Abstract
The c-Met protein, a transmembrane receptor tyrosine kinase, is the product of a proto-oncogene. Its only known ligand, hepatocyte growth factor (HGF), regulates cell growth, motility, migration, invasion, proliferation, and angiogenesis. The aberrant expression of c-Met is often associated with poor prognosis in multiple cancers, including renal cell carcinoma (RCC). Silencing or inactivation of c-Met leads to decreased viability of cancer cells, thereby making ablation of c-Met signaling an attractive concept for developing novel strategies for the treatment of renal tumors. Naturally-occurring products or substances are the most consistent source of drug development. As such, we investigated the functional impact of piperlongumine (PL), a naturally occurring alkaloid present in the Long pepper (Piper longum) on c-Met expression in RCC cells and demonstrated that PL and its analogs rapidly reduce c-Met protein and RNA levels in RCC cells via ROS-dependent mechanism. PL-mediated c-Met depletion coincided with the inhibition of downstream c-Met signaling; namely Erk/MAPK, STAT3, NF-κB and Akt/mTOR. As such, PL and PL analogs hold promise as potential therapeutic agents for the treatment of metastatic RCC and the prevention of postoperative RCC recurrence.
Keywords:
Abbreviations
| Erk | = | Extracellular signal-regulated kinase |
| FAK | = | Focal adhesion kinase |
| HGF | = | Hepatocyte growth factor |
| MAPK | = | Mitogen-activated protein kinase |
| mTOR | = | Mammalian target of rapamycin |
| NF-kB | = | Nuclear factor kappaB |
| PL | = | Piperlongumine |
| PL-Di | = | PL-Dimer |
| PL-FPh | = | PL-fluorophenyl |
| RCC | = | Renal cell carcinoma |
| RECIST | = | Response evaluation criteria in solid tumors |
| RNA | = | Ribonucleic acid |
| ROS | = | Reactive oxygen species |
| STAT | = | Signal transducer and activator of transcription |
| TKIs | = | Tyrosine kinase inhibitors |
| VEGFR | = | Vascular endothelial growth factor receptor |
Introduction
Renal cell carcinoma (RCC) is one of the most lethal urologic cancers. While significant advances in both extirpative and systemic approaches have been achieved, 2 distinct groups of patients are at risk of death from kidney cancer: those who present with metastatic disease and those who recur following surgery. At the time of initial presentation, approximately 20–25% of RCC patients already have radiographically detectable metastatic disease, while an additional 20% have locally advanced pT3 or N+ disease. Surgery is not curative in the former and is associated with 35–70% recurrence rates in the latter. Moreover, an additional 10–25% of patients with localized (pT1–2N0) tumors experience recurrence of the tumor despite incidental detection and complete surgical resection.Citation1-3 Current targeted molecular strategies including tyrosine kinase inhibitors (TKIs) have resulted in a doubling of progression-free survival and significant gains in overall survival (median 18–30 months), thereby fundamentally changing the treatment paradigm of advanced kidney cancer.Citation4,5 Unfortunately, targeted therapies do not produce durable responses and all individuals eventually become refractory/develop resistance to treatment.
The c-Met protein is a transmembrane receptor tyrosine kinase and the product of the proto-oncogene. Its only known ligand, hepatocyte growth factor (HGF), regulates cell growth, motility, migration, invasion, proliferation, and angiogenesis.Citation6-8 Dysregulation of c-Met signaling has been observed in both clear cell and non–clear cell renal cell carcinomas (RCCs).Citation7,9 The c-Met signaling activates various intracellular effectors and pathways including PI3K/Akt/mTOR, Ras/Raf/MEK/ERK, NF-κB, STAT3 and FAK.Citation6,10 The aberrant expression of c-Met often correlates with poor prognosis in multiple cancers including RCC.Citation6 In addition, c-Met plays a critical role in the development of resistance to targeted therapies.Citation8,11 Discovery of c-Met and HGF overexpression in RCCs of all subtypes and the correlation between overexpression with worse outcomes reinforce the HGF/c-Met pathway as a logical therapeutic target.Citation6,7,9 Several approaches to target Met and its signaling have been developed including antagonists of HGF/c-Met and c-Met/effector interaction as well as inhibitors of c-Met activity.Citation7 Yet, amplification or expression of constitutively active c-Met forms may ultimately limit the use of such inhibitors.Citation11,12 Therefore, agents capable of completely depleting c-Met in malignant cells might be superior to other categories of c-Met targeted therapies.
Naturally-occurring products or substances are the most consistent source of drug development. Many anticancer agents, including docetaxel, vindesine, vinorelbine, etoposide, and topotecan, have progressed to clinical use based on advances of natural products. Peppers, from the genus Piper (Piperaceae), are the most common spices consumed worldwide and have a wide array of biologically active secondary compounds.Citation13 Piperlongumine (PL), a natural alkaloid abundantly present in the fruit of the Long pepper (Piper longum), shows several noteworthy biological activities.Citation14,15 Recent literature points to the fact that PL is capable of hindering the growth of prostate, sarcoma, bladder, breast, melanoma and lung tumors in vitro and in vivo.Citation13,16,17 Our work demonstrates for the first time that PL and PL derivatives potently down-regulate expression of c-Met at RNA and protein levels in long-term cultured and patient-derived RCC cells. PL-mediated c-Met depletion coincides with the inhibition of downstream c-Met signaling; namely Erk/MAPK, STAT3, NF-κB and Akt/mTOR. These findings open a wide avenue for investigation of the scientific realm of novel therapeutic modalities for the treatment of renal malignancy.
Results
PL down-regulates c-Met expression in RCC cells
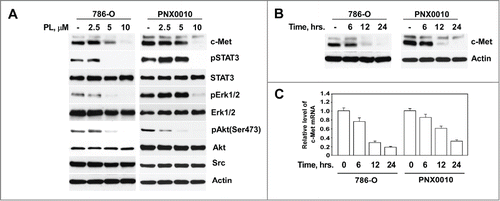
Our recent studies reveal that PL potently inhibits Akt and NF-κB signaling in tumor cells.Citation18,19 Given that c-Met is an upstream regulator of both these pathways,Citation6,10 we examined the potential effect of PL on c-Met protein expression in RCC cells. As demonstrated in , PL effectively depletes c-Met protein at low micromolar concentrations (>5 μM) in long-term cultured 786-O and patient-derived PNX0010 RCC cells. Importantly, PL-mediated down-regulation of c-Met coincides with the inhibition of its effectors, i.e. Erk1/2, STAT3, and Akt as was determined by examining phosphorylation status of these proteins (). Yet, PL-mediated effect is selective for c-Met since PL has no effect on the protein expression levels of other oncogenic kinases, namely Erk ½, Src and Akt (). Depletion of c-Met protein was evident as soon as 12 hours following PL administration (). Next, we examined whether treatment with PL modulates expression levels of c-Met mRNA. As shown in , PL induced time-dependent decrease of c-Met mRNA in both 786-O and PNX0010 cells.
Figure 1. PL reduces expression of c-Met in RCC cells. (A) PL induces dose-dependent reduction of c-Met protein levels in 786-O and PNX0010 RCC cells. Cells were treated with indicated concentrations of PL for 12 hours. Cell lysates were subjected to SDS-PAGE, blotted, and probed with specific antibodies. (B) Time course of c-Met protein depletion in 786-O and PNX0010 cells treated with PL at 10 μM for the indicated periods of time. (C) Levels of c-Met mRNA in 786-O and PNX0010 cells. Cells were treated with PL at 10 μM for the indicated periods of time. C-Met mRNA levels were detected by Real Time PCR using specific primers.
PL induces c-Met depletion via ROS-mediated proteasome-independent mechanism
Our recent studies reveal that PL rapidly reduces androgen receptor protein levels in LNCaP prostate cancer cells via proteasome-mediated mechanism.Citation16 To examine the potential role of proteasome-mediated pathway in PL-mediated c-Met depletion, 786-O and PNX0010 cells were pre-incubated with the proteasomal inhibitor bortezomib. The results presented in demonstrate that co-treatment with bortezomib did not restore the expression of c-Met protein. Additionally, treatment with bortezomib alone notably reduced c-Met protein levels in both tested RCC cell lines. It is important to note that these assays were performed long before the onset of cell death at a point when cell viability was greater than 95%.
Figure 2. PL induces c-Met depletion via ROS-mediated proteasome-independent mechanism. (A) Co-treatment with proteasomal inhibitor bortezomib failed to prevent PL-mediated depletion of c-Met protein. Cells were pre-incubated with bortezomib (1 μM) for 1 hour followed by treatment with PL (10 μM) for 12 hours. (B) PL induces ROS production in RCC cells. 786-O and PNX0010 cells were treated with PL (10 μM) with or w/o NAC (5 mM) for 1 hour, stained with CM-H2DCFDA and analyzed by flow cytometry as described in Materials and Methods. (C) Cells were treated with PL (10 μM) with or without NAC (5 mM) for 12 hours. Expression of c-Met and actin was detected by immunoblotting with specific antibodies. (D) Levels of c-Met mRNA in P786-O cells treated with PL with or without NAC. 786-O cells were treated with PL (10 μM) with or without NAC (5 mM) for 12 hours. C-Met mRNA levels were detected by Real Time PCR using specific primers.
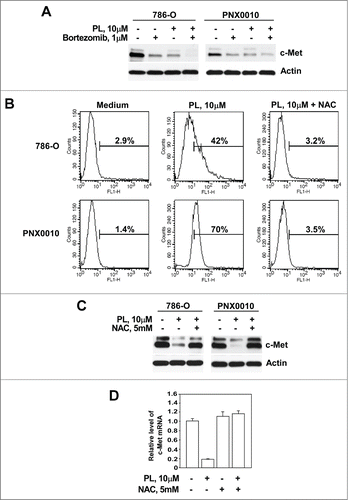
Several studies established that PL-mediated cytotoxicity is directly linked to elevated levels of reactive oxygen species (ROS).Citation16,17,20 Indeed, the addition of PL causes a marked rise in ROS levels in 786-O and PNX0010 cells (). N-acetyl-L-cysteine (NAC) is an anti-oxidant known to exert its effect on the cell via lowering levels of ROS. Co-administration of NAC and PL reversed PL-mediated increase of ROS and completely blocked depletion of c-Met at both mRNA and protein levels ().
PL derivatives demonstrate superior in vitro and in vivo antitumor activity compared with native PL
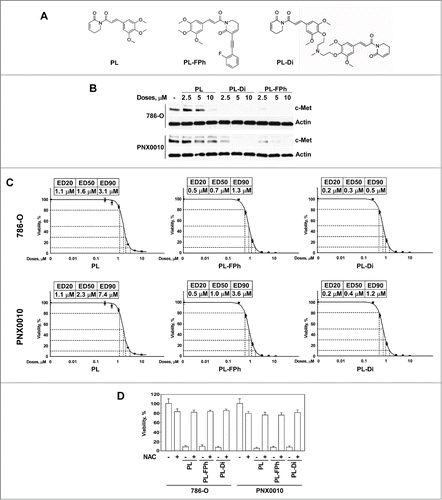
Studies by Adams et al revealed that PL analogs with specific chemical modifications demonstrate greatly enhanced antitumor activity compared with native PL.Citation21 To explore the potential therapeutic efficacy of PL derivatives, the effect of PL-fluorophenyl (PL-FPh) and PL-Dimer (PL-Di) () on the expression levels of c-Met protein in 786-O and PNX0010 cells was examined. As demonstrated in , both PL-FPh and PL-Di reduced c-Met protein levels at significantly lower concentrations than native PL. In addition, PL-FPh and PL-Di reduced viability of 786-O and PNX0010 cells at markedly lower concentrations compared with native PL (). Notably, PL-Di demonstrated strong cytotoxic effect against both 786-O and PNX0010 cells with ED50 values in the nanomolar range (). Administration of NAC completely abolished the inhibitory effect of all tested compounds on the viability of 786-O and PNX0010 cells, implicating that ROS generation plays a vital role in the antitumor efficacy of PL derivatives ().
Figure 3. PL derivatives, PL-FPh and PL-Di, deplete c-Met protein and reduce viability of RCC cells with greater efficiency than native PL. (A) Chemical structures of PL and PL derivatives. (B) The effect of PL derivatives on the expression of c-Met protein in 786-O and PNX0010 RCC cells. Cells were treated with indicated concentrations of either PL, PL-FPh or PL-Di for 12 hours. Cell lysates were subjected to SDS-PAGE, blotted, and probed with specific antibodies. (C) The effect of PL and PL derivatives on the viability of 786-O and PNX0010 cells. Cells were treated with escalating concentrations of PL, PL-FPh or PL-Di for 48 hours. Viability was analyzed as described in Materials and Methods. (D) Administration of NAC abolishes the inhibitory effect of PL and PL derivatives on the viability of 786-O and PNX0010 cells. Cells were treated at ED50 concentrations of PL, PL-FPh or PL-Di for 48 hours with or w/o NAC (5 mM). Viability was analyzed as described in Materials and Methods.
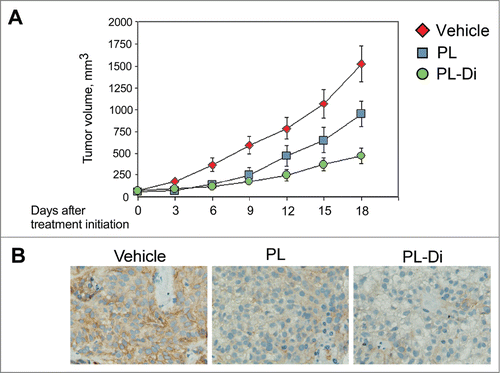
To corroborate our in vitro findings, we compared the effect of treatment with PL and PL-Di on in vivo tumor growth using xenograft tumors established from patient-derived PNX0010 RCC cells. As demonstrated in , animals treated with PL-Di showed a significant inhibition of tumor growth relative to control animals and animals treated with native PL. Suppression of tumor growth in animals treated with either PL or PL-Di coincided with the reduced expression of c-Met in tumor tissue specimens (). Importantly, treatment with both PL and PL-Di was well tolerated by all animals, with no apparent signs of toxicity.
Figure 4. The effect of the treatment with PL and PL-Di on the growth of PNX0010 xenograft tumors. (A) PNX0010 cells were inoculated s.c. in the flank region of 6 week old C.B17/lcr-scid mice. Animals were treated with either PL, PL-Di or vehicle as described in Materials and Methods. Values are means (n = 8) ± SEM. (B) Immunohistochemistry of representative sections of PNX0010 xenograft tumors stained for c-Met protein.
Discussion
Dysregulation of c-Met and its ligand, hepatocyte growth factor (HGF), have been implicated in tumor development, invasion, and angiogenesis for a range of malignancies.Citation6,10 Discovery of the correlation between c-Met overexpression in RCCs of all subtypes with worse outcomes and preclinical data demonstrating cancer control with c-Met inhibitors, underscore the importance of the c-Met/HGF pathway as a logical potential therapeutic target.Citation7,9 Multiple strategies to inhibit c-Met–dependent signaling are being extensively investigated in the laboratory and clinical settings. These include antagonists to c-Met ribozyme, HGF kringle variants/NK4, and decoy receptors, HGF-neutralizing antibodies, c-Met antagonist antibodies, and small-molecule c-Met inhibitors.Citation9,22-25 Modest clinical activity was seen with the anti-HGF antibody AMG 102, with only one partial response and stable disease in 43% of patients evaluated by RECIST criteria.Citation24 Recent studies suggest that direct targeting of c-Met might be a more effective antitumor treatment strategy. The results of phase II trial of c-Met and VEGFR2 inhibitor cabozantinib, demonstrated partial responses (RECIST criteria) in 24% of patients and some tumor regression in at least one post-baseline scan in 86% of patients.Citation26 Encouraging results were also obtained in the phase I trial of tivantinib (ARQ 197) for the treatment of patients with advanced or metastatic solid tumors refractory to standard therapy.Citation23 Despite its apparent efficacy, contemporary c-Met inhibitors predispose patients to certain toxicities including leukopenia, neutropenia, thrombocytopenia, vomiting, dehydration, hypophosphatemia, and diarrhea.Citation23,26
Results of our current study demonstrate that PL, a natural alkaloid abundantly present in the fruit of the Long pepper, potently down-regulates expression of c-Met at RNA and protein levels in RCC cells, which coincides with the inhibition of downstream c-Met signaling. Notably, recent studies demonstrate that PL does not cause any clinically significant side effects and has no major impact on biochemical, hematologic and histopathologic parameters in in vivo studies.Citation13,17 Our current work supports these findings. Indeed, treatment with PL appears to be well tolerated as no drug-related lethality, body weight loss or any other signs of adverse drug-related side effects were detected in experimental animals.
Recent studies reveal that PL is a potent inducer of ROS in tumor cells of various origins.Citation16,17,20 ROS can exert diverse effects on cellular function, by promoting either cell proliferation and tumor progression, or cell death and tumor regression.Citation27,28 Our data are in agreement with recent findings demonstrating that PL and PL derivatives inhibit growth of tumor cells by triggering increased ROS production.Citation17,21 On the contrary, studies by Adams et al. suggest that biological activity of certain PL analogs can be decoupled from their effect on ROS up-regulation.Citation21 However, antitumor activity of 2 tested PL derivatives, namely PL-Di and PL-FPh, were entirely dependent on ROS generation since administration of NAC completely abolished the inhibitory effect of these compounds on the viability of 786-O and PNX0010 cells. Interestingly, our current data along with the results of previous work also demonstrate that native PL as well as PL derivatives selectively affect viability of tumor cells but have minimal/an insignificant effect on normal cells.Citation17,21 Typically, cancer cells are under higher intrinsic levels of oxidative stress than normal cells. This phenomenon can be explained, at least in part, by hyperactive metabolism and mitochondrial malfunction in malignant cells required for their rapid growth.Citation29,30 It has been suggested that a therapy designed to increase ROS to a level above the threshold for cancer cell death, but at an adaptable level for normal cells, would be an attractive strategy to selectively destroy cancer cells.Citation31 Indeed, ROS-mediated cell death is an essential base for radiotherapy and many chemotherapeutic treatments.Citation27,30
Although our investigation suggests that PL-mediated depletion of c-Met parallels a loss of viability of RCC cells, the direct impact of PL on the other important cellular pathways cannot be excluded. Our recent studies demonstrate that PL also potently inhibits NF-κB and Akt/mTOR signaling pathways in tumor cells of various origins. These pathways play a critical role in development and progression of renal cancer.Citation32-37 Therefore, PL might potentially act as a multifocal inhibitor concurrently inhibiting several critical signaling pathways in RCC cells. Targeted therapies have often given disappointing results when used as single agents in solid tumors. Multiple survival mechanisms are disproportionally activated in malignant cells.Citation9,35,36,38,39 Thus, the most durable therapeutic benefit could be achieved with antitumor agents targeting diverse signaling pathways.
The development of new non-toxic regimens for the treatment of RCC represents a goal with enormous clinical and scientific merit. Our work describes original findings that naturally occurring alkaloid, piperlongumine induces c-Met depletion in RCC cells. Since PL is a non-toxic natural agent with potent antitumor activity both in vitro and in vivo, our current studies indicate that PL and PL derivatives hold significant promise as potential therapeutic agents for the treatment of metastatic RCC and the prevention of postoperative RCC recurrence.
Materials and Methods
Cells and culture conditions
The 786-O human RCC cell line was obtained from ATCC (Rockville, MD). The PNX0010 human RCC cell line, which was described previously,Citation40 was a kind gift of Dr. Vladimir Khazak, Ph.D. (Program in Biology, Priaxon Inc., Philadelphia, PA, USA). (Rockville, MD, USA) and Dr. Igor Astsaturov, MD, PhD. (Fox Chase Cancer Center, Philadelphia, PA, USA). Initial stocks were cryopreserved, and at every 6-month interval a fresh aliquot of frozen cells was used for the experiments. No authentication was done by the authors. Cells were cultured in RPMI 1640 (Bio-Whittaker, Walkersville, MD) supplemented with 10% FCS (Hyclone, Logan, UT), gentamicin (50 mg/l), sodium pyruvate (1 mM) and non-essential amino acids (0.1 mM) under conditions indicated in the figure legends.
Antibodies and reagents
Antibody against actin was obtained from Santa Cruz Biotechnology (Santa Cruz, CA); N-Acetyl Cysteine was obtained from Sigma (St. Louis, MO); Antibodies to c-Met, pSTAT3, STAT3, pErk 1/2, Erk 1/2, pAkt (Ser 473), Akt, and Src were obtained from Cell Signaling Technology (Beverly, MA); Piperlongumine was obtained from Indofine Chemical Company (Hillsborough, NJ); PL-fluorophenyl (PL-FPh) and PL-Dimer (PL-Di) were synthesized by Xcess Biosciences Inc. (San Diego, CA); and Bortezomib was obtained from Biomol (Plymouth Meeting, PA).
Western blot analysis
Whole cell lysates preparation and Western Blot Analysis were performed as described previously.Citation41
Real time PCR
Total RNA was isolated from 786-O and PNX0010 cells using Mini RNA isolation II Kit (Zymo Research, Orange, CA) and purified using/via RNA Clean and Concentrator Kit (Zymo Research). Total RNA (1 μg) was reverse transcribed in a final volume of 20 μl with 100 U of Superscript III Reverse Transcriptase (Invitrogen, Gaithersburg, MD) and 50 ng of random hexamer primers according to the manufacturer's instructions. After reverse transcription, cDNA samples were diluted 40 and 400 times respectively (for c-MET and 18S rRNA respectively) and 5 μl of diluted cDNA was amplified by real time PCR using MET TaqMan Gene Expression Assay (ID# Hs.PT.58. 3768320). Custom 18S rRNA Mini qPCR assay (IDT DNA Technologies, Coralville, IA) was used as an internal amplification control. The amplicon was detected with a forward primer 5′-GCTCTTTCTCGATTCCGT, reverse primer 5′-CCAGAGTCTCGTTCGTTATC and probe 5′-TTCTTAGTTG GTGGAGCGATTTGT labeled with 6-FAM and quenched with Jowa-Black FQ. Each sample was run in triplicate for both c-MET and 18S rRNA in 20 µl reaction mix using TaqMan Gene Expression Master Mix according to the manufacturer's instructions (Applied Biosystems, Foster City, CA, USA). Reactions were carried out/performed in an Applied Biosystems 7500 Real-Time PCR System. Analysis of relative c-MET expression was carried out/performed using the 2−ΔΔCt method.
Measurement of cell viability
Cell viability was analyzed by CellTiter Blue assay (Promega, Madison, WI, USA). Effective doses (ED) were calculated using XLfit, a Microsoft Excel add-in.
Analysis of ROS generation
Cells were re-suspended in PBS containing 5 μM of CM-H2DCFDA (Invitrogen, Carlsbad, CA), incubated at 37°C in the dark for 15 minutes and analyzed by flow cytometry. Analysis was performed using FACScan (Becton Dickinson, Franklin Lakes, NJ, USA). Individual fluorescent populations were determined through the use of acquisition and analysis software (Cell Quest, Becton Dickinson).
Assessment of in vivo tumor growth
For in vivo studies, 1 × 106 PNX0010 cells were inoculated s.c. in the flank region of 6 week-old male C.B17/Icr-scid mice using a 27-gauge needle (all animal procedures were done in accordance with institutional guidelines on animal care and with appropriate institutional certification). Animals were fed an autoclaved 2018SX diet (Harlan Teklad, Madison, WI, USA) and water ad libitum. Two weeks after the injection of tumor cells, animals were randomly assigned to the control or experimental groups (n = 8 mice/group). The mice were treated with either vehicle (0.3% DMSO in 0.9% NaCl solution), PL or PL-Di (20 mg/kg intraperitoneally, 3 times/week) as previously described.Citation19 Tumor volumes were calculated using the formula: (volume = 0.52 × (width)2 × length).
Statistical analysis
Statistical analysis was performed using a 2-sided Student's t-test. A P-value of <0.05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
These experiments were performed with the support of the Laboratory Animal and Flow Cytometry Facilities at Fox Chase Cancer Center.
Funding
This work was supported in part by the National Institutes of Health Grants (RO1 CA134463, RO3 CA167671), FCCC/Temple University Nodal Grant and FCCC/Temple University Interdisciplinary Translational Cancer Research Grant to VMK.
References
- Levy DA, Slaton JW, Swanson DA, Dinney CP. Stage specific guidelines for surveillance after radical nephrectomy for local renal cell carcinoma. J Urol 1998; 159(4):1163-7; PMID:9507823; http://dx.doi.org/10.1016/S0022-5347(01)63541-9
- Sorbellini M, Kattan MW, Snyder ME, Reuter V, Motzer R, Goetzl M, McKiernan J, Russo P. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol 2005; 173(1):48-51; PMID:15592023; http://dx.doi.org/10.1097/01.ju.0000148261.19532.2c
- Flanigan RC, Campbell SC, Clark JI, Picken MM. Metastatic renal cell carcinoma. Curr Treat Options Oncol 2003; 4(5):385-90; PMID:12941198; http://dx.doi.org/10.1007/s11864-003-0039-2
- Rini BI. Metastatic renal cell carcinoma: many treatment options, one patient. J Clin Oncol 2009; 27(19):3225-34; PMID:19470934; http://dx.doi.org/10.1200/JCO.2008.19.9836
- Rini BI. New strategies in kidney cancer: therapeutic advances through understanding the molecular basis of response and resistance. Clin Cancer Res 2010; 16(5):1348-54; PMID:20179240; http://dx.doi.org/10.1158/1078-0432.CCR-09-2273
- Gibney GT, Aziz SA, Camp RL, Conrad P, Schwartz BE, Chen CR, Kelly WK, Kluger HM. c-Met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann Oncol 2013; 24(2):343-9; PMID:23022995; http://dx.doi.org/10.1093/annonc/mds463
- Giubellino A, Linehan WM, Bottaro DP. Targeting the Met signaling pathway in renal cancer. Expert Rev Anticancer Ther 2009; 9(6):785-93; PMID:19496715; http://dx.doi.org/10.1586/era.09.43
- Graveel CR, Tolbert D, Vande Woude GF. MET: a critical player in tumorigenesis and therapeutic target. Cold Spring Harb Perspect Biol 2013; 5(7); PMID:23818496; http://dx.doi.org/10.1101/cshperspect.a009209
- Harshman LC, Choueiri TK. Targeting the hepatocyte growth factor/c-Met signaling pathway in renal cell carcinoma. Cancer J 2013; 19(4):316-23; PMID:23867513; http://dx.doi.org/10.1097/PPO.0b013e31829e3c9a
- Eder JP, Vande Woude GF, Boerner SA, LoRusso PM. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res 2009; 15(7):2207-14; PMID:19318488; http://dx.doi.org/10.1158/1078-0432.CCR-08-1306
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007; 316(5827):1039-43; PMID:17463250; http://dx.doi.org/10.1126/science.1141478
- Qi J, McTigue MA, Rogers A, Lifshits E, Christensen JG, Janne PA, Engelman JA. Multiple mutations and bypass mechanisms can contribute to development of acquired resistance to MET inhibitors. Cancer Res 2011; 71(3):1081-91; PMID:21266357; http://dx.doi.org/10.1158/0008-5472.CAN-10-1623
- Bezerra DP, Pessoa C, Moraes MO, Alencar NM, Mesquita RO, Lima MW, Alves AP, Pessoa OD, Chaves JH, Silveira ER, et al. In vivo growth inhibition of sarcoma 180 by piperlonguminine, an alkaloid amide from the Piper species. J Appl Toxicol 2008; 28(5):599-607; PMID:17975786; http://dx.doi.org/10.1002/jat.1311
- Morikawa T, Matsuda H, Yamaguchi I, Pongpiriyadacha Y, Yoshikawa M. New amides and gastroprotective constituents from the fruit of Piper chaba. Planta Med 2004; 70(2):152-9; PMID:14994194; http://dx.doi.org/10.1055/s-2004-815493
- Reddy L, Odhav B, Bhoola KD. Natural products for cancer prevention: a global perspective. Pharmacol Ther 2003; 99(1):1-13; PMID:12804695; http://dx.doi.org/10.1016/S0163-7258(03)00042-1
- Golovine KV, Makhov PB, Teper E, Kutikov A, Canter D, Uzzo RG, Kolenko VM. Piperlongumine induces rapid depletion of the androgen receptor in human prostate cancer cells. Prostate 2013; 73(1):23-30; PMID:22592999; http://dx.doi.org/10.1002/pros.22535
- Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 2011; 475(7355):231-4; PMID:21753854; http://dx.doi.org/10.1038/nature10167
- Ginzburg S, Golovine KV, Makhov PB, Uzzo RG, Kutikov A, Kolenko VM. Piperlongumine inhibits NF-kappaB activity and attenuates aggressive growth characteristics of prostate cancer cells. Prostate 2014; 74(2):177-86; PMID:24151226; http://dx.doi.org/10.1002/pros.22739
- Makhov P, Golovine K, Teper E, Kutikov A, Mehrazin R, Corcoran A, Tulin A, Uzzo RG, Kolenko VM. Piperlongumine promotes autophagy via inhibition of Akt/mTOR signalling and mediates cancer cell death. Br J Cancer 2014; 110(4):899-907; PMID:24434432
- Makhov PB, Golovine K, Kutikov A, Teper E, Canter DJ, Simhan J, Uzzo RG, Kolenko VM. Modulation of Akt/mTOR signaling overcomes sunitinib resistance in renal and prostate cancer cells. Mol Cancer Ther 2012; 11(7):1510-7; PMID:22532600; http://dx.doi.org/10.1158/1535-7163.MCT-11-0907
- Adams DJ, Dai M, Pellegrino G, Wagner BK, Stern AM, Shamji AF, Schreiber SL. Synthesis, cellular evaluation, and mechanism of action of piperlongumine analogs. Proc Natl Acad Sci U S A 2012; 109(38):15115-20; PMID:22949699; http://dx.doi.org/10.1073/pnas.1212802109
- Choueiri TK, Vaishampayan U, Rosenberg JE, Logan TF, Harzstark AL, Bukowski RM, Rini BI, Srinivas S, Stein MN, Adams LM, et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J Clin Oncol 2013; 31(2):181-6; PMID:23213094; http://dx.doi.org/10.1200/JCO.2012.43.3383
- Rosen LS, Senzer N, Mekhail T, Ganapathi R, Chai F, Savage RE, Waghorne C, Abbadessa G, Schwartz B, Dreicer R. A phase I dose-escalation study of Tivantinib (ARQ 197) in adult patients with metastatic solid tumors. Clin Cancer Res 2011; 17(24):7754-64; PMID:21976535; http://dx.doi.org/10.1158/1078-0432.CCR-11-1002
- Schoffski P, Garcia JA, Stadler WM, Gil T, Jonasch E, Tagawa ST, Smitt M, Yang X, Oliner KS, Anderson A, et al. A phase II study of the efficacy and safety of AMG 102 in patients with metastatic renal cell carcinoma. BJU Int 2011; 108(5):679-86; PMID:21156020
- Wagner AJ, Goldberg JM, Dubois SG, Choy E, Rosen L, Pappo A, Geller J, Judson I, Hogg D, Senzer N, et al. Tivantinib (ARQ 197), a selective inhibitor of MET, in patients with microphthalmia transcription factor-associated tumors: results of a multicenter phase 2 trial. Cancer 2012; 118(23):5894-902; PMID:22605650; http://dx.doi.org/10.1002/cncr.27582
- Choueiri TK, Pal SK, McDermott DF, Ramies DA, Morrissey S, Lee Y, Miles D, Holland JS, Dutcher JP. Activity of cabozantinib (XL184) in patients (pts) with metastatic, refractory renal cell carcinoma (RCC). J Clin Oncol 2012; suppl 5:abstr 364
- Wang J, Yi J. Cancer cell killing via ROS: to increase or decrease, that is the question. Cancer Biol Ther 2008; 7(12):1875-84; PMID:18981733; http://dx.doi.org/10.4161/cbt.7.12.7067
- Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog 2006; 5:14; PMID:16689993; http://dx.doi.org/10.1186/1477-3163-5-14
- Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science 2006; 312(5782):1882-3; PMID:16809515; http://dx.doi.org/10.1126/science.1130481
- Xu Y, Fang F, Miriyala S, Crooks PA, Oberley TD, Chaiswing L, Noel T, Holley AK, Zhao Y, Kiningham KK, et al. KEAP1 is a redox sensitive target that arbitrates the opposing radiosensitive effects of parthenolide in normal and cancer cells. Cancer Res 2013; 73(14):4406-17; PMID:23674500; http://dx.doi.org/10.1158/0008-5472.CAN-12-4297
- Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat 2004; 7(2):97-110; PMID:15158766; http://dx.doi.org/10.1016/j.drup.2004.01.004
- Cho D, Signoretti S, Regan M, Mier JW, Atkins MB. The role of mammalian target of rapamycin inhibitors in the treatment of advanced renal cancer. Clin Cancer Res 2007; 13(2 Pt 2):758s-63s; PMID:17255306; http://dx.doi.org/10.1158/1078-0432.CCR-06-1986
- Di Lorenzo G, Buonerba C, Biglietto M, Scognamiglio F, Chiurazzi B, Riccardi F, Carteni G. The therapy of kidney cancer with biomolecular drugs. Cancer Treat Rev 2010; 36 Suppl 3:S16-20; PMID:21129605; http://dx.doi.org/10.1016/S0305-7372(10)70015-3
- Hudes GR. Targeting mTOR in renal cell carcinoma. Cancer 2009; 115(10 Suppl):2313-20; PMID:19402072; http://dx.doi.org/10.1002/cncr.24239
- Husseinzadeh HD, Garcia JA. Therapeutic rationale for mTOR inhibition in advanced renal cell carcinoma. Curr Clin Pharmacol 2011; 6(3):214-21; PMID:21827395; http://dx.doi.org/10.2174/157488411797189433
- Morais C, Gobe G, Johnson DW, Healy H. The emerging role of nuclear factor kappa B in renal cell carcinoma. Int J Biochem Cell Biol 2011; 43(11):1537-49; PMID:21854869; http://dx.doi.org/10.1016/j.biocel.2011.08.003
- Oya M, Takayanagi A, Horiguchi A, Mizuno R, Ohtsubo M, Marumo K, Shimizu N, Murai M. Increased nuclear factor-kappa B activation is related to the tumor development of renal cell carcinoma. Carcinogenesis 2003; 24(3):377-84; PMID:12663495; http://dx.doi.org/10.1093/carcin/24.3.377
- Brenner W, Farber G, Herget T, Lehr HA, Hengstler JG, Thuroff JW. Loss of tumor suppressor protein PTEN during renal carcinogenesis. Int J Cancer 2002; 99(1):53-7; PMID:11948491; http://dx.doi.org/10.1002/ijc.10303
- Baldewijns MM, van Vlodrop IJ, Vermeulen PB, Soetekouw PM, van Engeland M, de Bruine AP. VHL and HIF signalling in renal cell carcinogenesis. J Pathol 2010; 221(2):125-38; PMID:20225241; http://dx.doi.org/10.1002/path.2689
- Kirsanov KI, Kotova E, Makhov P, Golovine K, Lesovaya EA, Kolenko VM, Yakubovskaya MG, Tulin AV. Minor grove binding ligands disrupt PARP-1 activation pathways. Oncotarget 2014; 5(2):428-37; PMID:24504413
- Golovine K, Makhov P, Uzzo RG, Shaw T, Kunkle D, Kolenko VM. Overexpression of the zinc uptake transporter hZIP1 inhibits nuclear factor-kappaB and reduces the malignant potential of prostate cancer cells in vitro and in vivo. Clin Cancer Res 2008; 14(17):5376-84; PMID:18765529; http://dx.doi.org/10.1158/1078-0432.CCR-08-0455
