Abstract
Purpose: Women with metastatic triple negative breast cancer (TNBC) can have a poor prognosis with treatment limited to cytotoxic chemotherapy. The identification of effective therapies that may limit exposure to cytotoxic chemotherapy and lead to prolonged survival is an unmet medical need. We tested an inhibitor of the epidermal growth factor receptor, panitumumab in combination with chemotherapy. Methods: We conducted a single arm clinical trial in women with metastatic or locally advanced TNBC to paclitaxel 80 mg/m2 and carboplatin AUC of 2 on days 1, 8, and 15 and panitumumab 6 mg/kg on days 1 and 15 for a cycle length of 28 days. The objectives were to evaluate the response rate and safety of the combination in comparison to historical controls. Results: Fourteen patients with TNBC were enrolled with a median age of 53 years. The majority of women were African American (64.3%) with visceral metastasis (64.2%). Hematologic toxicities, particularly neutropenia and thrombocytopenia, were a major cause of missed chemotherapy and delayed treatment in this study. The overall response rate (complete and partial response) of the 13 evaluable patients was 46%. The median time to best response was 2.4 months and the median time to disease progression was 3.6 months. We were able to perform the PAM50 analysis on tumors from 7 of our subjects. All the samples tested clustered within the basal-like subtype. Conclusions: In our experience the response rate of carboplatin, paclitaxel and panitumumab was consistent with other reports of response for cytotoxic chemotherapy in metastatic TNBC.
Abbreviations
| AUC | = | area under the curve |
| CR | = | complete response |
| EGFR | = | Epidermal growth factor inhibitor |
| FISH | = | Fluorescence in situ hybridization |
| g/dl | = | grams/deciliter |
| Hgb | = | hemoglobin |
| IHC | = | immunohistochemistry |
| IRB | = | Institutional Review Board |
| Mg/dl | = | milligram/deciliter |
| Mg/kg | = | milligram/kilogram |
| PD | = | progressive disease |
| PR | = | partial response |
| RECIST | = | response evaluation in sold tumors |
| TNBC | = | triple negative breast cancer |
Introduction
Triple negative breast cancer (TNBC) lacks the hormonal receptors for estrogen and progesterone and also lacks the human epidermal growth factor receptor 2 (HER2). It is a relatively rare form of breast cancer and accounts for approximately 15% of cases. The need for effective therapies for TNBC is tremendous because it affects a younger population of women as compared to breast cancer that is hormonally responsive. Furthermore, although TNBC is sensitive to cytotoxic chemotherapy, relapses are common and women with metastatic disease have a rapidly fatal disease course.Citation1 As a result there is interest in the identification of effective therapies that may improve survival. Until targeted therapies are identified, the standard of care is cytotoxic chemotherapy in the adjuvant and metastatic setting.
Potential targeted therapy for TNBC is directed at the epidermal growth factor receptor (EGFR). Amplification of the EGFR gene occurs commonly in metaplastic and basal-like breast cancer.Citation2 Monoclonal antibodies directed at EGFR are inhibitory to TNBC cell lines.Citation3 Cetuximab, a monoclonal antibody against EGFR, has shown modest improvement over chemotherapy with minimal improvement in progression free survival and response rate.Citation4-6 We studied panitumumab, a fully human immunoglobulin G2 monoclonal antibody directed against EGFR that competitively inhibits epidermal growth factor receptor binding. This is the first clinical trial reported of panitumumab for metastatic TNBC. Panitumumab has recently been shown to improve the pathologic complete response rate as neoadjuvant treatment for women with TNBC.Citation7
As in other trialsCitation8 for triple negative breast cancer we combined the biologic therapy with a platinum therapy. Platinum chemotherapy (carboplatin and cisplatin) directed at DNA strand cross-link breaks may be particularly important to TNBC biology.Citation9-11 The paclitaxel and carboplatin combination was reported to have high response rates in metastatic TNBC.Citation12 Cetuximab and carboplatin have been evaluated in TNBC with an acceptable toxicity profile.Citation5 Moreover, the combination of paclitaxel, carboplatin and panitumumab has been investigated in non-small cell lung cancer with manageable toxicity.Citation13 We investigated this novel biologic targeted therapy in combination with carboplatin and paclitaxel in patients with metastatic TNBC.
Methods
Treatment plan and study design
The study was designed to assess the response rate to combination paclitaxel, carboplatin and panitumumab for women with metastatic TNBC. This was a single arm study of the following regimen: paclitaxel 80 mg/m2 on days 1, 8, and 15; carboplatin AUC of 2 on days 1, 8, and 15; and panitumumab 6 mg/kg on days 1 and 15. The cycle length was 28 days. Participants had a history and physical exam at the beginning of each cycle to assess for disease response and toxicity to therapy. Amgen Inc. provided the panitumumab and funding for costs of the clinical trial.
All participants had a baseline imaging (CT scans, bone scan or PET-CT scan) and the first re-staging was planned for 3 months unless the patient had clinical evidence of disease progression. Those without progression remained on study with monthly clinical evaluations for toxicity and imaging every other month until progression. Best response was measured from the start of the treatment until disease progression. Per RECIST criteria, complete response (CR) was defined as radiographic resolution of all malignant lesions. Partial responses (PR) required at least a 30% decrease in the sum of the largest dimension of target lesions. Patients had progressive disease (PD) if they experienced a 20% or more increase in the sum of the largest dimensions of target lesions. Stable disease (SD) was defined as insufficient shrinkage to qualify for PR and insufficient increase to qualify for PD.
All patients enrolled on this study were evaluated for treatment-related toxicity prior to administration of every cycle using the Common Terminology Criteria for Adverse Events (CTCAE v3.0). For the expected skin toxicities of panitumumab, prophylactic antibiotics were prescribed only when a rash of any grade occurred. Study parameters required an ANC >1,000/uL and platelet count >75,000/uL on day 1 of each cycle to receive carboplatin and paclitaxel. Patients who remained neutropenic for 2 consecutive weeks received a 25% dose reduction in their carboplatin and paclitaxel doses. The use of growth factor, either granulocyte-stimulating factor or erythrocyte-stimulating factor, were left to investigator discretion.
Patients
This trial was approved by the Institutional Review Boards at Wake Forest University in Winston-Salem NC and also at Ochsner Medical Center in New Orleans and Baton Rouge, LA (NCI-2009-01257). It was open to accrual from March 26, 2010 through July 2, 2013. All patients provided written informed consent before enrollment. Inclusion criteria were age ≥18 years, Karnofsky performance status ≥80%, life expectancy longer than 3 months, and measurable (≥1 cm) invasive breast cancer. The breast cancer cases had to be pathologically confirmed and ER <10%, PR <10% by IHC, HER2 1+, 0, or FISH negative. At time of enrollment, patients were required to have measurable (≥1CM) disease either by imaging or physical exam. Biopsies of metastatic disease were performed per standard of care. Participants were allowed one or no prior therapies for metastatic breast cancer and less than 3 prior regimens for locally advanced breast cancer. The required initial laboratory data included absolute neutrophil count ≥ 1,500/μL, platelets ≥ 100,000/μL, bilirubin < 2 mg/dl, serum creatinine < 2.0 mg/dl, Hgb>9 g/dl. Exclusion criteria were leptomeningeal disease, history of interstitial lung disease, irreversible peripheral neuropathy, other malignancy, active uncontrolled infection, active pregnancy or breast feeding, or history of myocardial infarction.
Tumor sample gene expression
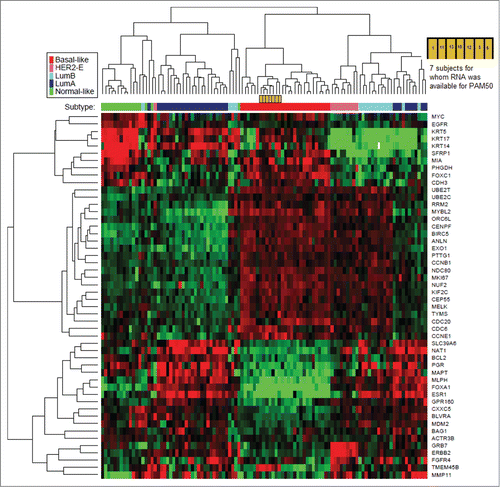
For each patient we requested a pre-trial, formalin-fixed paraffin embedded block of representative tumor. The sample could have been either from the primary breast cancer or from a metastatic lesion. Tumor blocks were sectioned using a microtome (5-10 microns x 10 sections) and RNA was extracted and purified for quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) amplification. Microarray data were then generated for expression of 50 genes, as per the Prediction Analysis of Microarray (PAM50). The PAM50 analysis was performed at University of North Carolina (Chapel Hill).Citation14
Statistical considerations
The primary endpoint for this study was efficacy defined as the proportion of partial and complete responders (according to RECIST criteria) among all evaluable patients. We intended to use the optimal (under H0) 2-stage phase II design proposed by Simon to test the null hypothesis H0: r = .25 versus the alternative hypothesis H1: r = .50 with type I and II errors of 10%. The plan involved accruing 10 patients during the first phase of the study and then, if 3 or more patients demonstrated response to therapy, accruing an additional 22 patients during the second stage. Toxicity, time to progression, and expression of EGFR and other gene markers were secondary outcome measures.
Results
Patient characteristics
Between March 2010 and July 2013, we enrolled 14 patients with metastatic TNBC onto this study. The baseline characteristics of these patients are summarized in . Median age of patients in this study was 53 years, with 71% of patients under age 60 at time of enrollment. The majority of women (64.3%) were African American and the majority (64.2%) had visceral metastasis. The majority of participants had breast cancer that progressed within 3 years of the completion of adjuvant chemotherapy. The study was closed early because of slow accrual and the lack of obvious improvement in efficacy in a high risk population.
Table 1. Demographics
Toxicity
The panitumumab associated toxicities were predictable, including rash, electrolyte abnormalities, and diarrhea as outlined in . The majority of patients in this study experienced some type of grade 1-2 skin toxicity. With regard to electrolyte abnormalities, one patient experienced grade 4 hypomagnesemia along with grade 3 hypocalcemia and hypokalemia which required discontinuation of panitumumab after cycle 2 of therapy. This patient received one additional cycle of chemotherapy and then was transitioned to hospice because of clinical deterioration. Two patients experienced grade 3 diarrhea and were subsequently removed from study, one patient because of concurrent clinical progression and the other because of prolonged thrombocytopenia.
Table 2. Panitumumab related toxicities by grade
Hematologic toxicities, particularly neutropenia and thrombocytopenia, were a major cause of missed chemotherapy and delayed treatment in this study. The proportion of patients unable to complete cycles of chemotherapy due to neutropenia and/or thrombocytopenia is outlined in . One patient had partial response to therapy, but was removed from the study because of prolonged neutropenia despite granulocyte-stimulating factor support. This particular patient was HIV positive.
Table 3. Ability to give study drug limited by cytopenias
Efficacy
Of the 14 patients initially enrolled in this trial, only 13 were evaluated for response as one patient was lost to follow-up. The overall response rate (complete and partial response) was 46%. The median time to best response was 2.4 months and the median time to disease progression was 3.6 months ( and ). There were enough patients accrued during the first phase of the study with adequate responses to not reject the null hypothesis and proceed to the second phase of the study. The study accrued slowly and was stopped before the accrual goal of 32 patients.
Table 4. Best response to therapy
Table 5. Time to Best Response
Two patients enrolled on this clinical trial achieved a complete response (CR). A CR was seen in one woman (patient 6) who participated on trial because she developed metastatic disease in the mediastinal lymph nodes less than one year after completing adjuvant therapy for stage IIIC breast cancer. She completed 9 cycles of therapy before experiencing radiographic progression of disease in the lungs. The other participant, patient 10, who experienced a CR had Stage IV disease at diagnosis with bone metastasis. Prior to enrollment on trial, she received 2 cycles of docetaxel therapy but experienced further disease progression in the skeleton and lymph nodes. She was enrolled on trial and received 6 cycles of therapy before developing progressive disease in the breast and bones. It should be noted that 2 of the patients on this trial experienced progression with brain only disease.
Gene expression profiling
We were able to extract enough RNA to perform the PAM50 analysis on tumors from 7 of our subjects. Our samples were analyzed concurrently with 4 controls of known subtypes: basal-like (n = 1), luminal A (n = 2), and luminal B (n = 1). The genetic clustering of our study patients were mapped along with 98 representative tumor samples. All our samples clustered within the basal-like subtype, based on the PAM50 subtype analysis (). This finding is concordant with our samples which were previously characterized as ER <10%, PR <10%, HER2 1+, 0, or FISH negative. With regard to EGFR, our samples and the other basal-like samples in this cohort had low expression on the microarray analysis. None of our samples exhibited distinctive pre-therapy genetic signatures that corresponded with their responses to therapy. Specifically, patients who experienced CR (patient 6 and patient 10) did not show a uniquely identifiable gene expression to explain their favorable response. We did not collect post-treatment samples from patients in this study to compare genetic expression profiles pre- and post- therapy.
Conclusion
We have shown preliminary efficacy for the combination of carboplatin, paclitaxel and panitumumab in TNBC. We found it difficult to administer full doses of therapy on schedule because of dose-limiting cytopenias among trial participants. The women on this trial showed rapid disease progression which was outstanding for breast cancer in general, but consistent with other reports of metastatic or refractory TNBC.Citation5 We did characterize the majority of our patients as basal-like by PAM 50, but our samples exhibited low expression of EGFR.
Other clinical trials have investigated targeted agents, such as PARP and EGFR inhibitors, along with a backbone of platinum based chemotherapy for relapsed TNBC.Citation5,8 Our response rate of 46% is consistent with other investigations of chemotherapy with targeted agents for triple negative breast cancer, which show response rates between 20-52%.Citation4,5,8,15 TNBC is recognized as a chemotherapy sensitive subtype of breast cancer although duration of response is disappointing. Our time to progression of 3.6 months is consistent with other studies of cetuxumab plus carboplatin or cisplatin.Citation4,5
Our clinical trial specifically aimed to target the EGFR receptor with panitumumab. Based on microarray data, it has been suggested that the EGFR receptor is over-expressed in the majority of basal-like breast cancers.Citation16,17 In this series, however, the tumor samples available for microarray analysis did not over-express EGFR as compared with normal-like tissue. Of the 2 previously published trials of cetuximab in TNBC, tumor expression of EGFR was not associated with clinical benefit in one and not reported in the other.Citation4,18 We did not screen our participant's tumors for EGFR expression prior to enrollment in this trial. It may be possible that an appropriate target for guiding who may benefit from panitumumab other than EGFR may be EGFR-related ligands or the KRAS gene.Citation19,20
The women in our clinical trial experienced prolonged neutropenia and thrombocytopenia that limited the intended dosing. In our experience, administering combination chemotherapy to patients with advanced TNBC is difficult because of dose-limiting bone marrow toxicity. A potentiation of chemotherapy toxicity, when combined with a targeted agent, has also been reported previously.Citation21 Particular dose-limiting toxicities of neutropenia and thrombocytopenia have been reported with other EGFR inhibitors when combined with platinum-based chemotherapy regimens.Citation22-24 The addition of bevacuzimab with single agent taxanes increased the incidence of neutropenia in patients with metastatic HER2 negative breast cancer (RIBBON-1).Citation25 These observations may be a function of targeted therapy plus chemotherapy potentiation; however, another consideration is the tolerance of women with metastatic TNBC. This population has low tolerance for therapy even for single-agent targeted therapy as demonstrated by Finn et al in their trial of dasatinib for TNBC.Citation26 Our trial was closed early for futility, with slow accrual and a further consideration that the combination therapy, in our view, was not as tolerable as other regimens for metastatic breast cancer.
A limitation of our study is that it is a single arm, so we could not discern the contribution of panitumumab with the carboplatin and paclitaxel. This study was stopped prematurely, when negative results of cetuximab trials were reported and decreased enthusiasm for EGFR inhibitors in TNBC. Although this clinical trial did not meet its primary endpoint, our experience was a valuable attempt to develop a thoughtful therapy for patients with TNBC.
Development of targeted therapies for patients with triple negative and basal-like breast cancer is an unmet need in oncology. Our study highlights the complexity of developing clinical trials for this group. We enrolled a small cohort of women who were young, primarily African American and had a rapid disease progression. The combination therapy of panitumumab, paclitaxel and carboplatin showed a similar response rate to other combination regimens used to treat metastatic TNBC.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The study was supported by Amgen, Inc., Thousand Oaks, CA.
References
- Kassam F, Enright K, Dent R, Dranitsaris G, Myers J, Flynn C, Fralick M, Kumar R, Clemons M. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer 2009; 9(1):29-33; PMID:19299237; http://dx.doi.org/10.3816/CBC.2009.n.005
- Natrajan R, Williams RD, Hing SN, Mackay A, Reis-Filho JS, Fenwick K, Iravani M, Valgeirsson H, Grigoriadis A, Langford CF, et al. Array CGH profiling of favourable histology Wilms tumours reveals novel gains and losses associated with relapse. J Pathol 2006; 210(1):49-58; PMID:16823893; http://dx.doi.org/10.1002/path.2021
- Baselga J, Norton L, Masui H, Pandiella A, Coplan K, Miller WH, Jr., Mendelsohn J. Antitumor effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies. J Natl Cancer Inst 1993; 85(16):1327-33; PMID:8340945; http://dx.doi.org/10.1093/jnci/85.16.1327
- Baselga J, Gomez P, Greil R, Braga S, Climent MA, Wardley AM, Kaufman B, Stemmer SM, Pego A, Chan A, et al. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol 2013; 31(20):2586-92; PMID:23733761; http://dx.doi.org/10.1200/JCO.2012.46.2408
- Carey LA, Rugo HS, Marcom PK, Mayer EL, Esteva FJ, Ma CX, Liu MC, Storniolo AM, Rimawi MF, Forero-Torres A, et al. TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol 2012; 30(21):2615-23; PMID:22665533; http://dx.doi.org/10.1200/JCO.2010.34.5579
- Nechushtan H, Vainer G, Stainberg H, Salmon AY, Hamburger T, Peretz T. A phase 1/2 of a combination of Cetuximab and Taxane for "triple negative" breast cancer patients. Breast 2014; 23(4):435-8; PMID:24836394; http://dx.doi.org/10.1016/j.breast.2014.03.003
- Nabholtz JM, Abrial C, Mouret-Reynier MA, Dauplat MM, Weber B, Gligorov J, Forest AM, Tredan O, Vanlemmens L, Petit T, et al. Multicentric neoadjuvant phase II study of panitumumab combined with an anthracycline/taxane-based chemotherapy in operable triple-negative breast cancer: identification of biologically defined signatures predicting treatment impact. Ann Oncol 2014; 25(8):1570-7; PMID:24827135; http://dx.doi.org/10.1093/annonc/mdu183
- O'Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, Koo IC, Sherman BM, Bradley C. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med 2011; 364(3):205-14; PMID:21208101; http://dx.doi.org/10.1056/NEJMoa1011418
- Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, Juul N, Leong CO, Calogrias D, Buraimoh A, et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol 2010; 28(7):1145-53; PMID:20100965; http://dx.doi.org/10.1200/JCO.2009.22.4725
- Staudacher L, Cottu PH, Dieras V, Vincent-Salomon A, Guilhaume MN, Escalup L, Dorval T, Beuzeboc P, Mignot L, Pierga JY. Platinum-based chemotherapy in metastatic triple-negative breast cancer: the Institut Curie experience. Ann Oncol 2011; 22(4):848-56; PMID:20924076; http://dx.doi.org/10.1093/annonc/mdq461
- Uhm JE, Park YH, Yi SY, Cho EY, Choi YL, Lee SJ, Park MJ, Lee SH, Jun HJ, Ahn JS, et al. Treatment outcomes and clinicopathologic characteristics of triple-negative breast cancer patients who received platinum-containing chemotherapy. Int J Cancer 2009; 124(6):1457-62; PMID:19065658; http://dx.doi.org/10.1002/ijc.24090
- Chia J, Ang P, See H, Wong Z, Soh L, Yay Y, Wong N. Triple- negative metatstatic/reurrent breast cancer: Treatment with paclitaxel/carboplatin combination chemotherapy. J Clin Oncol 2007; 25; (18S, Abstract # 1086)
- Crawford J, Swanson P, Schwarzenberger P, Sandler A, Prager D, Zhang K, Freeman DJ, Johnson CW, Krishnan K, Johnson D. A phase 2 randomized trial of paclitaxel and carboplatin with or without panitumumab for first-line treatment of advanced non-small-cell lung cancer. J Thor Oncol 2013; 8(12):1510-8; PMID:24389433; http://dx.doi.org/10.1097/JTO.0b013e3182a7d1da
- Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009; 27(8):1160-7; PMID:19204204; http://dx.doi.org/10.1200/JCO.2008.18.1370
- Ma CX, Ellis MJ, Petroni GR, Guo Z, Cai SR, Ryan CE, Craig Lockhart A, Naughton MJ, Pluard TJ, Brenin CM, et al. A phase II study of UCN-01 in combination with irinotecan in patients with metastatic triple negative breast cancer. Breast Cancer Res Treat 2013; 137(2):483-92; PMID:23242585; http://dx.doi.org/10.1007/s10549-012-2378-9
- Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 2004; 10(16):5367-74; PMID:15328174; http://dx.doi.org/10.1158/1078-0432.CCR-04-0220
- Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 2003; 100(14):8418-23; PMID:12829800; http://dx.doi.org/10.1073/pnas.0932692100
- Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006; 295(21):2492-502; PMID:16757721; http://dx.doi.org/10.1001/jama.295.21.2492
- Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, Wong TW, Huang X, Takimoto CH, Godwin AK, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 2007; 25(22):3230-7; PMID:17664471; http://dx.doi.org/10.1200/JCO.2006.10.5437
- Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pinter T, Lim R, Bodoky G, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009; 360(14):1408-17; PMID:19339720; http://dx.doi.org/10.1056/NEJMoa0805019
- Yadav BS, Sharma SC, Chanana P, Jhamb S. Systemic treatment strategies for triple-negative breast cancer. World J Clin Oncol 2014; 5(2):125-33; PMID:24829859; http://dx.doi.org/10.5306/wjco.v5.i2.125
- Lynch TJ, Patel T, Dreisbach L, McCleod M, Heim WJ, Hermann RC, Paschold E, Iannotti NO, Dakhil S, Gorton S, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol 2010; 28(6):911-7; PMID:20100966; http://dx.doi.org/10.1200/JCO.2009.21.9618
- Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, Vynnychenko I, Park K, Yu CT, Ganul V, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009; 373(9674):1525-31; PMID:19410716; http://dx.doi.org/10.1016/S0140-6736(09)60569-9
- O'Shaughnessy JA. Highlights in metastatic breast cancer from the 2013 San Antonio Breast Cancer Symposium (SABCS). Clin Adv Hematol Oncol 2014; 12(3 Suppl 7):3-17; PMID:24870974
- Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 2011; 29(10):1252-60; PMID:21383283; http://dx.doi.org/10.1200/JCO.2010.28.0982
- Finn RS, Bengala C, Ibrahim N, Roche H, Sparano J, Strauss LC, Fairchild J, Sy O, Goldstein LJ. Dasatinib as a single agent in triple-negative breast cancer: results of an open-label phase 2 study. Clin Cancer Res 2011; 17(21):6905-13; PMID:22028489; http://dx.doi.org/10.1158/1078-0432.CCR-11-0288