Abstract
Renal Cell Carcinoma (RCC) is a common malignancy world-wide that is rising in incidence. Up to 10% of RCC patients present with inferior vena cava (IVC) tumor thrombus (IVC-TT). Although surgery is the only treatment with proven efficacy for IVC-TT, the surgical management of advanced (level III and IV) IVC-TT is difficult with high morbidity and mortality, and offers a poor survival outcome. Currently, there are no treatment options in the setting of recurrent or unresectable RCC IVC-TT. Even though RCC may be resistant to conventionally fractionated radiation therapy, hypofractionated radiation has shown excellent control rates for both primary and metastatic RCC. We report our experience treating 2 RCC patients with Level IV IVC-TT —one recurrent and the other unresectable—with stereotactic ablative radiation therapy (SABR). The first patient is a 75-year-old gentleman with a level IV RCC IVC-TT who presented 9 months after his radical nephrectomy and thrombectomy with a growing level IV IVC-TT that became refractory to 4 targeted agents. He received SABR of 50Gy in 5 fractions and at 2-year follow-up is doing well with a significant decrease in the enhancement and size of the IVC-TT. The second patient is an 83-year-old gentleman who presented with metastatic RCC and level IV IVC-TT but was not a surgical candidate. After progression on temsirolimus, he received SABR of 36Gy in 4 fractions to his IVC-TT and survived 18 months post-SABR. Both patients improved symptomatically and did not experience any acute or late treatment-related toxicity. Their survival of 24 months and 18 months are comparable to the reported median survival of 20 months in patients with level IV IVC-TT that underwent surgical resection. Therefore, SABR can be a potentially safe treatment option in the unresectable setting for RCC patients with IVC-TT and should be further evaluated in prospective trials.
Introduction
Kidney Cancer is a common malignancy with over 63,900 new cases and 13,800 deaths predicted in the United States in 2014.Citation1 The incidence of RCC is rising in incidence at an annual rate of 4.1% since 2004.Citation1,2 Approximately 4-36% of RCCs are associated with renal vein or inferior vena cava (IVC) tumor thrombi (IVC-TT).Citation3 Standard of care for RCC tumor thrombus in the non-metastatic setting is resection of the primary tumor and tumor thrombus with curative intent.Citation4–6 Extension of the IVC-TT above the level of hepatic veins (level III-IV) makes the tumor thrombectomy significantly more complicated often requiring cardiopulmonary bypass (CBP) with hypothermic circulatory arrest (HCA), resulting in a peri-operative complication rate of 34% and a mortality rate of 10.8%.Citation7,8 Therefore these procedures are often limited to high-volume academic centers and many patients with comorbidities are often not candidates for this treatment.
RCC was reported to be radio-resistant to conventionally fractionated radiation therapy in vitro by Deschavanne et. al.Citation9 However, multiple reports both in vitro and in vivo has demonstrated the radio-sensitivity of RCC to hypo-fractionated radiation therapy.Citation10,11 In fact, the control rates of metastatic RCC sites treated with stereotactic ablative radiation therapy (SABR) has been excellent at 88–98%.Citation12 Therefore, we surmise that SABR may have utility in the treatment of RCC IVC-TT in the unresectable and recurrent setting, and perhaps even in the neoadjuvant setting where it may reduce pulmonary emboli or metastasis, which are the most common complications after RCC IVC-TT surgery.Citation7,8,13 Here we report 2 cases of unresectable RCC IVC-TT treated with SABR.
Clinical Case Report
Recurrent IVC-TT
A 74-year-old male was found to have a right renal mass and level IV IVC-TT with no distant metastases. Radical nephrectomy, IVC-TT thrombectomy with CBP, and HCA were performed. A 15cm TT was removed that had vascular wall invasion. Pathology was consistent with Fuhrman grade 3 clear-cell RCC. IVC-TT recurred 9 months post-operatively as identified on MRI () and progressed on 4 systemic agents of Sunitinib, Everolimus, Axitinib, and Pazopanib respectively in addition to anti-coagulation over the next year to extend beyond the diaphragm () toward the right atria. He developed shortness of breath with mild exertion. Refusing further surgery and being refractory to multiple systemic agents with continued progression of the IVC-TT, he was referred for SABR with a palliative intent. He underwent SABR of 50Gy in 5 fractions delivered via 11 non-coplanar photon beams of 10 MV over 2 weeks with the IVC-TT target delineated using MRI (). All the adjacent critical organ dose constraints were achieved (). The patient tolerated SABR well without any acute or late toxicity. His shortness of breath resolved over the next several months. As of 2-year follow-up, he is alive and the IVC-TT continued to show radiographic evidence of response with decrease in size and enhancement on serial MRIs ().
Figure 1. MRI of IVC Tumor Thrombus in clear cell RCC before and after SABR. Coronal (top) and axial (bottom) contrast enhanced MR images at different time points during the course of treatment. After nephrectomy and thrombectomy, the patient had an intraluminal recurrence of tumor thrombus, which was adherent to the IVC wall (arrowheads, A). The superior extent of the thrombus is inferior to the diaphragm (Level III; arrow, A). Note the size of the thrombus at the level of the right hepatic vein (arrow, B). After systemic targeted therapy (C) there was obvious disease progression with thrombus extending superior to the diaphragm (level IV, arrow) and increased enhancement (arrowhead, C). Note marked increased in transverse diameter (arrow, D). Two years after SABR therapy there is persistent thrombus extending above the diaphragm (arrow, E) although exhibiting clear decrease in enhancement (arrowhead, E) and marked reduction in transverse diameter (arrow, F).
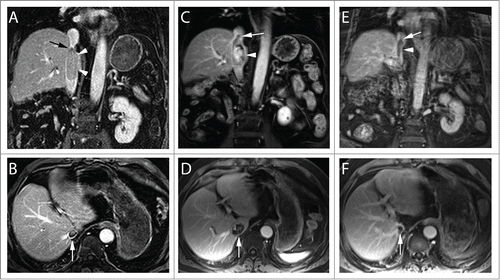
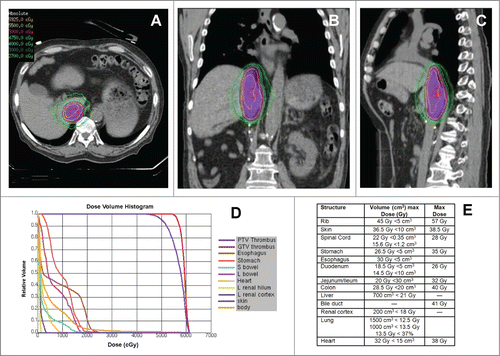
Figure 2. SABR Treatment of Recurrent IVC-TT. (A-C) Representative axial, sagittal, and coronal images of the SABR treatment plan with isodose lines showing dose distribution and coverage of the IVC-TT. Patient was immobilized with a vacuum bag in an Elekta body frame. Abdominal compression and 4D-CT with contrast was used for respiratory motion management and assessment respectively. Treatment planning MRI was fused for target delineation. The dose was prescribed to the 84% isodose line via 11 non-coplanar photon beams of 10 MV and 3D optimization ensuring >95% PTV coverage with a 0.5 cm margin on the TT. (D) Radiation dose volume histogram from SABR plan of 50 Gy in 5 fractions showing optimized doses to critical organs as well as target volume (PTV). (E) Radiation dose constraints used for treatment planning.
Unresectable IVC-TT
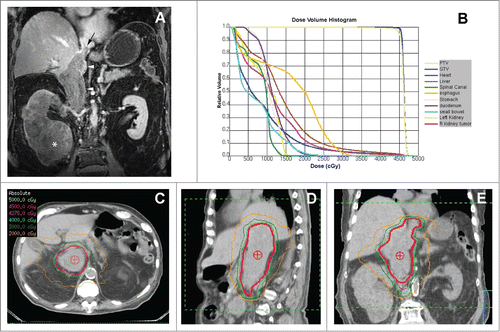
The second patient is an 83-year-old with multiple medical conditions who presented with an 11.6cm right lower pole kidney mass, periaortic lymphadenopathy, a 17cm level IV IVC-TT () and a T12 metastasis. He was in the intermediate-risk group based on the Heng criteria.Citation14 A CT-guided biopsy confirmed high-grade papillary RCC. He was high-risk for surgical intervention and was deemed not to be an operable candidate by 2 urologists due to his age, medical comorbidities and extent of resection necessary and was therefore started on temsirolimus. Two months post-treatment the patient was referred for RT with continued progression only at his retro-peritoneal lymphadenopathy, and IVC-TT which went from 5.5×4.7 cm to 6.5×5.6cm axially partially occluding the IVC. He developed lower extremity edema for which DVT was ruled out. His progressive IVC-TT on systemic therapy was thought to be his greatest site of morbidity with concerns for pulmonary embolus or Budd-Chiari syndrome, and therefore he was started on SABR targeting the IVC-TT alone with 45Gy in 5 fractions utilizing 13 non-coplaner 10MV photon beams (). The patient tolerated treatment well. However, after 4 fractions he decided to pursue palliative care refusing further treatment and follow-up scans. He survived 18 months post-SABR without any treatment-related toxicity.
Figure 3. SABR Treatment of Unresectable IVC-TT. (A) Coronal contrast-enhanced MRI during the venous phase demonstrates low level enhancement in a large renal mass (asterisk), which infiltrates the entire right kidney parenchyma and extends superiorly with a expansile tumor thrombus in the inferior vena cava (arrowheads). Note the superior extent of the tumor thrombus (black arrow) above the diaphragm (i.e. level IV thrombus). (B) Radiation dose volume histogram from SABR plan of 45 Gy in 5 fractions showing optimized doses to critical organs and target volumes. The patient set-up and target delineation was similar to the first case. The plan required 13 non-coplanar photon beams of 10 MV and IMRT optimization to ensure >95% PTV coverage with a 0.5 cm margin on the TT. C-E) Representative axial, sagittal, and coronal images of the SABR treatment plan with isodose lines showing dose distribution and coverage of the IVC-TT.
Discussion
SABR has been implemented successfully in the definitive management of several cancers including primary lung and prostate,Citation15-18 and is currently under investigation in many other sites including breast, pancreas and liver.Citation19-23 SABR has also been successfully implemented for the local control of metastatic lesions in multiple sites.Citation19,24-26 RCC has traditionally been considered a radioresistant tumor.Citation27 This conclusion was supported by a single study that examined the radiosensitivity of multiple human cancer cell lines in vitroCitation9 and examined one human RCC cell line, which happened to be the most radioresistant among all tested cell lines. Since then, multiple in vitro and in vivo studies have demonstrated that RCC is indeed radiosensitive, particularly at higher doses per fraction such as are used for SABR.Citation10,11 Clinical experience mimics this conclusion with SABR showing efficacy ranges of 90–100% and 82–95% for CNS and extra-CNS metastases respectively.Citation28-34 In fact, at hypofractionated dose levels, RCC may even be more radiosensitive than other primary sites. Lung, for example, requires 54Gy in 3 fractions compared to RCC, where 36Gy in 3 fractions appears to provide adequate control.Citation16,33,34 Despite the extensive and growing experience with SABR in multiple cancer sites, its application to primary renal cancers has been limited to a few retrospective reviews and 2 phase I studiesCitation34,35 all showing excellent local control rates. Three ongoing phase II clinical trials for SABR of primary RCC are currently underway (NCT 02141919, 01890590, 02138578)
The surgical management of level III or greater IVC-TT in RCC is difficult and requires extensive vascular control, with CBP and/or HCA often contributing to morbidity and mortality, and offers a poor survival outcome.Citation7,8 Even after a successful thrombectomy lung metastases and embolism develop frequently.Citation8,13 Progressing IVC-TT can lead to significant and life-threatening morbidity including pulmonary embolus or Budd-Chiari syndrome.Citation36 There has not been any report of the application of SABR for the treatment of RCC IVC-TT to our knowledge. Conventionally fractionated RT has been used for the treatment of HCC portal vein TT showing favorable response rates in multiple early phase clinical trials.Citation37 The application of SABR for the treatment of TT in general has been relatively rare and limited to a single institution series showing 41 patients with portal vein or IVC-TT for hepatocellular carcinoma (HCC) treated to a dose of 30–48 Gray (Gy) in 6 fractions to have a 36.6% complete response rate and 39% partial response rate.Citation38
Here we report the treatment of 2 large volume level IV RCC IVC-TTs — one recurrent and the other unresectable and both refractory to systemic therapy — with highly conformal SABR with no significant treatment-related toxicity. One patient had follow-up scans that showed clear evidence of local control, while both had better than expected clinical outcome. Both patients tolerated treatment well and improved symptomatically. Their survival of 24 months and 18 months are comparable to the reported median survival of 20 months in patients with level IV IVC-TT that underwent surgical resection.Citation7 Therefore, SABR may be further evaluated in prospective trials as a potentially safe treatment option in the setting of unresectable and/or refractory IVC-TT, not necessarily just with a palliative intent.
In addition, in the neo-adjuvant setting, SABR of IVC-TT may decrease local recurrences, lower the likelihood of embolic complications and pulmonary metastasis, which currently contribute significantly to the poor outcome of patients undergoing IVC tumor thrombectomy. A safety lead-in phase II clinical trial is being designed to test this hypothesis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
The authors acknowledge the contributions of Karen Roach, CMD toward this manuscript.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9-29; PMID:24399786; http://dx.doi.org/10.3322/caac.21208
- Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol 2010; 7:245-57; PMID:20448658; http://dx.doi.org/10.1038/nrurol.2010.46
- Kim HL, Zisman A, Han KR, Figlin RA, Belldegrun AS. Prognostic significance of venous thrombus in renal cell carcinoma. Are renal vein and inferior vena cava involvement different? J Urol 2004; 171:588-91; PMID:14713765; http://dx.doi.org/10.1097/01.ju.0000104672.37029.4b
- Lawindy SM, Kurian T, Kim T, Mangar D, Armstrong PA, Alsina AE, Sheffield C, Sexton WJ, Spiess PE. Important surgical considerations in the management of renal cell carcinoma (RCC) with inferior vena cava (IVC) tumour thrombus. BJU Int 2012; 110:926-39; PMID:22540179; http://dx.doi.org/10.1111/j.1464-410X.2012.11174.x
- Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, Patard JJ, Mulders PF, Sinescu IC; European Association of Urology Guideline Group. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol 2010; 58:398-406; PMID:20633979; http://dx.doi.org/10.1016/j.eururo.2010.06.032
- Neves RJ, Zincke H. Surgical treatment of renal cancer with vena cava extension. Br J Urol 1987; 59:390-5; PMID:3594097; http://dx.doi.org/10.1111/j.1464-410X.1987.tb04832.x
- Haddad AQ, Wood CG, Abel EJ, Krabbe LM, Darwish OM, Thompson RH, Heckman JE, Merril MM, Gayed BA, Sagalowsky AI, et al. Oncologic outcomes following surgical resection of renal cell carcinoma with inferior vena caval thrombus extending above the hepatic veins: a contemporary multicenter cohort. J Urol 2014; 192:1050-6; PMID:24704115; http://dx.doi.org/10.1016/j.juro.2014.03.111
- Abel EJ, Thompson RH, Margulis V, Heckman JE, Merril MM, Darwish OM, Krabbe LM, Boorjian SA, Leibovich BC, Wood CG. Perioperative outcomes following surgical resection of renal cell carcinoma with inferior vena cava thrombus extending above the hepatic veins: a contemporary multicenter experience. Eur Urol 2014; 66:584-92; PMID:24262104; http://dx.doi.org/10.1016/j.eururo.2013.10.029
- Deschavanne PJ, Fertil B. A review of human cell radiosensitivity in vitro. Int J Radiat Oncol Biol Phys 1996; 34:251-66; PMID:12118559; http://dx.doi.org/10.1016/0360-3016(95)02029-2
- Ning S, Trisler K, Wessels BW, Knox SJ. Radiobiologic studies of radioimmunotherapy and external beam radiotherapy in vitro and in vivo in human renal cell carcinoma xenografts. Cancer 1997; 80:2519-28; PMID:9406705; http://dx.doi.org/10.1002/(SICI)1097-0142(19971215)80:12+%3c2519::AID-CNCR26%3e3.0.CO;2-E
- Walsh L, Stanfield JL, Cho LC, Chang CH, Forster K, Kabbani W, Cadeddu JA, Hsieh JT, Choy H, Timmerman R, et al. Efficacy of ablative high-dose-per-fraction radiation for implanted human renal cell cancer in a nude mouse model. Eur Urol 2006; 50:795-800; discussion 800; PMID:16632182; http://dx.doi.org/10.1016/j.eururo.2006.03.021
- De Meerleer G, Khoo V, Escudier B, Joniau S, Bossi A, Ost P, Briganti A, Fonteyne V, Van Vulpen M, Lumen N, et al. Radiotherapy for renal-cell carcinoma. Lancet Oncol 2014; 15:e170-7; PMID:24694640; http://dx.doi.org/10.1016/S1470-2045(13)70569-2
- Sivaramakrishna B, Gupta NP, Wadhwa P, Hemal AK, Dogra PN, Seth A, Aron M, Kumar R. Pattern of metastases in renal cell carcinoma: a single institution study. Indian J Cancer 2005; 42:173-7; PMID:16391434
- Kroeger N, Xie W, Lee JL, et al: Metastatic non-clear cell renal cell carcinoma treated with targeted therapy agents: characterization of survival outcome and application of the International mRCC Database Consortium criteria. Cancer 2013; 119:2999-3006.
- Boike TP, Lotan Y, Cho LC, Brindle J, DeRose P, Xie XJ, Yan J, Foster R, Pistenmaa D, Perkins A, et al. Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J Clin Oncol 2011; 29:2020-6; PMID:21464418; http://dx.doi.org/10.1200/JCO.2010.31.4377
- Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone D, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303:1070-6; PMID:20233825; http://dx.doi.org/10.1001/jama.2010.261
- King CR, Freeman D, Kaplan I, Fuller D, Bolzicco G, Collins S, Meier R, Wang J, Kupelian P, Steinberg M, et al. Stereotactic body radiotherapy for localized prostate cancer: Pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol 2013; 109(2):217-21
- Katz AJ, Santoro M, Diblasio F, Ashley R. Stereotactic body radiotherapy for localized prostate cancer: disease control and quality of life at 6 years. Radiat Oncol 2013; 8:118; PMID:23668632; http://dx.doi.org/10.1186/1748-717X-8-118
- Timmerman RD, Kavanagh BD, Cho LC, Papiez L, Xing L. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol 2007; 25:947-52; PMID:17350943; http://dx.doi.org/10.1200/JCO.2006.09.7469
- Berber B, Sanabria JR, Braun K, Yao M, Ellis RJ, Kunos CA, Sohn J, Machtay M, Teh BS, Huang Z, et al. Emerging role of stereotactic body radiotherapy in the treatment of pancreatic cancer. Expert Rev Anticancer Ther 2013; 13:481-7; PMID:23560842; http://dx.doi.org/10.1586/era.13.19
- Tao C, Yang LX. Improved radiotherapy for primary and secondary liver cancer: stereotactic body radiation therapy. Anticancer Res 2012; 32:649-55; PMID:22287758
- Kwon JH, Bae SH, Kim JY, Choi BO, Jang HS, Jang JW, Choi JY, Yoon SK, Chung KW. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer 2010; 10:475; PMID:20813065; http://dx.doi.org/10.1186/1471-2407-10-475
- Bondiau PY, Courdi A, Bahadoran P, Chamorey E, Queille-Roussel C, Lallement M, Birtwisle-Peyrottes I, Chapellier C, Pacquelet-Cheli S, Ferrero JM. Phase 1 clinical trial of stereotactic body radiation therapy concomitant with neoadjuvant chemotherapy for breast cancer. Int J Radiat Oncol Biol Phys 2013; 85:1193-9; PMID:23332384; http://dx.doi.org/10.1016/j.ijrobp.2012.10.034
- Chang BK, Timmerman RD. Stereotactic body radiation therapy: a comprehensive review. Am J Clin Oncol 2007; 30:637-44; PMID:18091059; http://dx.doi.org/10.1097/COC.0b013e3180ca7cb1
- Lo SS, Fakiris AJ, Teh BS, Cardenes HR, Henderson MA, Forquer JA, Papiez L, McGarry RC, Wang JZ, Li K, et al. Stereotactic body radiation therapy for oligometastases. Expert Rev Anticancer Ther 2009; 9:621-35; PMID:19445579; http://dx.doi.org/10.1586/era.09.15
- Lo SS, Fakiris AJ, Chang EL, Mayr NA, Wang JZ, Papiez L, Teh BS, McGarry RC, Cardenes HR, Timmerman RD. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol 2010; 7:44-54; PMID:19997074; http://dx.doi.org/10.1038/nrclinonc.2009.188
- Blanco AI, Teh BS, Amato RJ. Role of radiation therapy in the management of renal cell cancer. Cancers (Basel) 2011; 3:4010-23; PMID:24213122; http://dx.doi.org/10.3390/cancers3044010
- Shuto T, Matsunaga S, Suenaga J, Inomori S, Fujino H. Treatment strategy for metastatic brain tumors from renal cell carcinoma: selection of gamma knife surgery or craniotomy for control of growth and peritumoral edema. J Neurooncol 2010; 98:169-75; PMID:20405309; http://dx.doi.org/10.1007/s11060-010-0170-4
- Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976) 2007; 32:193-9; http://dx.doi.org/10.1097/01.brs.0000251863.76595.a2
- Gerszten PC, Burton SA, Ozhasoglu C, Vogel WJ, Welch WC, Baar J, Friedland DM. Stereotactic radiosurgery for spinal metastases from renal cell carcinoma. J Neurosurg Spine 2005; 3:288-95; PMID:16266070; http://dx.doi.org/10.3171/spi.2005.3.4.0288
- Yamada Y, Bilsky MH, Lovelock DM, Venkatraman ES, Toner S, Johnson J, Zatcky J, Zelefsky MJ, Fuks Z. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys 2008; 71:484-90; PMID:18234445; http://dx.doi.org/10.1016/j.ijrobp.2007.11.046
- Nguyen QN, Shiu AS, Rhines LD, Wang H, Allen PK, Wang XS, Chang EL. Management of spinal metastases from renal cell carcinoma using stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010; 76:1185-92; PMID:19632064; http://dx.doi.org/10.1016/j.ijrobp.2009.03.062
- Ranck MC, Golden DW, Corbin KS, Hasselle MD, Liauw SL, Stadler WM, Hahn OM, Weichselbaum RR, Salama JK. Stereotactic Body Radiotherapy for the Treatment of Oligometastatic Renal Cell Carcinoma. Am J Clin Oncol 2012; 36(6):589-95
- Wersall PJ, Blomgren H, Lax I, Linder C, Lundell G, Nilsson B, Nilsson S, Näslund I, Pisa P, Svedman C. Extracranial stereotactic radiotherapy for primary and metastatic renal cell carcinoma. Radiother Oncol 2005; 77:88-95; PMID:15972239; http://dx.doi.org/10.1016/j.radonc.2005.03.022
- Beitler JJ, Makara D, Silverman P, Lederman G. Definitive, high-dose-per-fraction, conformal, stereotactic external radiation for renal cell carcinoma. Am J Clin Oncol 2004; 27:646-8; PMID:15577450; http://dx.doi.org/10.1097/01.coc.0000145289.57705.07
- Wotkowicz C, Wszolek MF, Libertino JA. Resection of renal tumors invading the vena cava. Urol Clin North Am 2008; 35:657-71; viii; PMID:18992619; http://dx.doi.org/10.1016/j.ucl.2008.07.013
- Hou JZ, Zeng ZC, Zhang JY, Fan J, Zhou J, Zeng MS. Influence of tumor thrombus location on the outcome of external-beam radiation therapy in advanced hepatocellular carcinoma with macrovascular invasion. Int J Radiat Oncol Biol Phys 2012; 84:362-8; PMID:22381903; http://dx.doi.org/10.1016/j.ijrobp.2011.12.024
- Xi M, Zhang L, Zhao L, Li QQ, Guo SP, Feng ZZ, Deng XW, Huang XY, Liu MZ. Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PLoS One 2013; 8:e63864; PMID:23737955; http://dx.doi.org/10.1371/journal.pone.0063864
