and rather a combination of triggering factors has been suggested (Dyer, Citation2004; White et al. Citation2016).
The aim of the present study was to evaluate the possibility of using the ex vivo rat precision-cut lung slice (PCLS) model to evaluate the specific impact of the nerve agent pretreatment with PB on airway function. The nerve agent VX was chosen based on previous in vivo and in vitro studies demonstrating conflicting results. The dose-dependent influence of PB and the nerve agent VX on airway contraction and relaxation was investigated using the PCLS model. In addition, the post-exposure therapeutic effect of atropine sulfate was evaluated on lung slices exposed to PB and VX. A physiological relevant and neuronal-dependent airway response was achieved by electric-field stimulation (EFS) of PCLS. EFS mainly results in the release of the neurotransmitter acetylcholine from the nerve endings and, consequently, responses are dependent on the activation of muscarinic and nicotinic receptors on the airway smooth muscle cells (Schlepütz et al. Citation2011; Wigenstam et al. Citation2021). The model has previously been utilized to evaluate the impact of the reversible AChE-inhibitor neostigmine on cholinergic responses (Schlepütz et al. Citation2011; Citation2012). However, the impact of PB-treatment on nerve agent-induced bronchoconstriction has never been evaluated in the PCLS-model.
2. Materials and methods
2.1. Chemicals
O-ethyl S-[2-(diisopropylamino)ethyl] methylphosphonothioate (VX; >97% pure measured by NMR; CAS No. 50782-69-9) was synthesized in-house. Pyridostigmine bromide (PB; ≥98% pure; CAS No. 101-26-8), atropine sulfate (hereafter named atropine; ≥97% pure; CAS No. 5908–99-6), acethylthiocholine iodide (≥98%; CAS No. 1866-15-5), 5,5′-dithiobis(2-nitrobenzoic acid) (≥98%; CAS No. 69-78-3), sodium phosphate (96%; CAS No. 7601-54-9), TritonTM X100 (CAS No. 9002-93-1) and substances for incubation medium and standard laboratory chemicals were obtained from Merck KGaA (Darmstadt, Germany). Low-melting agarose was purchased from Gerbu Biotechnik (Heidelberg, Germany; CAS No. 39346-81-1).
2.2. Animals
Sprague Dawley female rats (8–10 weeks old; 220–300 g) were obtained from Scanbur A/S (Karlslunde, Denmark). Animals were housed for at least one week prior to experiments to allow proper acclimatization. Animal care and housing conditions complied with Directive 2010/63/EU of the European Parliament and of the council on the protection of animals used for scientific purposes (EU, Citation2010). The regional ethics committee on animal experiments in Umeå, Sweden (A8-2020) has approved the experiments.
2.3. Precision-cut lung slices (PCLS)
PCLS were prepared as described accordingly to Wigenstam et al. (Wigenstam et al. Citation2021, Citation2022). Rats were euthanized with an overdose of a pentobarbital intraperitoneal injection (120 mg kg−1) and the aorta was cut off to ensure death. The lungs were filled with 1.5% low-melting agarose dissolved in an incubation medium, removed en bloc from the animal, and cooled on ice for 15 min to allow the agarose to solidify. Lung lobes were carefully separated and the left lobe was cut into a 10 mm wide core with a penetrating airway in the center using a coring tool (Alabama Research and Development, Munford, USA). Tissue slices of approximately 250 µm were prepared from the core using a Krumdieck tissue slicer (Alabama Research and Development; Munford, USA) filled with slicing medium (2 mM CaCl2, 1 mM MgSO4, 5 mM KCl, 116 mM NaCl, 1 mM NaH2PO4, 17 mM glucose, 26 mM NaHCO3, 25 mM HEPES; pH 7.2). PCLS were collected and cultured in a Petri dish in humidified conditions (37 °C, 5% CO2 atmosphere). Slices were washed to remove agarose and remaining erythrocytes from the tissue by changing the incubation medium (slicing medium with addition of supplements: 1 mM sodium pyruvate, 2 mM glutamine, 1:50 amino acids, 1:100 vitamins, 1% gentamycin; pH 7.2) every 30 min for 2 h and thereafter every hour for 4 h. Experiments were performed the day after PCLS preparation according to the separate protocols below.
Airways were imaged at specific time points using an inverted microscope (Leica DM IRB; Wetzlar, Germany) and a camera with the connected NIS-Elements D software (Nikon Instruments Europe BV; Amsterdam, the Netherlands). Only slices with airways free of agarose and with an intact smooth muscle layer were used. In order to measure airway areas, images were automatically analyzed using an in-house program developed in Matlab® (MathWorks; Kista, Sweden). Each condition was evaluated on 4-6 PCLS from at least three animals.
2.3.1. Airway contraction induced by EFS
EFS was performed as previously described (Schlepütz et al. Citation2011; Wigenstam et al. Citation2021). Prior to EFS, the PCLS was transferred to a 12-well plate with two ml incubation medium, placed between two platinum electrodes, and weighted down by a Teflon ring. The electric field was applied by a Stimulator C (Hugo Sachs Electronics; March-Hugstetten, Germany). The parameters to achieve EFS were set to a train rhythm of 60 s, train width of 2.5 s, pulse duration of 1 ms, frequency of 50 Hz, and a voltage of 50 V (Wigenstam et al. Citation2021). The EFS was continuously operating throughout the complete experimental time. Images were taken at three time-points during each train rhythm at 6, 30, and 50 s. The stimulation response on PCLS was followed for 5 min prior to adding substances.
The dose-dependent effects of VX-exposure (0.005–5 µM) on airway contraction and relaxation were investigated by adding the agent after the first round of EFS and incubated for 5 min prior to images were taken. Airways were also imaged between time-points 20–25 min and 30–35 min post-exposure to VX. In total, the response was followed for 40 min.
The impact on airway response by PB was evaluated using several protocols (). PB (1 or 10 µM) was added to the incubation medium after the first round of EFS followed by the addition of VX (0.5 µM) at time-point 35 min. To evaluate the individual effects of PB and VX, water was added as a substitute for either compound at the respective time point. In total, the response was followed for 55 min.
Figure 1. Protocol for evaluation of efficacy for pretreatment of pyridostigmine bromide (PB; 1 or 10 µM) followed by exposure to the nerve agent VX (0.5 µM). to quantify the separate impacts of PB or VX, water was added at the same time-points.
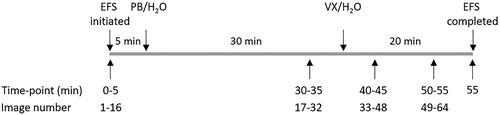
The therapeutic effect of atropine (1 µM) was evaluated by applying the compound to the incubation medium 10 min after the VX-addition and including pretreatment with 10 µM PB (as shown in ). In total, airway responses were followed for 55 min.
2.4. AChE-activity measurements
Rat lung tissue from the right lobes were prepared to PCLS as described in Section 2.3. Each sample was prepared by pooling five PCLS in phosphate-buffered saline and homogenized using a scissor and micro tissue pestle (Merck KGaA; Darmstadt, Germany). Samples were stored at −80 °C until use. The AChE-activity in PCLS-samples was monitored using a modified spectrophotometric Ellman assay (Ellman et al. Citation1961; Ekstrom et al. Citation2006). The assay mixture (1 ml) contained 0.2 mM 5,5′-Dithiobis (2-nitrobenzoic acid), 100 mM sodium phosphate pH 7.4, 0.1% Triton X100 and 1 mM acethylthiocholine iodide. AChE-activity was measured in two experimental set-ups: samples incubated with only PB (1 and 10 µM) and samples incubated with PB followed by the addition of VX (0.5 µM). The initial AChE-activity in PCLS was determined prior to the addition of PB and VX. The volume of PCLS-sample in each experiment was 15 µl and each set-up was performed in 3-6 replicates. Assays were performed at room temperature and the hydrolysis of acetylthiocholine iodide was monitored at 412 nm at time-point 0 min and up to 50 min (Lambda 650 UV/VIS spectrometer; PerkinElmer, Sweden).
2.5. Data analysis and statistics
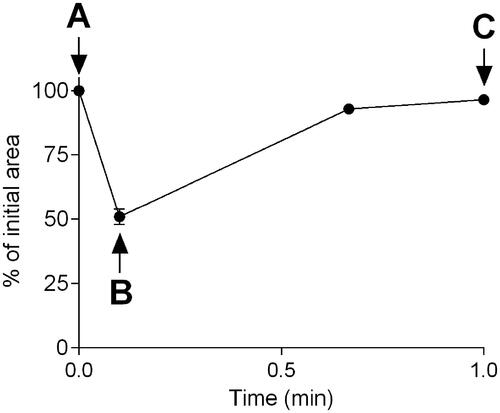
All results are presented as the mean ± the standard error of mean (SEM). The initial airway area (IAA) was determined prior to stimulation and was set to 100%. To quantify the airway response, data is expressed as a percent of IAA. The measuring of the IAA, maximum airway contraction after 6 s, and relaxation after 60 s in a single train rhythm is illustrated in .
Figure 2. Illustration of the bronchoconstriction pattern following a single train rhythm induced by electric-field stimulation (EFS). to evaluate the response, the initial airway area is set to 100% prior to EFS (A). the maximum airway contraction is determined 6 s after the EFS-pulse (B) and the maximum airway relaxation is measured at time-point 60 s (C). Thereafter, the % of the initial airway are is determined for point B and C for each train rhythm throughout the experimental time.
For the dose-response experiments including VX, the mean of the maximum airway contraction or relaxation following VX-exposure was calculated by merging the data from both time points (20–25 and 30–35 min). For experiments including pretreatment with PB and post-exposure treatment with atropine, the mean of the maximum airway contraction or relaxation was calculated for each time point (30–35, 40–45, and 50–55 min). The untreated control, as airway responses achieved by only EFS, was calculated as the mean of the maximum airway contraction or relaxation at time-point 0–5 min in all experimental set-ups.
In the biochemical evaluation of AChE-activity in PCLS, the initial activity prior to the addition of PB and VX was set to 100%. The enzyme activity following compound addition is presented as a percent of the initial AChE-activity.
All graphs were prepared and statistical analyses were performed using the GraphPad Prism program (version 6.07 GraphPad Software Inc., San Diego, USA). Statistical comparisons of the maximum airway response and relaxation in EFS experiments including VX- and/or PB- and atropine exposure were performed using one-way analysis of variance (ANOVA) followed by Tukeýs multiple comparison test. The statistical comparison of the AChE-activity after PB-addition was performed using a two-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. The effects on the AChE-activity after PB-pretreatment and addition of VX were assessed using one-way ANOVA followed by Dunnet’s multiple comparison test. The null hypothesis, defined as identical mean values of compared groups, was discarded if the probability of identical mean values were below 0.05 (*p < 0.05). Probabilities of 0.01–0.001 and below 0.001 were additionally indicated (** and ***, respectively).
3. Results
3.1. Dose-dependent airway contraction and relaxation following VX-exposure
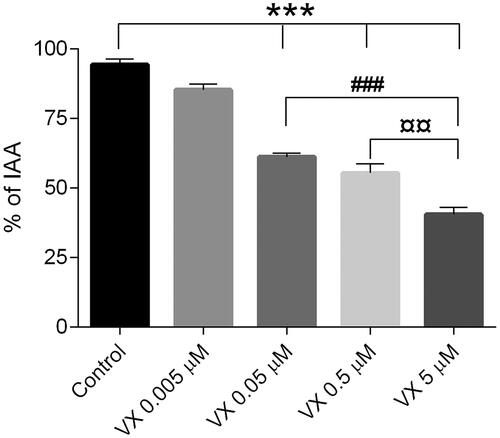
To evaluate the dose-dependent effects of VX on airway contractions induced by EFS, four concentrations of the nerve agent (0.005-5 µM) were added to the incubation medium following the initial round of EFS during the first five min of the experimental time (referred to as the control). The impact of VX exposure on both the maximum airway contraction () and relaxation () was quantified.
Figure 3. Dose-dependent maximum airway contraction following exposure to the nerve agent VX (0.005-5 µM). the control, as airway responses achieved by only EFS, was monitored during the first 5 min of the experimental time. Data is expressed as percent of the initial airway area (IAA) and presented as the mean ± the SEM (n = 4-5). ***p < 0.001 for increased maximum airway contraction of VX 0.05-5 µM versus the control or VX 0.005 µM (one-way ANOVA). ##p < 0.01 for increased maximum airway contraction of VX 5 µM versus VX 0.05 µM (one-way ANOVA).
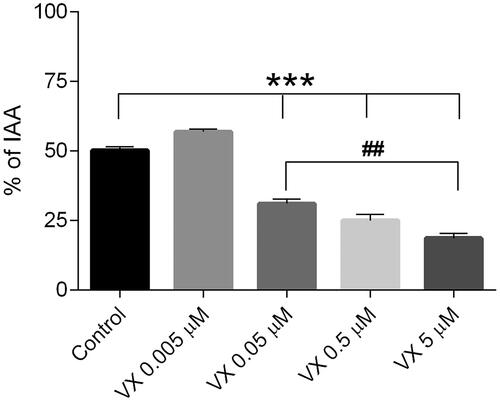
Figure 4. Dose-dependent maximum airway relaxation following exposure to the nerve agent VX (0.005–5 µM). the control, as airway responses achieved by only EFS, was monitored during the first 5 min of the experimental time. Data is expressed as percent of the initial airway area (IAA) and presented as the mean ± the SEM (n = 4–5). ***p < 0.001 for decreased airway relaxation of VX 0.05-5 µM versus the control or VX 0.005 µM (one-way ANOVA). ###p < 0.001 for decreased airway relaxation of VX 5 µM versus VX 0.05 µM (one-way ANOVA). ¤¤p < 0.01 for decreased airway relaxation of VX 5 µM versus VX 0.5 µM (one-way ANOVA).
VX exposed in concentrations between 0.05–5 µM significantly increased the maximum bronchoconstriction compared to both the control and the 0.005 µM concentration (p < 0.001). The highest VX concentration (5 µM) significantly increased the maximum airway contraction compared to the 0.05 µM VX concentration (p < 0.01). The lowest VX-concentration (0.005 µM) applied in the incubation medium did not significantly change the maximum airway contraction compared to the control.
VX exposed in concentrations between 0.05-5 µM significantly reduced the maximum airway relaxation compared to the control and the concentration of 0.005 µM concentration (p < 0.001). The highest VX-concentration (5 µM) significantly reduced the maximum airway relaxation compared to both the 0.05 µM and 0.5 µM VX-concentration (p < 0.001 and p < 0.01, respectively). The lowest VX-concentration applied (0.005 µM) did not significantly change the maximum airway relaxation compared to the control.
3.2. Impact of pretreatment by PB prior to VX-exposure
To evaluate the efficacy of pretreatment, PB (1 and 10 µM) was added to the incubation medium following the initial round of EFS (referred to as the control). After the 30 min PB-incubation, VX was applied and the maximum airway contraction ( and ) and relaxation ( and ) was followed during 55 min. The individual effects of PB and VX were determined by adding water instead of either compound at the same time point. The VX-concentration 0.5 µM was chosen based on the agent dose-dependent effects shown in Section 3.1.
Figure 5. Maximum airway contractions following pretreatment with pyridostigmine bromide (PB; 1 or 10 µM) and/or following exposure to the nerve agent VX (0.5 µM). PCLS were incubated with PB for 30 min prior to addition of VX. To evaluate the separate effects of PB and VX, water was added at the same time-points. The control, as airway responses achieved by only EFS, was monitored during the first 5 min of the experimental time. Data is expressed as percent of the initial airway area (IAA) and presented as the mean ± the SEM (n = 5–6).
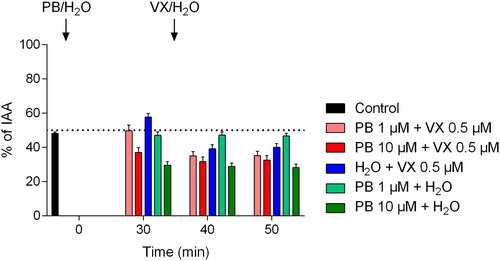
Figure 6. Maximum airway relaxation following pretreatment with pyridostigmine bromide (PB; 1 or 10 µM) and/or following exposure to the nerve agent VX (0.5 µM). PCLS were incubated with PB for 30 min prior to addition of VX. To evaluate the separate effects of PB and VX, water was added at the same time-points. The control, as airway responses achieved by only EFS, was monitored during the first 5 min of the experimental time. Data is expressed as percent of the initial airway area (IAA) and presented as the mean ± the SEM (n = 5–6).
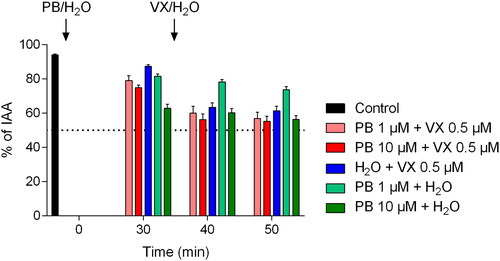
Table 1. Maximum airway contractions following pretreatment with pyridostigmine bromide (PB; 1 or 10 µM) and/or following exposure to the nerve agent VX (0.5 µM). PCLS were incubated with PB for 30 min prior to addition of VX. The control, as airway responses achieved by only EFS, was monitored during the first 5 min of the experimental time. Data is expressed as percent of the initial airway area (IAA) and presented as the mean ± the SEM (n = 5–6). *p < 0.05, **p < 0.01 and ***p < 0.001 for increased maximum airway contraction versus the control (one-way ANOVA). ##p < 0.01 and ###p < 0.001 for increased maximum airway contraction at time-point 40-45 min and 50–55 min versus the time-point 30–35 min within the same experiment (one-way ANOVA). ¤¤¤<p < 0.001 for increased maximum airway contraction at time-point 40–45 min and 50–55 min versus the 30–35 min in experiment including only VX-exposure (one-way ANOVA).
Table 2. Maximum airway relaxation following pretreatment with pyridostigmine bromide (PB; 1 or 10 µM) and/or following exposure to the nerve agent VX (0.5 µM). PCLS were incubated with PB for 30 min prior to addition of VX. The control, as airway responses achieved by only EFS, was monitored during the first 5 min of the experimental time. Data is expressed as percent of the initial airway area (IAA) and presented as the mean ± the SEM (n = 5-6). **p < 0.01 and ***p < 0.001 for decreased maximum airway relaxation versus the control (one-way ANOVA). ###p < 0.001 for decreased maximum airway relaxation at time-point 40–45 min and 50–55 min versus the time-point 30–35 min within the same experiment (one-way ANOVA). ¤¤¤<p < 0.001 for decreased maximum airway relaxation at time-point 40–45 min and 50–55 min versus the 30–35 min in experiment including only VX-exposure (one-way ANOVA).
Following incubation with 10 µM PB, significantly increased maximum airway contraction was observed at time-points 30–35 min, 40–45 min, and 50–55 min compared to the control (p < 0.01 for time-point 30-35 min and p < 0.001 for time-points 40–45 min and 50–55 min). Pretreatment with 1 µM PB did not significantly change the maximum airway contraction compared to the control. VX-exposure significantly increased the maximum airway contraction (p < 0.05 for time-points 40–45 min and 50–55 min) and following pretreatment with PB applied in the 1 µM concentration (p < 0.001 for time-point 40–45 min and p < 0.01 for time-point 50-55 min) compared to the control. Following pretreatment with PB in the 10 µM concentration, the application of VX did not significantly change the maximum airway contraction. No significant differences in maximum airway contraction were observed between experiments including both PB- and VX-exposure compared to VX-exposure alone at time points 40–45 min and 50–55 min.
Following incubation with PB (1 and 10 µM), significantly decreased maximum airway relaxation was observed at time-points 30–35 min, 40–45 min, and 50–55 min compared to the control (p < 0.01 for 30-35 min for 1 µM PB and p < 0.001 for 10 µM at 30–35 min and for all groups at time-points 40–45 min and 50–55 min). VX-exposure significantly decreased maximum airway relaxation (p < 0.001 for time-points 40–45 min and 50–55 min) and following pretreatment with PB applied in both concentrations (p < 0.001 for time-points 40–45 min and 50–55 min) compared to the control. Following pretreatment with PB in both concentrations, the application of VX significantly lowered the maximum airway relaxation compared to incubation with PB alone (p < 0.001). No significant differences in maximum airway relaxation were observed between experiments including both PB- and VX-exposure compared to VX-exposure alone at time points 40–45 min and 50–55 min.
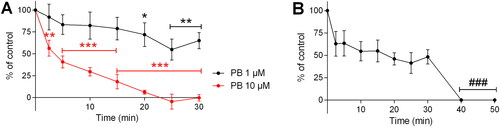
The specific impact of PB and VX on AChE-activity in PCLS was evaluated using the modified Ellman method (). The 10 µM concentration of PB significantly inhibited the AChE-activity after a 2.5 min incubation time (56.4 ± 9.1% remaining AChE-activity; p < 0.01). Complete enzyme inhibition of PB (10 µM) was achieved after approximately 25 min. PB applied in the 1 µM concentration resulted in significant inhibition of the AChE-activity at time-points between 20–30 min (65.2.4 ± 9.2% remaining AChE-activity at the 30 min time-point; p < 0.01). Following incubation with the 1 µM concentration of PB, complete AChE-inhibition was observed following the addition of VX at both time-point 40 and 50 min (0.5 µM; p < 0.001).
Figure 7. Effect of A) pyridostigmine bromide (PB; 1 or 10 µM) and B) pretreatment with 1 µM PB followed by addition of VX (0.5 µM) on the acetylcholineesterese (AChE) activity in precision-cut lung slices. VX was added 30 min post-exposure to PB. Data is expressed as percent of the initial AChE-activity set to 100% and presented as the mean ± the SEM (n = 3–6). *p < 0.05, **p < 0.01 and ***p < 0.001 for decreased AChE-activity following PB-incubation versus the control activity (two-way ANOVA). ###p < 0.001 for decreased AChE-activity after VX-addition compared to the remaining enzyme activity at the 30 min time-point (one-way ANOVA).
3.3. Treatment efficacy of atropine following PB- and VX-exposure
To evaluate the therapeutic effect of atropine (1 µM; ), the drug was added at a time-point 45 min post-exposure to PB (10 µM) and VX (0.5 µM). The maximum airway contraction and relaxation were determined at time-points 0–5 min (airway responses only induced by EFS), 30–35 min (post-exposure to PB), 40–45 min (post-exposure to PB and VX) and 50–55 min (post-exposure to PB, VX, and atropine).
Figure 8. Therapeutic efficacy of atropine (atr; 1 µM) following pretreatment with pyridostigmine bromide (PB; 10 µM) and exposure to VX (0.5 µM). A) Maximum airway contraction and B) maximum airway relaxation. PCLS were incubated with PB for 30 min prior to addition of VX and atropine was applied 10 min post-exposure to VX. The control, as airway responses achieved by only EFS, was monitored during the first 5 min of the experimental time. Data is expressed as percent of the initial airway area (IAA) and presented as the mean ± the SEM (n = 6). *p < 0.05 or ***p < 0.001 for atropine treatment versus PB and VX-exposure without treatment at time-point 50–55 min (one-way ANOVA).
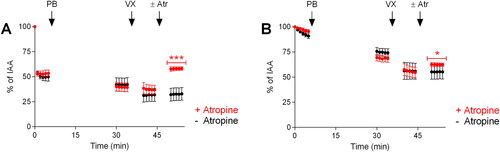
Following atropine treatment, the maximum airway contraction and relaxation levels were significantly changed compared to experiments including only exposure to PB and VX at time-point 50–55 min (p < 0.001 for maximum contraction and p < 0.05 for maximum relaxation).
4. Discussion
Prior to prospective nerve agent exposure, the only available pharmacological prophylactic treatment for military use is the carbamate PB and the drug was widely used during the Gulf War among U.S. and British soldiers. The main peripheral action is the protection of AChE, which should be of significance to avoid the life-threatening respiratory effects following severe nerve agent exposure (Peng et al. Citation2014). However, the ambivalent broad-spectrum nerve agent efficacy, the deficit ability of PB to reach the central nervous system, and potential long-term adverse effects has limited its use (Koplovitz et al. Citation1992; Koplovitz & Stewart, Citation1994; Dyer, Citation2004; Myhrer & Aas, Citation2016). In the present study, the efficacy of pretreatment with PB against VX-induced bronchoconstriction was evaluated using an ex vivo rat PCLS model. The PCLS-model enabled studies of the specific impact of pharmacological prophylaxis on airway function.
Previously, VX-concentrations between 1–10 µM have been used in PCLS-studies to assess respiratory nerve agent effects (Herbert et al. Citation2017, Citation2019; Wigenstam et al. Citation2021). To enable the evaluation of pretreatment efficacy with PB on nerve agent-induced bronchoconstriction, the dose-dependent VX effects on maximum airway contraction and relaxation were determined in the present study. A significant nerve agent effect and partial reduction of risk for re-inhibition of AChE was achieved at the VX-concentration of 0.5 µM which was used in the subsequent experiments. The lower concentration of PB (1 µM) was selected based on a previous in vitro study in which an approximately two-fold PB-concentration in comparison to the nerve agent concentration provided protection and maintained a critical level of AChE-activity (Herkert et al. Citation2011). The higher PB-concentration (10 µM) was included to evaluate the greatest carbamate response on PCLS. During prophylactic use of PB, the aim is to inhibit the AChE-activity to approximately 20% to partly remain normal enzyme function, and to avoid complete inhibition by the nerve agent (Center for Drug Evaluation and Research, Citation2003). To enable comparison with previous in vitro studies, PB was used as a pretreatment 30 min prior to VX-exposure (Herkert et al. Citation2011). In the in vivo studies performed for clinical approval, PB-pretreatment was administered 45 min prior to the nerve agent by intramuscular injections resulting in a rapid absorption to the bloodstream and consequently avoidance of first-pass metabolism (Center for Drug Evaluation and Research, Citation2003). However, the time for distribution to the target organ tissue in vivo is deducted in ex vivo-models such as the PCLS. In the biochemical evaluation of AChE-inhibition for the two selected PB-concentrations and within the 30 min incubation time, the lower concentration met the criteria of adequate residual enzyme activity while the higher concentration completely inhibited the AChE-activity.
Bronchoconstriction induced by EFS was significantly increased by the higher PB-concentration and both concentrations decreased the ability of airways to recover following contraction. Using a similar PCLS experimental set-up, the reversible AChE-inhibitor neostigmine correspondingly increased the maximum airway contraction and prolonged the recovery time following stimulation (Schlepütz et al. Citation2011; Citation2012). Incubation of VX following pretreatment resulted in decreased airway relaxation compared to PB-exposure alone. However, VX-exposure without pretreatment demonstrated similar effects on both airway contraction and relaxation as experiments including pretreatment prior to nerve agent exposure. Overall, no beneficial impact of pretreatment prior to nerve agent exposure was recognized, and the enhanced effects on airway contraction are of concern. It is previously reported that adverse effects of PB mainly involve depressed cardiovascular function, suggested to be caused by interactions between the anticholinesterase compound and nicotinic and muscarinic receptors (McIsaac & Albrecht, Citation1975; Backman et al. Citation1993; Worek et al. Citation1995; Worek & Szinicz, Citation1995; Kuca et al. Citation2018). The increased bronchoconstriction induced by PB may also implicate actions on muscarinic receptors, which is the main post-junctional target on airway smooth muscle cells (Ehlert, Citation2003). Data on the efficacy of pretreatment with PB prior to VX-intoxication has been conflicting between previous in vivo studies, which may be a result of the different animal species, experimental end-points (LD50 compared to specific physiological responses), and post-exposure antidotes used (Koplovitz et al. Citation1992; Koplovitz & Stewart, Citation1994; Worek & Szinicz, Citation1995). In PCLS-studies using human and different animal lung tissues, the impact of neostigmine exposure has also been varying as a result of species-specific airway pharmacology (Schlepütz et al. Citation2012). On the other hand, PB-prophylaxis prior to soman exposure has generally been found efficient and has resulted in improved post-exposure treatment (Koplovitz et al. Citation1989; Koplovitz & Stewart, Citation1994; Center for Drug Evaluation and Research, Citation2003; Haigh et al. Citation2010; Myhrer et al. Citation2013). Putatively, the agent-specific interactions with the target enzyme could influence the pretreatment efficacy (Worek et al. Citation1998). In the in vitro study demonstrating protective measures for PB against GD exposure, the experimental set-up included discontinuation of both AChE-inhibitors that do not correspond to the conditions in a poisoned patient, in which both anticholinesterase compounds may cause re-inhibition of the enzyme (Herkert et al. Citation2011). In addition, mechanistic studies are needed that aim at elucidating all targets involved in the PB-effects demonstrated in the present PCLS-model, such as other enzyme systems or receptors.
Post-exposure treatment with atropine demonstrated the ability to restore airway function, although, the efficacy of the antimuscarinic drug was slightly lower compared to previous studies including only VX-exposure and atropine treatment (Herbert et al. Citation2017; Wigenstam et al. Citation2021). This is consistent with in vivo studies in rodents, showing that PB-pretreatment resulted in reduced or no significant effect on antidotal efficacy, including antimuscarinics and oxime reactivation, following VX-exposure (Koplovitz et al. Citation1992; Worek & Szinicz, Citation1995).
In conclusion, the PCLS model was found to be suitable for studies of the airway-specific impact of nerve agent prophylactic treatment. Furthermore, the complete management following nerve agent exposure, including both prophylaxis and post-exposure treatment, could be evaluated using the PCLS model. The pretreatment with PB was demonstrated to adversely impact on airway function in PCLS and no beneficial effects were observed following VX exposure. Post-exposure treatment with atropine significantly reduced the bronchoconstriction induced by PB- and VX-exposure, although the efficacy was slightly lowered compared to previous experiments including only VX-exposure. Preferably, other prophylactic measures should be prioritized for clinical development such as stoichiometric or catalytic scavengers, which will inactivate the nerve agent prior to inhibiting the AChE (Lenz et al. Citation2007; McGarry et al. Citation2020; Kohler et al. Citation2021). Although improved pretreatment would be beneficial, timely administered post-exposure therapeutics will remain of the highest importance to prevent nerve agent intoxication (Amend et al. Citation2020).
Acknowledgements
Authors would like to thank Karin Wallgren for her excellent technical assistance.
Disclosure statement
The authors declare that they have no conflict of interest.
Data availability statement
The data presented in this work are available on request from the corresponding author.
Additional information
Funding
References
- Amend N, Niessen KV, Seeger T, Wille T, Worek F, Thiermann H. 2020. Diagnostics and treatment of nerve agent poisoning-current status and future developments. Ann N Y Acad Sci. 1479(1):13–28. doi:10.1111/nyas.14336.
- Aracava Y, Pereira EF, Akkerman M, Adler M, Albuquerque EX. 2009. Effectiveness of donepezil, rivastigmine, and (+/−)huperzine A in counteracting the acute toxicity of organophosphorus nerve agents: comparison with galantamine. J Pharmacol Exp Ther. 331(3):1014–1024. doi:10.1124/jpet.109.160028.
- Backman SB, Bachoo M, Polosa C. 1993. Mechanism of the bradycardia produced in the cat by the anticholinesterase neostigmine. J Pharmacol Exp Ther. 265(1):194–200. https://www.ncbi.nlm.nih.gov/pubmed/8474006.
- Beck KD, Zhu G, Beldowicz D, Brennan FX, Ottenweller JE, Moldow RL, Servatius RJ. 2001. Central nervous system effects from a peripherally acting cholinesterase inhibiting agent: interaction with stress or genetics. Ann N Y Acad Sci. 933:310–314. doi:10.1111/j.1749-6632.2001.tb05833.x.
- Bonhage MR, Chilcoat CD, Li Q, Melendez V, Flournoy WS. 2009. Evaluation of two scopolamine and physostigmine pretreatment regimens against nerve agent poisoning in the dog. J Vet Pharmacol Ther. 32(2):146–153. doi:10.1111/j.1365-2885.2008.01013.x.
- Carpenter JM, Brown KA, Diaz AN, Dockman RL, Benbow RA, Harn DA, Norberg T, Wagner JJ, Filipov NM. 2021. Delayed treatment with the immunotherapeutic LNFPIII ameliorates multiple neurological deficits in a pesticide-nerve agent prophylactic mouse model of Gulf War Illness. Neurotoxicol Teratol. 87:107012. doi:10.1016/j.ntt.2021.107012.
- Center for Drug Evaluation and Research. 2003. Clinical pharmacology and biopharmaceutics review(s). Pyridostigmine bromide. Application number: 20–414.
- Dyer O. 2004. US and UK scientists disagree about causes of Gulf war syndrome. BMJ. 329(7472):940. doi:10.1136/bmj.329.7472.940-b.
- Ehlert FJ. 2003. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal, airway and urinary bladder smooth muscle. Life Sci. 74(2-3):355–366. doi:10.1016/j.lfs.2003.09.023.
- Ekstrom F, Pang YP, Boman M, Artursson E, Akfur C, Borjegren S. 2006. Crystal structures of acetylcholinesterase in complex with HI-6, Ortho-7 and obidoxime: structural basis for differences in the ability to reactivate tabun conjugates. Biochem Pharmacol. 72(5):597–607. doi:10.1016/j.bcp.2006.05.027.
- Ellman GL, Courtney KD, Andres V, Feather-Stone RM. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 7:88–95. doi:10.1016/0006-2952(61)90145-9.
- EU. 2010. Directive 2010/63/EU on the protection of animals used for scientific purposes. OJEU. 276/33.
- US Food and Drug Administration. 2003. Pyridostigmine Bromide Tablets, USP 30 mg. 1–17.
- Haigh JR, Adler M, Apland JP, Deshpande SS, Barham CB, Desmond P, Koplovitz I, Lenz DE, Gordon RK. 2010. Protection by pyridostigmine bromide of marmoset hemi-diaphragm acetylcholinesterase activity after soman exposure. Chem Biol Interact. 187(1–3):416–420. doi:10.1016/j.cbi.2010.02.003.
- Herbert J, Thiermann H, Worek F, Wille T. 2017. Precision cut lung slices as test system for candidate therapeutics in organophosphate poisoning. Toxicology. 389:94–100. doi:10.1016/j.tox.2017.07.011.
- Herbert J, Thiermann H, Worek F, Wille T. 2019. COPD and asthma therapeutics for supportive treatment in organophosphate poisoning. Clin Toxicol. 57(7):644–651. doi:10.1080/15563650.2018.1540785.
- Herkert NM, Thiermann H, Worek F. 2011. In vitro kinetic interactions of pyridostigmine, physostigmine and soman with erythrocyte and muscle acetylcholinesterase from different species. Toxicol Lett. 206(1):41–46. doi:10.1016/j.toxlet.2011.03.004.
- Joyce MR, Holton KF. 2020. Neurotoxicity in Gulf War Illness and the potential role of glutamate. Neurotoxicology. 80:60–70. doi:10.1016/j.neuro.2020.06.008.
- Keeler JR, Hurst CG, Dunn MA. 1991. Pyridostigmine used as a nerve agent pretreatment under wartime conditions. JAMA. 266(5):693–695. doi:10.1001/jama.1991.03470050093029.
- Kohler A, Escher B, Job L, Koller M, Thiermann H, Skerra A, Worek F. 2021. Catalytic activity and stereoselectivity of engineered phosphotriesterases towards structurally different nerve agents in vitro. Arch Toxicol. 95(8):2815–2823. doi:10.1007/s00204-021-03094-0.
- Koplovitz I, Harris LW, Anderson DR, Lennox WJ, Stewart JR. 1992. Reduction by pyridostigmine pretreatment of the efficacy of atropine and 2-PAM treatment of sarin and VX poisoning in rodents. Fundam Appl Toxicol. 18(1):102–106. doi:10.1016/0272-0590(92)90201-r.
- Koplovitz I, Romano JA, Stewart JR. 1989. Assessment of motor performance decrement following soman poisoning in mice. Drug Chem Toxicol. 12(3–4):221–235. doi:10.3109/01480548908999155.
- Koplovitz I, Stewart JR. 1994. A comparison of the efficacy of HI6 and 2-PAM against soman, tabun, sarin, and VX in the rabbit. Toxicol Lett. 70(3):269–279. doi:10.1016/0378-4274(94)90121-x.
- Kuca K, Karasova JZ, Soukup O, Kassa J, Novotna E, Sepsova V, Horova A, Pejchal J, Hrabinova M, Vodakova E, et al. 2018. Development of small bisquaternary cholinesterase inhibitors as drugs for pre-treatment of nerve agent poisonings. Drug Des Devel Ther. 12:505–512. doi:10.2147/DDDT.S133038.
- Layish I, Krivoy A, Rotman E, Finkelstein A, Tashma Z, Yehezkelli Y. 2005. Pharmacologic prophylaxis against nerve agent poisoning. Isr Med Assoc J. 7(3):182–187. https://www.ncbi.nlm.nih.gov/pubmed/15792266.
- Lenina OA, Zueva IV, Zobov VV, Semenov VE, Masson P, Petrov KA. 2020. Slow-binding reversible inhibitor of acetylcholinesterase with long-lasting action for prophylaxis of organophosphate poisoning. Sci Rep. 10(1):16611. doi:10.1038/s41598-020-73822-6.
- Lenz DE, Yeung D, Smith JR, Sweeney RE, Lumley LA, Cerasoli DM. 2007. Stoichiometric and catalytic scavengers as protection against nerve agent toxicity: a mini review. Toxicology. 233(1–3):31–39. doi:10.1016/j.tox.2006.11.066.
- Lorenzoni PJ, Kay CSK, Ducci RD, Fustes OJH, Werneck LC, Scola RH. 2020. Celebrating the 70 years of pyridostigmine on therapy of Myasthenia Gravis: historical aspects of the preliminary trials. Arq Neuropsiquiatr. 78(3):179–181. doi:10.1590/0004-282x20190189.
- Macht VA, Woodruff JL, Burzynski HE, Grillo CA, Reagan LP, Fadel JR. 2020. Interactions between pyridostigmine bromide and stress on glutamatergic neurochemistry: insights from a rat model of Gulf War Illness. Neurobiol Stress. 12:100210. doi:10.1016/j.ynstr.2019.100210.
- McGarry KG, Lalisse RF, Moyer RA, Johnson KM, Tallan AM, Winters TP, Taris JE, McElroy CA, Lemmon EE, Shafaat HS, et al. 2020. A Novel, Modified human butyrylcholinesterase catalytically degrades the chemical Warfare nerve agent, Sarin. Toxicol Sci. 174(1):133–146. doi:10.1093/toxsci/kfz251.
- McIsaac RJ, Albrecht E. 1975. Depression of transmission through the isolated superior cervical ganglion of the rat by physostigmine sulphate. Neuropharmacology. 14(2):439–445. doi:10.1016/0028-3908(75)90036-2.
- Miller SA, Blick DW, Kerenyi SZ, Murphy MR. 1993. Efficacy of physostigmine as a pretreatment for organophosphate poisoning. Pharmacol Biochem Behav. 44(2):343–347. doi:10.1016/0091-3057(93)90472-6.
- Myhrer T, Aas P. 2016. Pretreatment and prophylaxis against nerve agent poisoning: are undesirable behavioral side effects unavoidable? Neurosci Biobehav Rev. 71:657–670. doi:10.1016/j.neubiorev.2016.10.017.
- Myhrer T, Enger S, Aas P. 2013. Determination of anti-convulsant and life-preserving capacities of three types of auto-injector therapies against soman intoxication in rats. Drug Test Anal. 5(8):693–701. doi:10.1002/dta.1414.
- Park JH, Lee JY, Kim KT, Joe HE, Cho HJ, Shin YK, Kim DD. 2018. Physostigmine-loaded liposomes for extended prophylaxis against nerve agent poisoning. Int J Pharm. 553(1-2):467–473. doi:10.1016/j.ijpharm.2018.10.053.
- Peng X, Perkins MW, Simons J, Witriol AM, Rodriguez AM, Benjamin BM, Devorak J, Sciuto AM. 2014. Acute pulmonary toxicity following inhalation exposure to aerosolized VX in anesthetized rats. Inhal Toxicol. 26(7):371–379. doi:10.3109/08958378.2014.899410.
- Schlepütz M, Rieg AD, Seehase S, Spillner J, Perez-Bouza A, Braunschweig T, Schroeder T, Bernau M, Lambermont V, Schlumbohm C, et al. 2012. Neurally mediated airway constriction in human and other species: a comparative study using precision-cut lung slices (PCLS). PLoS One. 7(10):e47344. doi:10.1371/journal.pone.0047344.
- Schlepütz M, Uhlig S, Martin C. 2011. Electric field stimulation of precision-cut lung slices. J Appl Physiol (1985). 110(2):545–554. doi:10.1152/japplphysiol.00409.2010.
- Nam JH, Kim MS, Song YJ, Kim CH, Kim WS, Yu CH, Joe HE, Hur GH, Seo MR, Kim Y, et al. 2023. Pretreatment of rhesus monkeys with transdermal patches containing physostigmine and procyclidine: implications of the delivery system for the potential application against VX nerve agent intoxication in humans. Arch Toxicol. 97(3):697–710. doi:10.1007/s00204-022-03438-4.
- Thiermann H, Worek F. 2022. Pro: oximes should be used routinely in organophosphate poisoning. Br J Clin Pharmacol. 88(12):5064–5069. doi:10.1111/bcp.15215.
- Wetherell J, Hall T, Passingham S. 2002. Physostigmine and hyoscine improves protection against the lethal and incapacitating effects of nerve agent poisoning in the guinea-pig. Neurotoxicology. 23(3):341–349. doi:10.1016/s0161-813x(02)00082-7.
- White RF, Steele L, O’Callaghan JP, Sullivan K, Binns JH, Golomb BA, Bloom FE, Bunker JA, Crawford F, Graves JC, et al. 2016. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: effects of toxicant exposures during deployment. Cortex. 74:449–475. doi:10.1016/j.cortex.2015.08.022.
- Wigenstam E, Artursson E, Bucht A, Thors L. 2022. Supplemental treatment to atropine improves the efficacy to reverse nerve agent induced bronchoconstriction. Chem Biol Interact. 364:110061. doi:10.1016/j.cbi.2022.110061.
- Wigenstam E, Forsberg E, Bucht A, Thors L. 2021. Efficacy of atropine and scopolamine on airway contractions following exposure to the nerve agent VX. Toxicol Appl Pharmacol. 419:115512. doi:10.1016/j.taap.2021.115512.
- Von Bredow J, Corcoran K, Maitland G, Kaminskis A, Adams N, Wade J. 1991. Efficacy evaluation of physostigmine and anticholinergic adjuncts as a pretreatment for nerve agent intoxication. Toxicol Sci. 17(4):782–789. doi:10.1093/toxsci/17.4.782.
- Worek F, Kleine A, Szinicz L. 1995. Effect of pyridostigmine pretreatment on cardiorespiratory function in tabun poisoning. Hum Exp Toxicol. 14(8):634–642. doi:10.1177/096032719501400803.
- Worek F, Szinicz L. 1995. Cardiorespiratory function in nerve agent poisoned and oxime + atropine treated guinea-pigs: effect of pyridostigmine pretreatment. Arch Toxicol. 69(5):322–329. doi:10.1007/s002040050178.
- Worek F, Widmann R, Knopff O, Szinicz L. 1998. Reactivating potency of obidoxime, pralidoxime, HI 6 and HLo 7 in human erythrocyte acetylcholinesterase inhibited by highly toxic organophosphorus compounds. Arch Toxicol. 72(4):237–243. doi:10.1007/s002040050495.
