Abstract
Background: The Veridex CellSearch is an FDA-approved technology for enumerating circulating tumor cells in blood samples of metastatic colorectal cancer mCRC) patients and has prognostic value. It is important to understand how counts of circulating tumor cells (CTCs), which are advocated to be tools for “liquid biopsy” of tumors, correlate with clinical and pathologic variables of significance in these patients. In this study, we have attempted to make such correlations along with evaluating how CTC counts change during the course of chemotherapy. Patients and methods: Following an IRB-approved protocol, blood samples were collected from 24 patients with mCRC along with relevant clinico-pathological data. Blood was collected at defined time-points both prior to as well as during the course of treatment with combination chemotherapy, and CTC counts were enumerated from7.5 ml of blood. Results: Seventeen out of 24 patients with mCRC showed a CTC count of 2 or less cells in 7.5 ml of blood at base-line assessment before chemotherapy while 7 patients showed 3 or more cells in 7.5 ml of blood at that point. A correlation was found between high carcino-embryonic antigen (CEA) levels and high CTC counts (P = 0.018) although it was also found that some patients had elevated CTCs without an elevated CEA. No correlation with the time interval between detection of primary tumor and appearance of secondary (metastatic) tumor(s) was found. CTC counts did not correlate with the presence of lung or liver metastases, i.e. a number of mCRC patients with lung or liver metastases had a count of zero CTCs at baseline. We also noted no correlation between CTC number and the status of KRAS or BRAF mutation. CTC counts dropped immediately after the start of chemotherapy in 11 out of 21 patients, and also reduced from the baseline at the end of chemotherapy in 5 out of 10 patients. Six of 7 patients who started with 3 or more CTCs in 7.5 ml at baseline also showed a final CTC reduction at the end of the therapy assessment. Conclusions: Analysis of circulating tumor cells may be of use in monitoring response to therapy in mCRC, either in combination with CEA monitoring or alone when CTCs are elevated but CEA level is not.
Abbreviations
| CTC | = | circulating tumor cell |
| CEA | = | carcinoembryonic antigen |
| FDA | = | Food and Drug Administration |
| mCRC | = | metastatic colorectal cancer |
| IRB | = | Institutional Review Board |
| ECOG | = | Eastern Cooperative Oncology Group |
Introduction
Colorectal cancer is the third leading cause of cancer-related death in the US and cytotoxic chemotherapy (with or without biologic agents such as bevacizumab or cetuximab) is the mainstay of treatment. Circulating tumor cells reflect disease burden in mCRC and higher counts adversely affect outcome in terms of disease-free survival and overall survival. Circulating tumor cells have been previously shown in a prospective study to correlate with overall survival as well as progression-free survival in mCRCCitation1 and are currently approved by the FDA for use in clinical practice. We hypothesized that CTC count changes during the course of treatment reflect response to therapy and thus can be used to assess the efficacy of ongoing treatment. Thus in non-responding patients alternative treatment strategies may be formulated early in the course of treatment. The present study was designed to investigate circulating tumor cells from peripheral blood of Stage IV CRC patients who were being treated with combination chemotherapy. Blood was collected at defined points during chemotherapy in defined volumes for CTC enumeration. During the course of the trial we also recorded relevant clinical and pathological characteristics, namely the number and sites of metastases, tumor histology, tumor genetics, and the time interval between appearance of primary tumor and detection of secondary metastatic tumors. CTC detection and enumeration was performed using the FDA-approved Veridex CellSearch system from Johnson&Johnson and all counts reported CTC numbers from 7.5 ml of blood.
Patients and methods
Patients: The prospective single-institution study enrolled patients with the following criteria:
Confirmed diagnosis of metastatic colorectal cancer
Patient will undergo chemotherapy
Documented histopathology of tumor
Radiologically measurable metastases
Age > 18 y
ECOG performance status 0–3
Patient signed informed consent
A patient meeting any of the following criteria was however excluded:
Prior chemotherapy in the past 5 weeks
Previous irradiation of metastatic tumor
Concurrent other solid tumor
Second malignancy in the previous 3 y
Life expectancy < 6 weeks
We gathered information about the number and sites of metastases, baseline CEA levels in serum, tumor genetics (BRAF, KRAS mutation status of the tumor), the time interval between detection of primary and appearance of secondary metastatic lesions. Complete clinical and pathological data was available from 24 patients, which is reported here.
CTC detection
Blood samples were collected at defined time-points during chemotherapy () for CTC detection purposes. The protocol allowed for drawing 2 or 3 tubes of blood (7.5 ml/tube) () at each time, though for patients who showed zero CTC counts, we only collected 1 tube at each subsequent time-point due to high costs and long processing time. All CTC detection was performed by the FDA-approved Veridex CellSearch technology, which uses immunomagnetic detection of EpCAM+, DAPI+, CK+, CD45- CTC cells. Tubes were processed within 96 hours of collection of blood as per recommendations of the manufacturer. The samples were handled and the procedure run by certified personnel trained in the CTC detection system. The results were expressed as CTC counts/7.5 ml of blood.
Table 1. Blood draw protocol for patients on FOLFOX/FOLFIRI regimes. Blood can be drawn upto 7 times during the course of chemotherapy.
Table 2. Blood draw protocol for patients on XELODA (capecitabine)-based chemotherapy.
Results
Blood samples for CTC assessment were taken from 33 mCRC patients seen at the Penn State Hershey Cancer Institute. CTC counts with complete clinical and pathologic data were available in 24 patients. Representative pathology images are shown in .
Figure 1. A (H&E) and 1B (EpCAM). Primary tumor of a case with baseline CTC of 56.33 ± 7 .06. (H&E) and 1D (EpCAM). Metastatic tumor of a different case with baseline CTC of 0.
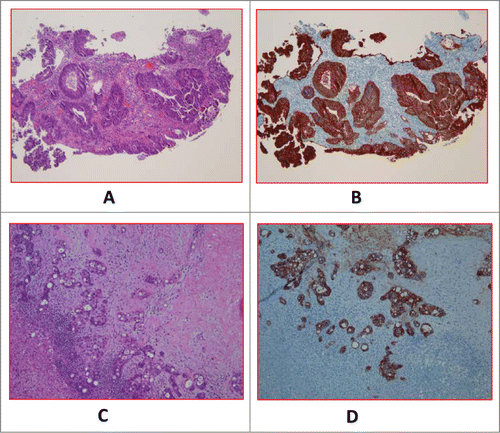
Out of the 24 patients, 17 patients (Group A) had 0–2 CTCs while 7 patients (Group B) had 3 or more CTCs at baseline in 7.5 ml of blood (). We noted that patients with extensive liver metastases did not necessarily have high CTC counts. Representative radiological images from patients with high metastatic burden from liver metastases along with CTC count are shown in .
Figure 2. The burden of metastatic disease in the liver does not correlate with CTC count as determined by the CellSearch method. Shown are CT scans of the abdomen from 2 patients (A and B) with diffuse colorectal cancer with hepatic metastases and high baseline CTC numbers. CT scans were performed before initiation of chemotherapy (baseline). The scans in panel C provide illustrative examples of the observed inter-patient heterogeneity from 4 patients. The numbers in black depict the ratio of total liver volume: volume of disease free liver, and the numbers in red depict the mean CTC count from replicates +/− standard error of the mean.
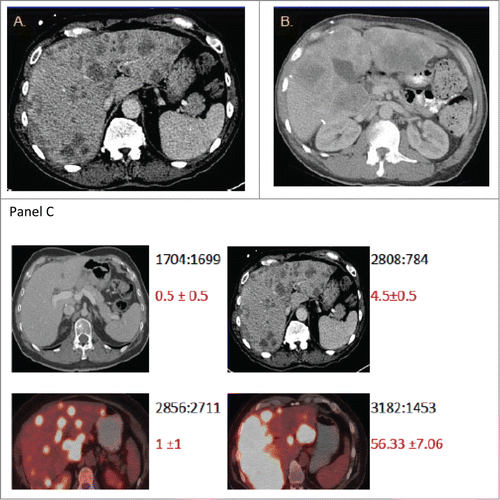
Table 3A. Comparison of incidence of liver and lung metastases between groups with low baseline CTC count (A) at 0–2 cells/7.5ml of blood and high baseline counts (B) of 3 or more CTCs in 7.5ml of blood (p = 0.39, Fisher's exact test).
We further analyzed (using relevant biostatistical methods) how the variables of organ-specific metastases, serum CEA levels, tumor KRAS and BRAF mutation status, and how the time interval between the diagnosis of primary and detection of secondary tumor(s) correlated with CTC counts and compared between the 2 groups ().
Table 3B. Comparison of CEA levels between patient groups with low and high CTC counts, respectively (p=0 .018, Fisher's exact test).
Table 3C. Comparison of tumor mutational status between patient groups with low and high CTC counts, respectively (p = 0.67, Fisher's exact test).
Table 3D. Comparison of time interval between the diagnosis of the primary tumor and the appearance of metastases between lowland high baseline CTC count groups (p = 0.35, Fisher's exact test).
Table 4A. Change in CTC counts at first assessment after start of chemotherapy as compared to the counts at baseline assessment prior to treatment.
Table 4B. Change in CTC counts at end-of-chemotherapy assessment as compared to the counts at baseline assessment prior to treatment.
Table 4C. Change in CTC counts at end-of-chemotherapy assessment compared to the counts at baseline assessment prior to treatment in the patients with high CTC counts of 3 or more cells in 7.5 ml of blood at baseline.
As part of the study, we measured CTC counts in the patients during successive cycles of chemotherapy and analyzed the changes in CTC counts. We compared the change in counts from baseline (pre-chemotherapy) counts (A) to the first available counts after chemotherapy had started. Out of 21 patients, 11 patients showed a reduced count, 3 showed an increase, while in 7 patients the CTC counts remained constant at this time-point (Table 4A). We compared CTC counts at the end-of-chemotherapy assessment (C) to the baseline CTC counts (A)in 10 patients for whom the data was available: 5 patients showed reduced counts, the numbers remained the same in 4, while a single patient was found to have an increase (Table 4B). In the 7 patients who had CTC counts of 3 or more cells at baseline (i.e., above the FDA-cutoff), CTC counts in 4 patients came down to 2 or less cells, the counts in 2 patients decreased but remained at 3 or more cells, while in one patient the counts went up at the first assessment after chemotherapy had started.
Discussion
There has been considerable interest in the concept of “liquid biopsy” of solid tumors and the potential use of CTC counts in blood as surrogate biomarkers to assess the patient's disease state and predict outcome.Citation2 In order to validate CTCs as prognostic markers of significance, it is crucial to investigate how CTC counts in patients with tumors correlate with established clinical and pathological parameters. Sastre et al. reported a correlation between tumor stage and elevated CTCs but no correlation was found between the presence of CTCs and the location of primary tumor, increased CEA level, increased lactate dehydrogenase level, or grade of differentiation.Citation3 Cohen et al. demonstrated a correlation between CTC numbers and overall survival and progression-free survival.Citation2
In our study we found that a high CTC count did not necessarily correlate with the presence or absence of liver and lung metastases (p = 0.39) but high serum CEA levels were accompanied by higher CTC counts (p = 0.018). We found no significant correlation between the status of KRAS or BRAF mutation and CTC counts (p = 0.68). CEA levels are established markers in clinical practice for prognostic purposes and the correlation of high CEA levels with higher CTC counts should add to the body of evidence emphasizing the predictive utility of measuring tumor cells in the blood.Citation4 We did not find that absence of expression of EpCAM in primary tumors could explain why some patients with high liver metastatic burden still had very low or undetectable CTC counts with the CellSearch system. It is possible that EMT could impact on CTC recovery from some patients although there is precedent for mixed epithelial and mesenchymal features of CTCs (i.e. even with EMT tumor cells can retain EpCAM expression). As a future direction, technologies that can capture CTCs and successfully study marker expression need tobe investigated to determine if higher expression of CEA or other markers in such cells correlates with higher disease burden and more advanced stage of the cancer. The available literature also emphasizes the importance of combining CTC enumeration with biomarker analysis to be effective as prognostic and disease-monitoring tools.Citation5,6
As a prognostic tool in oncology, a change in CTC counts should be observed in response to treatment. With this intent, we studied post-chemotherapy changes in CTC counts and found that in approximately 52% of cases the CTC counts dropped immediately at the first assessment after initiation of chemotherapy. In 50% of cases, the final CTC counts at the end of chemotherapy decreased from the baseline. It will be important in the future to determine whether the post-therapy change in counts correlated with the overall survival of these patients. Interestingly, in patients having CTC counts higher than the FDA cut-off of 3 or more cells, the counts came down to below 3 cells at end of therapy assessment in about 60% cases.
In conclusion, we believe enumeration of circulating tumor cells holds potential of impacting on cancer management and monitoring therapy through the “liquid biopsy” technique. It is important to evaluate the correlation of CTCs in blood with clinical and laboratory parameters that relate to the disease process and extent, as attempted in the study. Serial measurement of CTC counts during the course of treatment should offer clues to patterns of change of potential clinical significance. In the future, molecular markers whether they are prognostic or stem cell markersCitation7 or genomic analysis of CTCs will have relevance in understanding an individual patient's cancer better and help to personalize treatment. Biology of CTCs appears to be important too and our group had earlier reported that apoptotic CTCs (and not intact CTCs) in peripheral blood of mCRC patients were found to correlate with liver metastases.Citation8 Large trials investigating the combined impact of CTC counts along with appropriate biomarker analysis will be essential for formulating guidelines and defining rational criteria in clinical oncology that will enable us to better utilize CTC technology for improved management of primary and secondary cancers.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary_Data.docx
Download MS Word (12.8 KB)Acknowledgments
We thank the enrolled patients for their altruism and making this work possible. We also thank the infusion room personnel, nurses and physician-assistants at PSHCI for their support.
Funding
The REDCap database is supported by funds from NIH/NCATS (Grant NumberUL1 TR000127) to the Penn State Clinical & Translational Research Institute.
Supplemental data
Supplemental data for this article can be accessed on the publisher's website.
References
- Cohen SJ, Punt CJA, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et al. Relationship of Circulating Tumor Cells to Tumor Response, Progression-Free Survival, and Overall Survival in Patients With Metastatic Colorectal Cancer. J Clin Oncol 2008 July 1, 2008;26(19): 3213-21; http://dx.doi.org/10.1200/JCO.2007.15.8923
- Alix-Panabieres C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem 2013 January; 59(1): 110-8; http://dx.doi.org/10.1373/clinchem.2012.194258
- Sastre J, Maestro ML, Puente J, Veganzones S, Alfonso R, Rafael S, Garcia-Saenz JA, Vidauretta M, Martin M, Arroyo M, et al. Circulating tumor cells in colorectal cancer: correlation with clinical and pathological variables. Ann Oncol 2008; 19: 935-938.
- Iinuma H, Watanabe, Mimori K, Adachi M, Hayashi N, Tamura J, Matsuda K, Fukushima R, Okinaga K, Sasako M, et al. Clinical Significance of Circulating Tumor Stem Cells, Including Cancer Stem-like Cells, in Peripheral Blood for Recurrence and Prognosis in Patients with Dukes' Stage B and C Colorectal Cancer. J Clin Oncol 2011; 29(12): 1547-1555; http://dx.doi.org/10.1200/JCO.2010.30.5151
- Faltas B, Zeidan A, Peters K, Das A, Joudeh J, Navaraj A, Dolloff NG, Harvey HA, Jiang Y, Allen JE, et al. Identifying Circulating Tumor Stem Cells That Matter: The Key to Prognostication and Therapeutic Targeting. J Clin Oncol 2011; 29(21): 2946-2947; PMID: 21690466; http://dx.doi.org/10.1200/JCO.2011.36.6179
- Peters KL, Allen JE, Dicker DT, Das A, Joudeh J, El-Deiry WS. CTC profiling: Integration of biomarker analysis with current VeridexCellSearch technology. Cancer Res 2011; 71 (8 supplement) 5248-5248; http://dx.doi.org/10.1158/1538-7445.AM2011-5248
- Das A, Allen JE, Dicker DT, Peters KL, Joudeh J, El-Deiry WS. Quantum dot multiplexing of prognostic marker and stem cell marker expression in colorectal cancer circulating tumor cells. Cancer Res 2011; (8 supplement) 3818-3818.
- Allen JE, Saroya BS, Kunkel M, Dicker DT, Das A, Peters KL, Joudeh J, Zhu J, El-Deiry WS. Apoptotic circulating tumor cells (CTCs) in the peripheral blood of metastatic colorectal cancer patients are associated with liver metastasis but not CTCs. Oncotarget 2013( Vol 5 No 7).
