Abstract
We present the case of a 43-year-old-man with wild-type KRAS and BRAF colorectal adenocarcinoma that was metastatic to the liver and lung. The patient initially received neoadjuvant chemotherapy with FOLFOX and bevacizumab, followed by surgical resection of the primary tumor and hepatic metastases. His disease recurred shortly after surgery and he was treated with FOLFIRI plus the anti-EGFR antibody cetuximab. After this regimen failed to arrest his disease progression, treatment with FOLFIRI in combination with another anti-EGFR antibody, panitumumab was started. While on this therapy, the patient's lung nodules remained largely stable but metastatic lesions within the liver continued to progress. Our case highlights the differences between panitumumab and cetuximab, and contemplates the possible explanations for this patient's apparently heterogeneous disease progression within the liver despite stabilization of multiple pulmonary nodules.
Introduction
Colorectal adenocarcinoma is the fourth most common cancer diagnosis in the United States, with nearly 150,000 new cases occurring each year.Citation1 Fortunately, there exists a bevy of screening tests for this disease, permitting most colorectal cancers (CRC) to be discovered at an early stage. Such patients are often eligible for potentially curative surgery. Roughly 20% of patients, however, have metastatic disease at initial presentation. The prognosis for these patients is considerably less favorable, with fewer than 10% surviving beyond 5 yCitation2.
The management of unresectable metastatic CRC has evolved in recent years, particularly with the development of biologic agents that target the epithelial growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF). Currently, the NCCN guidelines recommend combination chemotherapy with FOLFOX (leucovorin, 5-fluorouracil, oxaliplatin), or CAPOX (capecitabine, oxaliplatin) initially, followed by FOLFIRI (leucovorin, 5-fluorouracil, irinotecan) after first progression.Citation3 These regimens are commonly augmented with a monoclonal antibody that targets either VEGF or EGFR. Bevacizumab, the VEGF inhibitor, is generally offered as a component of first line therapy since the mutational status of KRAS has historically been unavailable at the time of therapy initiation.
The anti-EGFR antibodies, cetuximab and panitumumab, are most often administered as second line agents but are approved as first line therapy for colorectal tumors that contain wild-type KRAS. Through competitive antagonism at the EGF receptor, these targeted biologic agents inhibit the activation of intracellular downstream signaling by the RAS/RAF/MEK and the PI3K/AKT/mTOR pathways. These pathways are often exploited by malignant cells to promote angiogenesis,Citation4 progression through the cell cycle, and local invasion via the synthesis of matrix metalloproteinases.Citation5
As cetuximab and panitumumab exert influence over downstream events through inhibition at a cell surface receptor, their efficacy is contingent upon EGFR being the primary stimulus for pathway activation. Unfortunately, some tumor cells harbor activating mutations downstream from the receptor. The most common of these are activating mutations of KRAS at codons 12, 13, or 61.Citation6 Tumors that contain these mutations generally do not respond to EGFR antagonists and thus patients with KRAS (or NRAS) mutated CRC should not receive cetuximab or panitumumab.Citation7
In the treatment of CRC, EGFR antagonists are typically offered to patients with disease that is either metastatic or refractory to first-line chemotherapy. Cetuximab, the older of these 2 agents, has been shown to improve overall response rate and overall survival when used in conjunction with FOLFOX,Citation8,9 or FOLFIRI.Citation10,11 Panitumumab, has demonstrated improved overall survival when administered with FOLFIRICitation12 and in May, 2014 was approved by the FDA in combination with FOLFOX for metastatic colorectal cancer. As a monotherapy, it is also associated with improved progression free survival and objective response rate in metastatic colorectal cancer patients, but without a consistent overall survival benefit.Citation13,14 Emerging research suggests that the benefits of EGFR blockade may surpass that of the VEGF inhibition. FIRE-3, a randomized phase 3 trial evaluated this by comparing FOLFIRI plus cetuximab to FOLFIRI plus bevacizumab as first line treatment for patients with metastatic colorectal cancer.Citation15 While the response rates were similar, patients in the cetuximab arm appeared to have improved overall survival compared to those treated with bevacizumab.
An area of active research is whether patients who fail cetuximab benefit from a trial of panitumumab. Results from a small studyCitation16 suggest that this approach does provide patients with some measure of benefit but the absence of data from a major clinical trial leaves this matter unresolved. Case report data presented at the 2014 ASCO meeting by Montagut et al. demonstrated the emergence of an ecto-domain S492R mutation in EGFR that was detected in circulating tumor DNA following treatment with a cetuximab-containing regimen.Citation17 After therapy with a regimen that contained panitumumab, the amount of circulating tumor DNA containing this S492R EGFR ecto-domain mutation was reduced. As clinical resistance to panitumumab developed, however, circulating tumor DNA containing KRAS mutations appeared. These observations demonstrate the state-of-the-art in clinical therapy, while also illuminating mechanisms by which resistance to targeted therapeutics develops.
Clinical Case Report
A 43-year-old man presented to the emergency department with an ischemic CVA of the basal ganglion in 2010. In the ED, a CT scan incidentally revealed multiple hepatic lesions along with a non-obstructing rectal mass. Biopsy of the rectal lesion showed adenocarcinoma of the rectum with wild-type KRAS and BRAF.
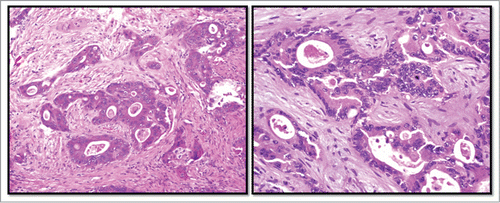
Neoadjuvant chemotherapy with FOLFOX plus bevacizumab was administered for 5 cycles. The patient then underwent right portal vein embolization and multiple surgical resections of hepatic metastases, followed by concurrent chemoradiation therapy with capecitabine. This was followed by an extensive sigmoid colon and rectum resection. Pathology from this procedure found moderately differentiated invasive adenocarcinoma with minimal treatment response, as demonstrated in . Metastatic disease was presented in 7 of 12 lymph nodes sampled.
Figure 1. Histopathology of rectal adenocarcinoma. Legend: Hematoxylin and eosin stained sections of the patient's primary rectal tumor (left) and hepatic metastases (right).
One month later, PET scan showed multiple new FDG-avid lesions within the liver and lung, as well as FDG-avidity along the rectal anastomosis that was concerning for residual or recurrent disease. He resumed treatment with modified FOLFOX plus bevacizumab, which produced a favorable response despite the persistence of residual hepatic disease. Later that year, the patient underwent a repeat small bowel resection with construction of an end loop ileostomy. This was followed by 9 cycles of FOLFIRI plus cetuximab for residual PET-avid disease and rising CA19.9. Unfortunately, his disease continued to progress and he was subsequently treated with FOLFIRI plus bevacizumab.
A repeat PET scan in October, 2013 showed continued growth of metastatic disease in the liver and lung, prompting a change of therapy that now included FOLFIRI plus panitumumab. Following 2 cycles, repeat CT showed stabilization of the pulmonary metastases but continued increase in density and size of hepatic metastases. FOLFIRI plus panitumumab was continued and in January of 2014, the patient also received intrahepatic Y90 radioembolization. Follow-up CT scan one month later demonstrated improvement of the hepatic disease, but with a slight increase in the size of metastatic lesions in the lung. The patient received a total of 7 cycles of FOLFIRI plus panitumumab before imaging showed convincing evidence of his pulmonary disease progressing.
Discussion
This patient presented with metastatic colorectal adenocarcinoma. He received neoadjuvant chemotherapy with FOLFOX and bevacizumab, followed by surgical resection of the primary tumor and hepatic metastases. Unfortunately, his disease showed minimal pathologic response to treatment. As his tumor was wild-type for KRAS and BRAF, he then received cetuximab in combination with FOLFIRI. This arrested the progression of his disease for approximately one year before continued tumor growth necessitated a change of therapy. The patient was then treated with FOLFIRI plus panitumumab.
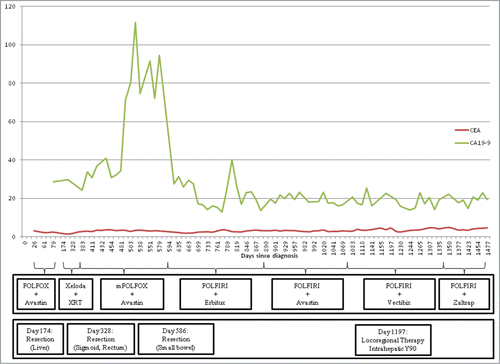
The response to this last treatment with FOLFIRI plus panitumumab was quite mixed. The patient's tumor markers, which are shown graphically in , remained largely stable during treatment with panitumumab. Radiographically, however, his response was considerably more complex. Multiple CT scans demonstrated relative stability of the patient's multiple pulmonary nodules despite continued expansion of the size and number of hepatic metastases. CT images of the patient's hepatic and pulmonary nodules at the start of treatment with panitumumab, approximately 1 y into treatment, and status post intrahepatic radioembolization are provided in below.
Figure 2. Response of tumor markers CEA and CA19–9 to systemic and locoregional treatments Legend: FOLFOX (5-fluorouracil, leucovorin, oxaliplatin), Avastin (bevacizumab), Xeloda (capecitabine), XRT (radiotherapy) mFOLFOX (modified FOLFOX), Erbitux (cetuximab), FOLFIRI (5-fluorouracil, leucovorin, irinotecan ), Vectibix (panitumumab), Zaltrap (aflibercept).
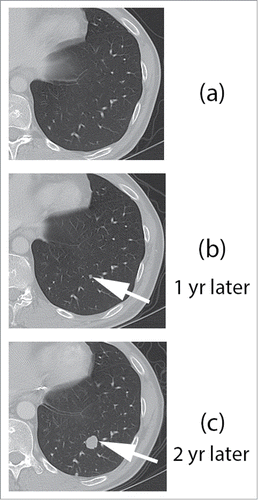
During the same time period, contrast enhanced CT images through the left lower lobe shows no pulmonary nodules (). Imaging 1 y later showed interval development of a small, sub cm left lower pulmonary nodule (, arrow). Follow up CT scan performed 10 months following Y90 SIRSpheres showed disease progression, with marked interval growth of the left lower pulmonary nodule (, arrow). Multiple additional nodules were present through both lungs (not shown).
Figure 3. Response of hepatic lesions to treatment with FOLFIRI plus panitumumab, and Y-90 intrahepatic radioembolization.
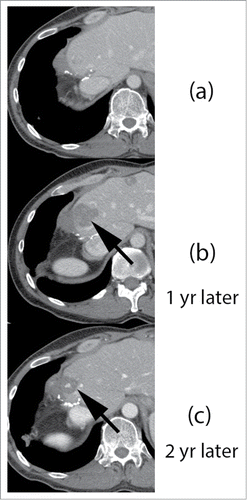
Figure 4. Response of hepatic lesions to treatment with FOLFIRI plus panitumumab, and Y-90 intrahepatic radioembolization. Legend: 42 y old male with metastatic colorectal carcinoma to the liver, underwent low anterior resection and trisegmentectomy of liver metastasis. Contrast enhanced CT () shows post surgical changes following partial hepatectomy. Follow up imaging 1 y later (, arrow) shows interval progression of liver metastasis near the resection margin. Additional liver metastases were present (not shown). Patient subsequently underwent treatment with Y90 SIRSpheres, and follow up imaging 10 months after treatment shows good, long lasting response to targeted liver therapy, with marked interval decrease in liver metastasis (, arrow).
Although certainty is impossible in the absence of biopsies from both a hepatic and pulmonary metastasis, there are several potential explanations for this patient's continued progression of disease in the liver despite stabilization within the lung. Perhaps the simplest explanation is that this patient's lung nodules did not contain metastatic CRC, but were instead inflammatory nodules or another non-malignant process. In this scenario, the general decrease in cell proliferation produced by FOLFIRI would likely suppress the inflammatory response within the lung, thereby limiting the uptake of radio-labeled glucose. In the liver, however, the EGFR inhibitor-resistant tumor continued to grow and thus remained FDG-avid on PET scan. While this explanation is certainly plausible, it is made considerably less likely by the eventual growth of these lung nodules approximately 9 months after starting panitumumab.
The far more intriguing explanation is that this patient's liver and lung contained cetuximab-resistant metastatic CRC with differing degrees of susceptibility to panitumumab. This would likely occur as a consequence of heterogeneity within the primary tumor, thereby enabling it to seed biologically distinct metastatic lesions at different organ sites. While cetuximab and panitumumab are both monoclonal antibodies that bind to the same cell surface receptor, these drugs are not identical. Evidence regarding cross-resistance between these 2 EGFR inhibitors has been limited and mixed. Citation16,18
Structurally, panitumumab is a fully humanized IgG2 antibody,Citation19 while cetuximab contains chimeric human-mouse IgG1 antibodies.Citation20 This difference, though subtle, influences the immunogenicity of the drug. Infusion reactions as a result of anti-chimeric antibodies occur in approximately 2–5% of patients treated with cetuximabCitation21 but are slightly less common with panitumumab. Citation22 In addition to causing infusion reactions, it can be reasonably surmised that these antibodies may also reduce the efficacy of cetuximab by forming complexes that are unable to block EGFR. Several case reports Citation23,24 and a single studyCitation25 suggest that panitumumab is not affected by the presence of anti-cetuximab antibodies.
A second possible mechanism of differential resistance relates to small differences in the binding sites of these drugs. A recent study by Montagut et. al. found that 2 out of 10 patients with cetuximab-resistant but panitumumab-susceptible CRC harbored a specific mutation of EGFR.Citation26 This mutation of the receptor's ecto-domain specifically impairs the binding of cetuximab but not panitumumab. As such, there is likely a subset of patients with wild-type KRAS tumors that develop this mutation and are therefore resistant to cetuximab but may respond to panitumumab. Although the patient described herein was not specifically tested for the panitumumab-sensitive cetuximab-resistant S492R ecto-domain EGFR mutation, it is clear that in the coming era of personalized medicine, such testing may be done routinely to determine which patients with wild-type RAS are most likely to benefit from panitumumab after progressing on cetuximab containing regimens.
With respect to the distribution of panitumumab-sensitive disease in the lung and panitumumab-resistant tumor in the liver, the current body of research does not offer any convincing explanation. Although uncommon, it is not unheard of for different sites of metastatic colorectal carcinoma to differ in their KRAS genetic sequence. Prior studies have demonstrated approximately a 77–96% concordance rate between KRAS mutation status in differing sites of metastatic CRC.Citation27-30 Interestingly, KRAS heterogeneity within a primary CRC tumor has also been well reported,Citation31–33 indicating that instances of non-concordance between metastatic lesions may be the result of a founder effect rather than new mutations arising within a metastasis. When comparing sites of metastatic disease, there does not appear to be a preference for KRAS mutation to develop within the microenvironment of the liver as compared to the lung, nor does there appear to be a tendency for cells with KRAS mutations to preferentially metastasize to one site as compared to others.Citation34 It is clear from emerging knowledge Citation17,35,36 that KRAS mutations are not only acquired after exposure to cetuximab but may also arise following sequential exposure to panitumamab after earlier exposure to cetuximab that led to initial resistance through the EGFR S492R ecto-domain mutation. While molecular testing was not done to confirm our hypothesis in this case, it is conceivable and indeed possible that the lung lesions of this patient may have retained sensitivity to panitumumab while the liver lesions acquired a mutation of RAS that conferred resistance to anti-EGFR therapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Rebecca Siegel M, Deepa Naishadham M, MS, Ahmedin Jemal D, PhD. Cancer Statistics, 2013. CA: A Cancer Journal for Clinicians 2013:11-30.
- Sanoff HK SD, Campbell ME, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Goldberg RM. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol 2008:5721-7; PMID:19001325; http://dx.doi.org/10.1200/JCO.2008.17.7147
- Benson A, Bekaii-Saab T, Chan E, Chen Y-J, Choti MA, Cooper HS, Engstrom PF, Enzinger PC, Fakih M, Fenton MJ et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Colon Cancer National Comprehensive Cancer Network, Inc. Version 3.2013. 2013: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines).
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007; 26:3291-310; PMID:17496923; http://dx.doi.org/10.1038/sj.onc.1210422
- Spano JP, Fagard R, Soria JC, Rixe O, Khayat D, Milano G. Epidermal growth factor receptor signaling in colorectal cancer: preclinical data and therapeutic perspectives. Ann Oncol 2005; 16:189-94; PMID:15668269; http://dx.doi.org/10.1093/annonc/mdi057
- Ciardiello F TG. EGFR antagonists in cancer treatment. Eng J Med 2008:1160-74; PMID:18337605; http://dx.doi.org/10.1056/NEJMra0707704
- Amado RG WM, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008:1626-34; PMID:18316791; http://dx.doi.org/10.1200/JCO.2007.14.7116
- Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, Celik I, Schlichting M, Koralewski P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol 2011; 22:1535-46; PMID:21228335; http://dx.doi.org/10.1093/annonc/mdq632
- Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2009; 27:663-71; PMID:19114683; http://dx.doi.org/10.1200/JCO.2008.20.8397
- Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011; 29:2011-9; PMID:21502544; http://dx.doi.org/10.1200/JCO.2010.33.5091
- Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009; 360:1408-17; PMID:19339720; http://dx.doi.org/10.1056/NEJMoa0805019
- Peeters M PT, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F, Punt CJ, Strickland AH, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010:4706-13; PMID:20921462; http://dx.doi.org/10.1200/JCO.2009.27.6055
- Giusti RM SK, Pilaro AM, Fuchs C, Cordoba-Rodriguez R, Koti K, Rothmann M, Men AY, Zhao H, Hughes M, Keegan P, et al. U.S. Food and Drug Administration approval: panitumumab for epidermal growth factor receptor-expressing metastatic colorectal carcinoma with progression following fluoropyrimidine-, oxaliplatin-, and irinotecan-containing chemotherapy regimens. Clin Cancer Res 2008:1296-302; PMID:18316547; http://dx.doi.org/10.1158/1078-0432.CCR-07-1354
- Van Cutsem E PM, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007; 25:1658-64; PMID:17470858; http://dx.doi.org/10.1200/JCO.2006.08.1620
- Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014; 15:1065-75; PMID:25088940; http://dx.doi.org/10.1016/S1470-2045(14)70330-4
- Wadlow RC, Hezel AF, Abrams TA, Blaszkowsky LS, Fuchs CS, Kulke MH, Kwak EL, Meyerhardt JA, Ryan DP, Szymonifka J, et al. Panitumumab in patients with KRAS wild-type colorectal cancer after progression on cetuximab. The oncologist 2012; 17:14; PMID:22210091
- Montagut C, Bellosillo B, Gonzalez I, Martinez A, Dalmases A, Iglesias M, et al. Evolution of heterogeneous mechanisms of acquired resistance to cetuximab-based therapy in colorectal cancer. J Clin Oncol 2014; 32: Suppl; abstr 3526. http://meetinglibrary.asco.org/content/133355-144
- Sonoda H, Mekata E, Tomoharu S, Endo Y, Tani T. Safety and efficacy of panitumumab therapy after metastatic colorectal cancer progression with cetuximab: Experience at a single Japanese institution. Oncol Lett 2013:1331-4; PMID:23599789
- Peeters M PT, Van Laethem JL. Anti-epidermal growth factor receptor monotherapy in the treatment of metastatic colorectal cancer: where are we today? Oncologist 2009:29-39; PMID:19144681; http://dx.doi.org/10.1634/theoncologist.2008-0167
- Vincenzi B SD, Tonini G. New issues on cetuximab mechanism of action in epidermal growth factor receptor - negative colorectal cancer: the role of vascular endothelial growth factor. J Clin Oncol 2006:1957-8; PMID:16622275; http://dx.doi.org/10.1200/JCO.2005.05.0450
- Squibb B-M. Erbitux (Cetuximab) package insert. New York, NY, and Princeton, NJ: ImClone Systems Incorporated, 2006.
- Inc. A. Vectibix (Panitumumab) package insert. Thousand Oaks, CA, 2006.
- Heun J, Holen K. Treatment with panitumumab after a severe infusion reaction to cetuximab in a patient with metastatic colorectal cancer: a case report. Clin Colorectal Cancer 2007; 6:529-31; PMID:17553202; http://dx.doi.org/10.3816/CCC.2007.n.019
- Helbling D, Borner M. Successful challenge with the fully human EGFR antibody panitumumab following an infusion reaction with the chimeric EGFR antibody cetuximab. Ann Oncol. England, 2007:963-4; PMID:17488734, http://dx.doi.org/10.1093/annonc/mdm130
- Resch G, Schaberl-Moser R, Kier P, Kopetzky G, Scheithauer W, Sliwa T, Greil R, Nösslinger T, Mayrbäurl B, Thaler J. Infusion reactions to the chimeric EGFR inhibitor cetuximab–change to the fully human anti-EGFR monoclonal antibody panitumumab is safe. Ann Oncol. England, 2011; 22:486-7; PMID:21239398
- Montagut C, Dalmases A, Bellosillo B, Crespo M, Pairet S, Iglesias M, Salido M, Gallen M, Marsters S, Tsai SP, et al. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat Med 2012:221-3; PMID:22270724; http://dx.doi.org/10.1038/nm.2609
- Santini D, Loupakis F, Vincenzi B, Floriani I, Stasi I, Canestrari E, Rulli E, Maltese PE, Andreoni F, Masi G, et al. High concordance of KRAS status between primary colorectal tumors and related metastatic sites: implications for clinical practice The Oncologist 2008:1270-5; PMID:19056857; http://dx.doi.org/10.1634/theoncologist.2008-0181
- Baldus SE, Schaefer K-L, Engers R, Hartleb D, Stoecklein NH, Gabbert HE. Prevalence and heterogeneity of KRAS, BRAF, and PIK3CA mutations in primary colorectal adenocarcinomas and their corresponding metastases. Clin Cancer Res 2010:790-9; PMID:20103678; http://dx.doi.org/10.1158/1078-0432.CCR-09-2446
- Artale S, Sartore-Bianchi A, Veronese SM, Gambi V, Sarnataro CS, Gambacorta M, Lauricella C, Siena S. Mutations of KRAS and BRAF in Primary and Matched Metastatic Sites of Colorectal Cancer J Clin Oncol 2008:4217-9; PMID:18757341
- Knijn N, Mekenkamp LJM, Klomp M, Vink-Börger ME, Tol J, Teerenstra S, Meijer JW, Tebar M, Riemersma S, van Krieken JH, et al. KRAS mutation analysis: a comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br J Cancer 2011:1020-6; PMID:21364579; http://dx.doi.org/10.1038/bjc.2011.26
- Ishii M, Sugai T, Habano W, and S.-i. Nakamura S-i. Analysis of Ki-ras gene mutations within the same tumor using a single tumor crypt in colorectal carcinomas. J Gastroenterol 2014; 39:544-549. http://link.springer.com/article/10.1007/s00535-003-1340-3
- Ishii M, Sugai T, Habano W, Nakamura S. Analysis of Ki-ras gene mutations within the same tumor using a single tumor crypt in colorectal carcinomas. J Gastroenterol 2004; 39:544-9; PMID:15235871; http://dx.doi.org/10.1007/s00535-003-1340-3
- Al-Mulla F, Going JJ, Sowden ET, Winter A, Pickford IR, Birnie GD. Heterogeneity of mutant versus wild-type Ki-ras in primary and metastatic colorectal carcinomas, and association of codon-12 valine with early mortality. J Pathol 1998; 185:130-8; PMID:9713338; http://dx.doi.org/10.1002/(SICI)1096-9896(199806)185:2%3c130::AID-PATH85%3e3.0.CO;2-M
- Oudejans JJ SR, Zoetmulder FA, Mooi WJ, Rodenhuis S. Differential activation of ras genes by point mutation in human colon cancer with metastases to either lung or liver. Int J Cancer 1991:875-9; PMID:1959991; http://dx.doi.org/10.1002/ijc.2910490613
- Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, Valtorta E, Schiavo R, Buscarino M, Siravegna G, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 2012; 486:532-6; PMID:22722830
- Diaz LA, Jr., Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, Allen B, Bozic I, Reiter JG, Nowak MA, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 2012; 486:537-40; PMID:22722843
