Abstract
Resistant maltodextrin Fibersol-2 is a soluble and fermentable dietary fiber that is Generally Recognized As Safe (GRAS) in the United States. We tested whether Fibersol-2 contains anti-tumor activity. Human colorectal cancer cell line, HCT116, and its isogenic cells were treated with FIbersol-2. Tumor growth and tumorigenesis were studied in vitro and in vivo. Apoptotic pathway and generation of reactive oxygen species (ROS) were investigated. We discovered that Fibersol-2 significantly inhibits tumor growth of HCT116 cells by inducing apoptosis. Fibersol-2 strongly induces mitochondrial ROS and Bax-dependent cleavage of caspase 3 and 9, which is shown by isogenic HCT116 variants. Fibersol-2 induces phosphorylation of Akt, mTOR in parental HCT116 cells, but not in HCT116 deficient for Bax or p53. It prevents growth of tumor xenograft without any apparent signs of toxicity in vivo. These results identify Fibersol-2 as a mechanism-based dietary supplement agent that could prevent colorectal cancer development.
Abbreviations
| BAX | = | Bcl-2-associated X protein |
| MFI | = | mean fluorescence intensity |
| mTOR | = | mammalian target of rapamycin |
| PARP | = | poly ADP reibose polymerase |
| ROS | = | reactive oxygen species |
Introduction
Colorectal cancer is one of the most common malignant diseases of Western societies and its incidence appears to be strongly influenced by environmental factors.Citation1 Diets that are low in fat and high in fruits, vegetables, and fiber, are associated with a lower cancer risk.Citation1-3
Physical and chemical properties of dietary fibers have been extensively investigated using epidemiological methods but have not been confirmed conclusively.Citation4,5 It has been implicated that fermentation of dietary fiber into short-chain fatty acids subsequently decreases soluble bile acids in the large bowel.Citation5-8 Other possible mechanisms are an increase in fecal bulking, which could dilute carcinogenic components in the feces in transit time.Citation9-12 However, not all dietary fibers indicate the same biological effects on fermentation and fecal bulking.Citation12-15 Thus, biological activities of dietary fiber to lower colon cancer risk remains to be conclusive.
Resistant starch is insoluble and an undigested starch that reaches the colon undigested, just like dietary fiber.Citation16 It is a potential source of fermentable substrate and decreases the fecal bile acidsCitation17,18 and fecal pH,Citation19 increases total fecal bulking Citation18,19 and excretion of short-chain fatty acid.Citation18,19,20 Thus, resistant starch may have similar activities with dietary fiber to regulate fermentation and fecal bulking. Recent clinical studies have shown that the healthy volunteers could take resistant starch up to 6 times of the daily amount in a normal Western diet, indicating that resistant starch is possible alternative for dietary fiber.Citation18
Distinct from resistant starch, Fibersol-2 is a soluble and fermentable dietary fiber made from corn starch. It is a nonviscous low-calorie bulking fiber and has been used with a variety of food applications. It contains numerous starch linkages that remain undigested by enzymes in the digestive tract. Resistant maltodextrin is not digested or absorbed in the human small intestine, and thus passes to the large intestine where it is fermented by the colonic bacteria producing short-chain fatty acids, lower the pH, gaseous byproducts and beneficial bacteria.Citation5 Clinical studies have shown that postprandial blood concentration of glucose, insulin and serum lipids are decreased and fecal volume is increased after consumption of Fibersol-2.Citation21 Continuous consumption of Fibersol-2 also decreases the risk factors of metabolic syndrome with improved glucose and lipid metabolism.Citation22
In the current studies, we investigated whether Fibersol-2 contains anti-tumor activity in cell culture and mouse model systems. We found that Fibersol-2 significantly increases mitochondrial reactive oxygen species (ROS). Isogenic cell lines of HCT116 variants indicate that cleavage of PARP and caspase 3 by Fibersol-2 is p53 and Bax dependent. Anti-tumor activity in vivo was demonstrated by the decreased tumor development in mouse xenograft. These results establish the usefulness of Fibersol-2 for the prevention human colorectal cancer.
Results
Fibersol-2 induces apoptosis and suppresses the anchorage independent HCT116 p53(-) cells growth
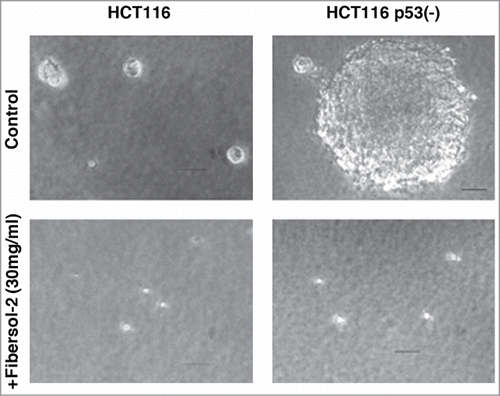
Inhibition of tumor growth by Fibersol-2 was explored by colony formation assay in soft agar. HCT116 and HCT116 p53(-) cells Citation23 were plated in soft agar, and colony formation was observed. As shown in , both HCT116 and HCT116 p53(-) cells formed colonies in 7 to 10 days. Diameter of colonies of HCT116 p53(-) cells was much larger than those of HCT116 cells. Significantly, when Fibersol-2 was provided, both of these cells did not grow in soft agar, indicating that Fibersol-2 directly inhibits tumorigenecity.
Figure 1. Colony formation in soft agar is strongly inhibited by Fibersol-2. Parental HCT116 and p53(-) cells (1 × 104 cells/60 mm plate) were treated with 3% Fibersol-2 in soft agar culture. Colonies were photographed at day 10. Data were presented as representative of 3 independent experiments.
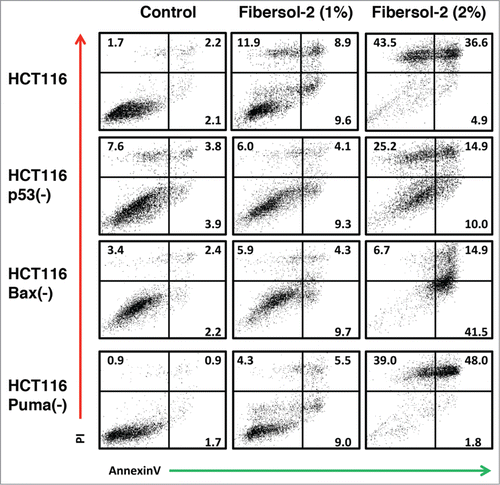
Anti-tumor activities of Fibersol-2 were studied by FACS/Annexin V analysis (). Isogenic cell lines, HCT116, HCT116 p53(-), HCT116 Bax(-) and HCT116 Puma(-),Citation24,25 were treated with PBS or Fibersol-2 (final concentration 1% or 2%) for 24 h. Apoptosis was determined by Annexin V/PI double staining. As shown in , apoptosis induced by Fibersol-2 (1%) was 30.4%, 19.4%, 19.9%, and 18.8%, and apoptosis induced by Fibersol-2 (2%) was 85%, 50.1%, 63.1%, and 88.8% in HCT116, HCT116 p53(-), HCT116 Bax(-) cells, and HCT116 Puma(-) cells, respectively. These results show that p53 or Bax, but not Puma, is essential for the maximal induction of apoptosis by Fibersol-2. Besides, HCT116 p53(-) and HCT116 Bax(-) cells still underwent lower levels of apoptosis, suggesting that FIbersol-2 can induce apoptosis in p53/Bax-independent manner.
Figure 2. Fibersol-2 induces apoptosis of Isogenic HCT116 cell lines. Parental and 3 different isogenic knockout HCT116 cell lines (p53(-), Bax(-), and Puma(-)) were treated with vehicle only (1 × PBS, control), 1% or 2% Fibersol-2 (in 1X PBS). After 24 h, cells were harvested and analyzed of apoptotic cell population by double staining with PtdIns/annexinV as described in M&M. Figure was representative of 3 independent experiments.
Fibersol-2 regulates cell survival signals
We next studied the biochemical analysis of pro-apoptotic proteins when cells are treated with Fibersol-2 (1% or 2%) for 24 hours (). Cleaved forms of PARP, caspase 3 and caspase 9 are detected in HCT116 and HCT116 Puma(-) cells, but not in HCT116 p53(-) and HCT116 Bax(-) cells. Together with the results of Annexin V staining of apoptotic cells (), these results indicate that cleavage of these proteins in HCT116 cells is essential for the maximal induction of apoptosis by Fibersol-2.
Figure 3. Fibersol-2 treatment activates both pro-apoptotic and anti-apoptotic signaling pathways. 1% and 2% Fibersol-2 (10 and 20 mg/ml in PBS) were added to culture media of parental and isogenic knockout HCT116 cell lines (p53(-), Bax(-), and Puma(-)) (A & B). After 24 h, total lysates were prepared and subjected to western blot analysis (30μg/sample). Doxorubicin (1 μM) was used as positive control to induce DNA damage signaling pathway (B).
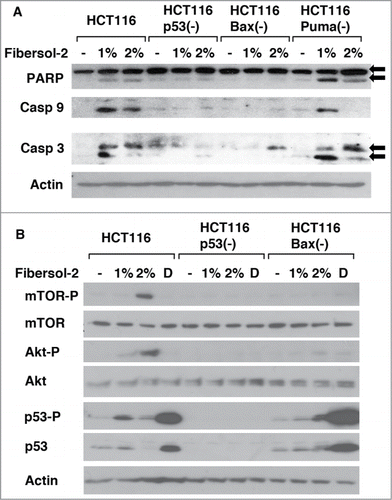
Regulation of cell survival by mTOR/Akt pathway has been well illustrated.Citation26-28 Interestingly, treatment of HCT116 cells with Fibersol-2 induced phosphorylation of mTOR at Ser2481, Akt at Ser473 and p53 at Ser15 in dose dependent manner (). In these experiments, doxorubicin was used as a positive control to induce p53 phosphorylation. Of note, phosphorylation of mTOR and Akt is p53-dependent since it is not observed in HCT116 p53(-) cells. Furthermore, phosphorylation of these proteins is not induced in HCT116 Bax(-) cells, although p53 is similarly phosphorylated in those cells. These results suggest that p53/Bax axis is important to induce phosphorylation of mTOR and Akt when exposed to Fibersol-2.
Fibersol-2 induces mitochondrial ROS
It has been extensively studied that Bax plays crucial roles for mitochondria-mediated apoptosis.Citation29 We studied whether Fibersol-2 regulates mitochondria homeostasis. We examined mitochondrial integrity and ROS production in HCT116 and HCT116 p53(-) cells (). JC-1 was used as a fluorescence marker of mitochondrial transmembrane potential (ΔΨμ) as previously described.Citation30,31 When mitochondria functions normally, the JC-1 concentrates inside mitochondrial membrane. A cytotoxic event that dissipates the mitochondrial membrane potential prevents the accumulation of the JC-1 dye in the mitochondrial matrix and thus, JC-1 remains as a monomeric form (green fluorescent) in cytoplasm. Fibersol-2 treatment induced increased JC-1 monomeric signals in HCT116, indicating that Fibersol-2 perturbs mitochondrial membrane potential. Basal levels of JC-1 signals were higher in HCT116 p53(-) cells, and they did not change after Fibersol-2 treatment in those cells, suggesting that mitochondria function is continuously perturbed in HCT116 p53(-) cells.
Figure 4. Fibersol-2 increases mitochondrial ROS of HCT116 and HCT116 p53(-) cells. Parental and p53(-) HCT116 were incubated in 2% or 4% Fibersol-2 containing cDMEM for 24 h, and then stained with JC-1 dye at 2.5 μg/ml for 10 min (upper panel). Mitochondrial ROS levels in parental and p53(-) HCT116 cells were detected by MitoSox (lower panel). Quantitative flow cytometry data were expressed as ΔMFI. Data were representative of 2 independent experiments.
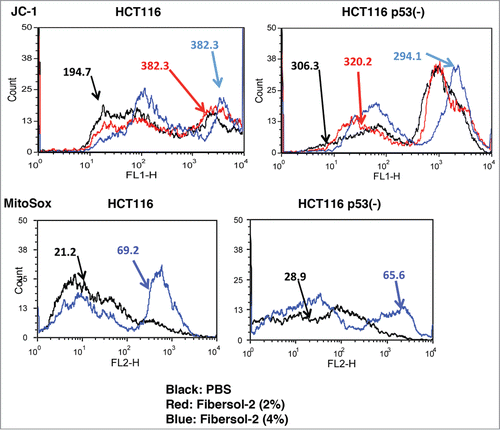
Next, we measured levels of mitochondrial ROS with MitoSox, which are indicated by MFI (mean fluorescence intensity). Treatment with Fibersol-2 resulted in a high increment in MitoSox fluorescence intensity in HCT116 (3.3 fold), compared to HCT116 p53(-) (2.3 fold). These results indicate that treatment with Fibersol-2 induces perturbation of mitochondria homeostasis, such as change in mitochondrial membrane potential and increase in mitochondrial ROS.
Fibersol-2 decreases tumor development of HCT116 in xenograft study
Inhibition of tumor growth by Fibersol-2 was further investigated with HCT116 xenograft model. On day 7 when small size of tumors was observed, we started s.c. injection of Fibersol-2 (1% and 5% in water) with these mice, and tumor growth was measured at indicated day. As demonstrated in , both 1% and 5% of Fibersol-2 significantly decreased tumor growth during this time course. Tumor growth of xenograft of a breast cancer cell line, MDA-MB-468 cells, was also delayed, although this inhibition was not as effective as that seen in HCT116 cells (data not shown). Given the results showing that FIbersol-2 induces caspase-mediated apoptosis (), it is assumed that injected Fibersol-2 directly decreases tumor growth by activating apoptosis pathway.
Figure 5. Inhibition of tumor growth by Fibersol-2. HCT116 cells were transplanted into nude mice as described in M&M. 1% or 5% Fibersol-2 solution (in ddH2O) was injected (s.c.) around tumor. Tumor size was measured at indicated times.
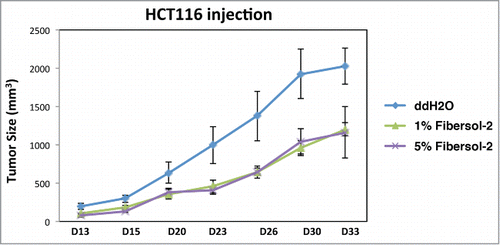
These in vivo results demonstrate that Fibersol-2 contains a mechanism-based anti-tumor activity, and suggest that it can be considered for clinical application for colorectal tumors.
Discussion
It has been debated whether fiber consumption can reduce colorectal cancer incidence.Citation32,33 At least 2 possible mechanisms of how dietary fiber inhibits colorectal cancer development have been implicated; (a) it maintains digestive movement of the bowels to minimize the exposure to carcinogens, and (b) metabolites (e.g. butyrate) from bacterial fermentation in intestine inhibit tumor cell growth.Citation34 However, it has not been well explored whether dietary fiber directly affects cancer cell proliferation. The present study first describes about anti-cancer effects of specific dietary fiber (resistant maltodextrin), Fibersol-2. The treatment with Fiberso-2 resulted in apoptosis and reduction in tumor development in xenograft model.
Regulation of apoptosis by p53 has been extensively studied.Citation35 To understand the roles of p53 pathway in inducing apoptosis by Fibersol-2, we used isogenic colon cancer cell lines generated from HCT116 cells in which p53-associated genes are disrupted individually. Compared to the parental HCT116 cells, HCT116 p53(-) and HCT116 Bax(-) cells showed less apoptosis when treated with Fibersol-2, suggesting the Fiberso-2's apoptotic activities are at least in part p53/Bax dependent. We also observed that Fibersol-2 induced activation of caspase 3 and 9 in the parental HCT116 cells, but not in HCT116 p53(-) and HCT116 Bax(-) cells, indicating that cleavage of caspase 3 and 9 induced by Fibersol-2 is p53/Bax dependent. These results suggest that FIbersol-2's cytotoxicity is mediated by cleaved caspase 3 and 9.
We found that Fibersol-2 reduces mitochondrial membrane potential, causing mitochondrial malfunction, and increases mitochondrial ROS production (). These results, together with other biochemical analyses (), suggest a model that increase in levels of ROS activates p53, resulting in induction of Bax. Elevated Bax induces translocation of cytochrome c and Apaf-1, causing formation of apoptosome containing caspase 9.Citation36
Colony formation of HCT116 p53(-) cells in soft agar was strongly inhibited by Fibersol-2, showing p53 independent mechanism of cytotoxicity. Consistent with this model, HCT116 p53(-) cells underwent apoptosis when treated with Fibersol-2, although efficiency is lower compared to the parental HCT116 cells. These results support a hypothesis that Fibersol-2 can induce apoptosis by p53 dependent and independent manner. Puma is one of the transcriptional targets of p53 and it interacts with anti-apoptotic Bcl2 family member proteins, releasing pro-apoptotic proteins, Bax and Bak, leading to cell death through mitochondrial apoptosis pathway.Citation37 Our results indicating Puma-independent Fibersol-2's activity are consistent with the recent study showing stress-induced cell death by Puma-independent pathway characterized by rapid cell shrinkage and nuclear condensation.Citation38
It has been well illustrated that Akt/mTOR signaling is involved in cell proliferation and anti-apoptosis.Citation26-28 However, recent reports described that single or combination treatment of anti-cancer drugs induces apoptosis through ROS/Akt signaling in colorectal cancer cells.Citation39,40 In fact, Akt regulates tumor cell death induced by nutritional starvation and ROS production.Citation41 In our experiments, although Fibersol-2 induced mitochondrial ROS in both HCT116 and HCT116 p53(-) cells (), phosphorylation of Akt/mTOR was observed only in HCT116 cells in which p53 is wild type (). Furthermore, phosphorylation of mTOR and Akt is p53/Bax-dependent since it was not observed in HCT116 p53(-) and Bax(-) cells. Therefore, our findings suggest that p53/Bax axis is involved in activation of mTOR/Akt pathway during Fibersol-2-induced CRC cell apoptosis, although the mechanism of Akt activation by p53 remains to be elucidated. Given that Fibersol-2 induces apoptosis of these isogenic HCT116 variants (), it is suggested that Fibersol-2 produces ROS and subsequent apoptosis in p53/Akt-dependent and independent manner. Doxorubicin-induced DNA damage strongly induced p53 phosphorylation, but not phosphorylation of Akt, suggesting that Fibersol-2-induced activation of Akt is not due to DNA damage.
In summary, our study first characterized the novel effect of dietary fiber on cellular growth and tumor development by in vitro and in vivo experiments using colorectal cancer cell line. In addition, on-going comparison study between Fibersol-2 and other commercial dietary fibers shows that Fibersol-2 has more suppressive effects than the other on the growth of HCT116 cells (in progress). It has been shown that approximately 10% of Fibersol-2 is digested and absorbed in the small intestineCitation42,43 , therefore, it is suggested that this fraction of Fibersol-2 induces anti-tumor activity after circulation in the blood. On the basis of our results, a protocol of the Phase II clinical trial for the high risk colorectal cancer patients has been just approved at Roswell Park Cancer Institute, in which oral administration of Fibersol-2 will be studied.
Further biochemical analysis using Fibersol-2 fractions will contribute to a better understanding how Fibersol-2 directly suppresses cancer cell growth. Finding from these studies can assist the development of an anti-tumor adjuvant, and potential application against human colorectal cancer.
Materials and Methods
Cells and reagents
HCT116 (human colorectal carcinoma) cells were purchased from ATCC (CCL-247, Manassas, VA), HCT116 p53(-), HCT116 Bax(-), and HCT116 Puma(-) cells were kindly provided by Dr. B. Vogelstein (Johns Hopkins University, Baltimore, MD) who had originally established these cell lines. All of these cells were grown in MoCoy's 5a media (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Invitrogen), penicillin/streptomycin (100 units/ml, Invitrogen). Fibersol-2 was provided by Matsutani America (Itasca, IL), and solubilized in 1X PBS for cells or water (w/v) (for mouse feeding). Mitochondrial superoxide indicator (MitoSOX) was purchased from Invitrogen. JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbeznimidazolylcarbocyanine iodide) was obtained from eBioscience (San Diego, CA). Doxorubicin was purchased from Sigma-Aldrich (St. Louis, MO).
Soft Agar Assay for anchorage independent cell growth
DMEM supplemented with 10% calf serum and 0.4% agarose containing 1 × 10Citation4 of either HCT116 and HCT116 p53(-) cells were plated on DMEM supplemented with 0.5% agar, 10% calf serum and 3% of Fibersol-2. Cell culture was maintained for 7 to 10 days and colony formation was studied under a microscope. Photographs were taken under 40× magnifications using a SPOT Insight mosaic microscope camera (SPOT Imaging Solutions, Sterling Heights, MI) attached to Leica DM IRB microscope (Buffalo Grove, IL).
Flow cytometry for apoptosis analysis
HCT116, HCT116 p53(-), HCT116 Bax(-), and HCT116 Puma(-) cells were treated with or without Fibersol-2 (1% or 2%). After 24 h culture, the rate of apoptotic cells were examined after double staining with Annexin V-FITC/Propidium Iodide (PI) by a flow cytometer (LSR II, BD Biosciences, Franklin Lakes, NK). The data were analyzed with FCS Express 4 program (De Novo software, Los Angeles, CA).
Western blot assay
Cells were treated Fibersol-2 (1% or 2%) or Doxorubicin (1 μM) for 24 h, then lysed in ice-cold lysis buffer (50 mM Tris-HCl (pH 7.6),150 mM NaCl, 1 mM EDTA (pH 8.0), 20 mM NaF, 1 mM Na3VO4, 1% NP40, 0.5 mM dithiothreitol) in the presence of protease–inhibitors (leupeptin, aprotinin, and PMSF, 10 mg/ml, respectively). Total cell lysate (30 μg) was loaded in 15 or 6% SDS-PAGE, and transferred to a PVDF membrane (Immobilon-P, Millipore, Billerica, MA). Primary antibodies used in this study were anti-Akt, anti-mTOR, anti-PARP, anti-cleaved caspase 3 (Asp175), anti-cleaved caspase 9 (Asp330) (Cell signaling Technology, Danvers, MA), and anti-p53 (Santa Cruz Biotechnology, Santa Cruz, CA). Also specific anti-phosphorylation antibodies were used against phospho-Akt (Ser473), phosphor-mTOR (Ser2448), and phosphor-p53 (Ser15) (Cell signaling). Actin was detected as internal loading control.
Measurement of mitochondrial membrane potential and ROS
HCT116 and HCT116 p53(-) cells were treated with 2% or 4% Fibersol-2 for 24 h. To determine mitochondrial membrane potential (ΔΨm) , cells were incubated with JC-1 (2.5 μg/ml) for 10 mim at room temperature and then analyzed as described.Citation31 For measurement of mitochondrial ROS, cells were cultured in media containing MitoSox (5 μM) for 10 min at 37°C. Single cell suspensions were quantitatively analyzed by flow cytometry. Data were expressed as histogram for Green (FL1, JC-1) or Red fluorescence (FL2, MitoSox), indicated by MFI (mean fluorescence intensity).
Tumor formation in nude mice
Female athymic nude mice (n = 10) were purchased from Jackson Lab (Bar Harbor, Maine), and housed in a pathogen-free facility of Roswell Park Cancer Institute. A total 5 × 106 HCT116 cells were subcutaneously (s.c.) injected into the flanks. Fibersol-2 was solubilized in water (1% and 5%) and autoclaved. When small size of tumors was developed (200 ∼ 400 mm3), tumor was s.c. injected with Fibersol-2. Tumor growth was measured at day 13, 15, 20, 26, 30 and 33 after injection and tumor volume was determined using the equation:= (width)2 × length/2. These procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Roswell Park Cancer Institute.
Acknowledgment
We thank all the members of the Ouchi Laboratory for helpful discussion. We particularly thank Dr. Bert Vogelstein at Johns Hopkins University for providing us with isogenic HCT116 cell lines.
Author Contributions
E.Y.S., M.O., S.C.S., S.L.O. and T.O. planned and generated all the data. E.Y.S, D.R., K.S., and T.O. discussed the results, and E.Y.S. and T.O. wrote the paper.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work is supported by Matsutani America Cancer Research Fund, R01CA79892, R01CA90631, and 5P30CA16056 from National Institutes of Health, and Breast Cancer Research Grant from Susan G. Komen Foundation.
References
- Potter JD. Nutrition and colorectal cancer. Cancer Causes Control 1996; 7(1):127-46; PMID:8850441; http://dx.doi.org/10.1007/BF00115644
- Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: a review. J Am Dietetic Assoc 1996; 96(10):1027-39; PMID:8841165; http://dx.doi.org/10.1016/S0002-8223(96)00273-8
- Hill MJ. Cereals, cereal fibre and colorectal cancer risk: a review of the epidemiological literature. Eur J Cancer Prevention 1997; 6(3):219-25; PMID:9306072; http://dx.doi.org/10.1097/00008469-199706000-00002
- Kim YI. AGA technical review: impact of dietary fiber on colon cancer occurrence. Gastroenterology 2000; 118(6):1235-57; PMID:10833499; http://dx.doi.org/10.1016/S0016-5085(00)70377-5
- Asp NG. Dietary fibre–definition, chemistry and analytical determination. Mol Aspects Med 1987; 9(1):17-29; PMID:3031413; http://dx.doi.org/10.1016/0098-2997(87)90014-8
- Song CW, Lee H, Dings RP, Williams B, Powers J, Santos TD, Choi BH, Park HJ. Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Scientific reports 2012; 2:362; PMID:22500211
- Goodwin PJ, Stambolic V. Obesity and insulin resistance in breast cancer–chemoprevention strategies with a focus on metformin. Breast 2011; 20 Suppl 3:S31-35; PMID:22015290; http://dx.doi.org/10.1016/S0960-9776(11)70291-0
- Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med 2011; 9:33; PMID:21470407; http://dx.doi.org/10.1186/1741-7015-9-33
- Cummings JH, Hill MJ, Jenkins DJ, Pearson JR, Wiggins HS. Changes in fecal composition and colonic function due to cereal fiber. Am J Clin Nutr 1976; 29(12):1468-73; PMID:998555
- Cummings JH, Branch W, Jenkins DJ, Southgate DA, Houston H, James WP. Colonic response to dietary fibre from carrot, cabbage, apple, bran. Lancet 1978; 1(8054):5-9; PMID:74533; http://dx.doi.org/10.1016/S0140-6736(78)90357-4
- Fleming SE, O'Donnell AU, Perman JA. Influence of frequent and long-term bean consumption on colonic function and fermentation. Am J Clin Nutr 1985; 41(5):909-18; PMID:2986447
- Haack VS, Chesters JG, Vollendorf NW, Story JA, Marlett JA. Increasing amounts of dietary fiber provided by foods normalizes physiologic response of the large bowel without altering calcium balance or fecal steroid excretion. Am J Clin Nutr 1998; 68(3):615-22; PMID:9734738
- Fleming SE, Marthinsen D, Kuhnlein H. Colonic function and fermentation in men consuming high fiber diets. J Nutr 1983; 113(12):2535-44; PMID:6317826
- Reddy B, Engle A, Katsifis S, Simi B, Bartram HP, Perrino P, Mahan C. Biochemical epidemiology of colon cancer: effect of types of dietary fiber on fecal mutagens, acid, and neutral sterols in healthy subjects. Cancer Res 1989; 49(16):4629-35; PMID:2545348
- Chen HL, Haack VS, Janecky CW, Vollendorf NW, Marlett JA. Mechanisms by which wheat bran and oat bran increase stool weight in humans. Am J Clin Nutr 1998; 68(3):711-9; PMID:9734752
- Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr 1992; 46 Suppl 2:S33-50; PMID:1330528
- Hylla S, Gostner A, Dusel G, Anger H, Bartram HP, Christl SU, Kasper H, Scheppach W. Effects of resistant starch on the colon in healthy volunteers: possible implications for cancer prevention. Am J Clin Nutr 1998; 67(1):136-42; PMID:9440388
- van Munster IP, Tangerman A, Nagengast FM. Effect of resistant starch on colonic fermentation, bile acid metabolism, and mucosal proliferation. Dig Dis sciences 1994; 39(4):834-42; PMID:8149850; http://dx.doi.org/10.1007/BF02087431
- Phillips J, Muir JG, Birkett A, Lu ZX, Jones GP, O'Dea K, Young GP. Effect of resistant starch on fecal bulk and fermentation-dependent events in humans. Am J Clin Nutr 1995; 62(1):121-30; PMID:7598054
- Cummings JH, Beatty ER, Kingman SM, Bingham SA, Englyst HN. Digestion and physiological properties of resistant starch in the human large bowel. Br J Nutr 1996; 75(5):733-47; PMID:8695600; http://dx.doi.org/10.1079/BJN19960177
- ToKunaga K, Matsuoka A. Effects of a food for specified health use (FOSHU) which contains indigestible dextrin as an effective ingredient on glucose and lipid metabolism. J Japan Diabetes Soc 1999; 42:61-5
- Fastinger ND, Karr-Lilienthal LK, Spears JK, Swanson KS, Zinn KE, Nava GM, Ohkuma K, Kanahori S, Gordon DT, Fahey GC Jr. A novel resistant maltodextrin alters gastrointestinal tolerance factors, fecal characteristics, and fecal microbiota in healthy adult humans. J Am Nutr 2008; 27(2):356-66; PMID:18689571; http://dx.doi.org/10.1080/07315724.2008.10719712
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 1998; 282(5393):1497-501; PMID:9822382; http://dx.doi.org/10.1126/science.282.5393.1497
- Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science 2000; 290(5493):989-92; PMID:11062132; http://dx.doi.org/10.1126/science.290.5493.989
- Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci U S A 2003; 100(4):1931-6; PMID:12574499; http://dx.doi.org/10.1073/pnas.2627984100
- Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene 2005; 24(50):7465-74; PMID:16288293; http://dx.doi.org/10.1038/sj.onc.1209096
- Steelman LS, Abrams SL, Whelan J, Bertrand FE, Ludwig DE, Basecke J, Libra M, Stivala F, Milella M, Tafuri A, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia 2008; 22(4):686-707; PMID:18337767; http://dx.doi.org/10.1038/leu.2008.26
- Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet 2009; 43:95-118; PMID:19659442; http://dx.doi.org/10.1146/annurev-genet-102108-134850
- Vaseva AV, Moll UM. The mitochondrial p53 pathway. Biochim Biophys Acta 2009; 1787(5):414-20; PMID:19007744; http://dx.doi.org/10.1016/j.bbabio.2008.10.005
- Perelman A, Wachtel C, Cohen M, Haupt S, Shapiro H, Tzur A. JC-1: alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis 2012; 3:e430; PMID:23171850; http://dx.doi.org/10.1038/cddis.2012.171
- So EY, Ouchi T. BRAT1 deficiency causes increased glucose metabolism and mitochondrial malfunction. BMC Cancer 2014; 14(1):548; PMID:25070371; http://dx.doi.org/10.1186/1471-2407-14-548
- Mathers JC, Movahedi M, Macrae F, Mecklin JP, Moeslein G, Olschwang S, Eccles D, Evans G, Maher ER, Bertario L, et al. Long-term effect of resistant starch on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet Oncol 2012; 13(12):1242-9; PMID:23140761; http://dx.doi.org/10.1016/S1470-2045(12)70475-8
- Skeie G, Braaten T, Olsen A, Kyro C, Tjonneland A, Nilsson LM, Landberg R, Lund E. Whole grain intake and survival among scandinavian colorectal cancer patients. Nutrition and cancer 2014; 66(1):6-13; PMID:24274588; http://dx.doi.org/10.1080/01635581.2014.847472
- Bultman SJ. Molecular pathways: gene-environment interactions regulating dietary fiber induction of proliferation and apoptosis via butyrate for cancer prevention. Clin Cancer Res 2014; 20(4):799-803; PMID:24270685; http://dx.doi.org/10.1158/1078-0432.CCR-13-2483
- Cavallo F, Feldman DR, Barchi M. Revisiting DNA damage repair, p53-mediated apoptosis and cisplatin sensitivity in germ cell tumors. Int J Dev Biol 2013; 57(2-4):273-80; PMID:23784838; http://dx.doi.org/10.1387/ijdb.130135mb
- Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995; 80(2):293-9; PMID:7834749; http://dx.doi.org/10.1016/0092-8674(95)90412-3
- Ming L, Wang P, Bank A, Yu J, Zhang L. PUMA Dissociates Bax and Bcl-X(L) to induce apoptosis in colon cancer cells. J Biol Chemistry 2006; 281(23):16034-42; PMID:16608847; http://dx.doi.org/10.1074/jbc.M513587200
- Tuffy LP, Concannon CG, D'Orsi B, King MA, Woods I, Huber HJ, Ward MW, Prehn JH. Characterization of Puma-dependent and Puma-independent neuronal cell death pathways following prolonged proteasomal inhibition. Mol And Cell Biol 2010; 30(23):5484-501; PMID:20921277; http://dx.doi.org/10.1128/MCB.00575-10
- Wan J, Liu T, Mei L, Li J, Gong K, Yu C, Li W. Synergistic antitumour activity of sorafenib in combination with tetrandrine is mediated by reactive oxygen species (ROS)/Akt signaling. Br J Cancer 2013; 109(2):342-50; PMID:23807172; http://dx.doi.org/10.1038/bjc.2013.334
- Luo H, Yang Y, Duan J, Wu P, Jiang Q, Xu C. PTEN-regulated AKT/FoxO3a/Bim signaling contributes to reactive oxygen species-mediated apoptosis in selenite-treated colorectal cancer cells. Cell Death Dis 2013; 4:e481; PMID:23392169; http://dx.doi.org/10.1038/cddis.2013.3
- Bruno P, Calastretti A, Priulla M, Asnaghi L, Scarlatti F, Nicolin A, Canti G. Cell survival under nutrient stress is dependent on metabolic conditions regulated by Akt and not by autophagic vacuoles. Cell Signal 2007; 19(10):2118-26; PMID:17643959; http://dx.doi.org/10.1016/j.cellsig.2007.06.008
- Hashizume C, Kazuhiro O. Fiber ingredients: food application and health benefits. Food Applications and Health Benefits, CRC press 2009
- Tsuji K, Gordon DT. Energy value of a mixed glycosidic linked dextrin determined in Rats. J Agric Food Chem 1998; 46(6):2253-9; http://dx.doi.org/10.1021/jf9708453
