Summary
It is still uncertain if targeted therapy-based regimens in advanced gastric cancer actually produce survival benefit. To shed light on this important question, we performed a systematic review and meta-analyses on each relevant targeted-pathway. By searching literature databases and proceedings of major cancer meetings in the time-frame 2005–2014, 22 randomized clinical trials exploring targeted therapy for a total of 7022 advanced gastric cancer patients were selected and included in the final analysis. Benefit was demonstrated for antiangiogenic agents in terms of overall survival (HR 0.759; 95%CI 0.655–0.880; p < 0.001). Conversely no benefit was found for EGFR pathway (HR 1.077; 95%CI 0.847–1.370; p = 0.543). Meta-analysis of HER-2 pathway confirmed improvement in terms of survival outcome, already known for this class of drugs (HR 0.823; 95%CI 0.722–0.939; p = 0.004). Pooled analysis demonstrated a significant survival benefit (OS: HR 0.823; PFS: HR 0.762) with acceptable tolerability profile for targeted-based therapies as compared to conventional treatments. This finding conflicts with the outcome of most individual studies, probably due to poor trial design or patients selection. In conclusion, our findings demonstrate a significant survival benefit for targeted therapy in its whole, which can be ascribed to anti-angiogenic and anti-HER2 agents.
Abbreviations
| Ab | = | monoclonal antibody |
| ADME | = | absorption, distribution, metabolism, and excretion |
| aGC | = | advanced gastric cancer |
| BSC | = | best supportive care |
| CHT | = | chemotherapy |
| EGFR | = | epidermal growth factor receptor |
| GC | = | gastric cancer |
| HER2 | = | human epidermal growth factor receptor 2 |
| HER3 | = | human epidermal growth factor receptor 3 |
| MET | = | mesenchymal epithelial transition factor |
| mTOR | = | mammalian target of rapamycin |
| mTORC | = | mTOR complex |
| NGS | = | next generation sequencing |
| NSCLC | = | non-small cell lung cancer |
| OR | = | odds-ratio |
| OS | = | overall survival |
| PARP | = | poly ADP ribose polymerase |
| PFS | = | progression free survival |
| PI3K | = | phosphatidylinositide 3-kinases |
| PRISMA | = | preferred reporting items for systematic reviews and meta-analyses |
| RAF | = | rapidly accelerated fibrosarcoma |
| RAS | = | rat sarcoma viral oncogene homolog |
| RCTs | = | randomized clinical trials |
| RR | = | response rate |
| TKI | = | tyrosine kinase inhibitor |
| VEGF | = | vascular endothelial growth factor |
| VEGFR | = | VEGF receptor |
Background
Description of epidemiology and clinical management
Gastric cancer (GC) is an aggressive malignancy and prognosis for advanced disease (aGC) is poor.Citation1,2 Due to its heterogeneity, GC usually include different subgroups based on histological, anatomical, epidemiological and more recently, genomic, or molecular classifications.Citation3,4 Adenocarcinoma is the most frequent histotype, representing 90% of GCs. Histologically, an intestinal type (well-differentiated) with better prognosis and response to treatment, and a diffuse type (or undifferentiated) more frequent in young patients and characterized by worse outcome are recognized.Citation5 As a further difference, intestinal GC seems to result from a multistep carcinogenesis process, while diffuse GC is characterized by de novo lesions frequently associated to E-cadherin loss.Citation6-8
Conventional treatment
Regardless of pathological subtype, the treatment of GC is based on a multidisciplinary approach that includes surgery, radiotherapy and chemotherapy.Citation9 Usually, aGC patients receive diagnosis when the tumor is not suitable for radical surgery.Citation10 At this stage, the mainstay of treatment is palliative systemic chemotherapy.Citation10,11 So far, several small randomized clinical trials (RCTs) provided evidence that systemic chemotherapy improves survival in aGC patients, but at the cost of relevant toxicity.Citation12,13 Whether fluoropyrimidines are the most common agents in this disease as single agent or in combination schedules, several other drugs showed efficacy as single agent such as docetaxel, irinotecan, anthracyclines, and platinum compounds (oxaliplatin and cisplatin).Citation14,15 Currently, combination chemotherapy schedules including fluoropyrimidines and platinum salts, with possible addition of docetaxel in fit patients, represent the landmark of first-line treatment for aGC with potential benefits in terms of quality of life and overall survival (OS).Citation10,16-18 Concerning the second-line treatment, available data on the safety and efficacy of therapy are limited.Citation19 In particular COUGAR-02, a phase III randomized clinical trial, reported a significant OS improvement for docetaxel versus best supportive care (BSC) with a median advantage of 1.5 months.Citation20 Regarding subsequent lines of treatment, BSC or recruitment in clinical trials (fit patients only) is considered as the best choice.Citation12,21
Molecular pathways and targeted therapy
Several pathways appear to act as “drivers” in different aGC subtypes. In particular non-diffuse cancers seem to depend on different alterations in epidermal growth factor or other peptide growth factor signaling (HER2, EGFR, MET) or in angiogenesis-related signaling, while in diffuse cancers beta-catenin, PI3K/Akt/mTOR pathway and HER3 activity play a predominant role.Citation22-24
Recently, RCTs investigated the efficacy of the targeted therapy alone or in combination with chemotherapy, but results were mostly unsatisfactory.Citation25-38 While several RCTs demonstrated an improvement in terms of response rate (RR), and progression free survival (PFS) only one study reported a significant increase in terms of OS in a selected subgroup of patients in front-line treatment.Citation25 In that trial, patients were selected according to HER2 status (resulted to be overexpressed in 16–34% of patients with intestinal type and 2–7% of diffuse aGC), and subsequently treated with trastuzumab plus standard chemotherapy with a significant 2.7 months advantage in OS. To date, the addition of trastuzumab to conventional chemotherapy represents the best treatment choice for aGC overexpressing HER2.Citation25 Serum VEGF concentration, EGFR overexpression and PI3K/Akt/mTOR pathway alterations have been shown to be related to vascular involvement, metastases and poor outcome, thus representing potential targets in this disease.Citation23 Indeed, different antiangiogenic agents showed interesting activity in terms of response rate. Furthermore, as in many other cancers, it has been demonstrated the reliance of GC on angiogenesis, with the arrest of tumor growth in the absence of neovascularization.Citation39 In particular, 3 phase II studies that investigated the effect of bevacizumab-based therapy showed an encouraging RR (65–68%), subsequently confirmed in a phase III trial in the absence, however, of significant benefit in OS.Citation39-41 Recently, a meta-analysis confirmed the benefit of anti-VEGF target therapy in aGC on all endpoints evaluated (OS, PFS, RR).Citation42 Despite EGFR overexpression is observed in 27–44% of all GC, different trials evaluating the role of anti-EGFR agents failed to demonstrate any improvement in either PFS, OS, or RR.Citation30,43 The role of targeted therapy in aGC remains therefore mostly undefined.
On this basis, we performed a systematic review to analyze the weight of each targeted pathway in aGC management through one by one meta-analysis.
Results
Studies selection
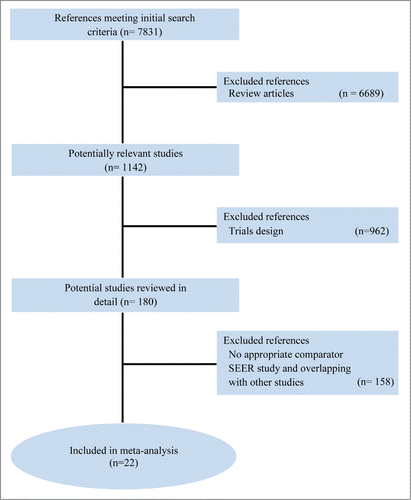
In , the PRISMA chart related to RCTs selection and search strategy is shown. In the time-frame covered by the present systematic review (2005–2014), 7831 studies were reported as full papers or meeting abstracts, while 6689 studies were initially excluded because reviews and 962 were excluded for trial design. Subsequently, we examined in detail the remaining 180 trials. Among them, 158 were excluded because selection criteria were not met. Further, one study was excluded because reported data about a major trial previously examined and already included.Citation28,44 One trial was excluded due to missing retrievable data, as already reported.Citation45 Six studies couldn't be evaluated because still ongoing.Citation46-50 Twenty-two trials for a total of 7022 patients were selected and included in the final analysis.Citation25-37,44,51-59 The TYTAN trial missed data about PFS. One trial missed results about OS. Two trials were analyzed only for RR and toxicity for missing data on survival endpoints.Citation33,51 Moreover, 3 trials, both designed for multiple arms comparison, were analyzed for single comparison considering an aggregate arm of different drug concentrations.Citation33,34,59 One trial was evaluated only for survival endpoints, because missing RR and toxicity data, as reported, an abstract in the meeting ASCO 2011.Citation27 At least one data-comparison in terms of survival, RR, or toxicity was reported in all selected RCTs, which were therefore deemed eligible for the end-point analysis. Summarizing the 22 trials included in final analyses: 19 were eligible for OS analysis (among them, we underlined, that: 9 were included in anti-angiogenic analysis; 4 studies were included in anti-EGFR analysis; 3 studies were included in anti-HER2 analysis; single trials respectively investigating a MET inhibitor, mTOR inhibitor and a PARP inhibitor), 19 were eligible for PFS analysis (among them, we underlined, that: 10 were included in anti-angiogenic analysis; 4 studies were included in anti-EGFR analysis; 2 studies were included in anti-HER2 analysis; single trials respectively investigating a MET inhibitor, mTOR inhibitor and a PARP inhibitor), and 19 were evaluable for RR analysis (among them, we underlined, that: 9 were included in anti-angiogenic analysis; 4 studies were included in anti-EGFR analysis; 3 studies were included in anti-HER2 analysis; single trials respectively investigating a Hedgehog inhibitor, a mTOR inhibitor and a PARP inhibitor). Twenty studies were evaluable for toxicity.
Study characteristics
Our analysis included 13 front-line (involving 4268 patients), 4 second-line (1140 patients), and 5 beyond second-line (1614 patients) trials in which targeted therapy-based treatment was compared to other conventional treatment. In particular, we evaluated 10 trials involving anti-angiogenic agents in experimental arm, 3 studies involving HER2 targeting agents, 5 trials involving anti-EGFR agents in experimental arm, single trials respectively investigating a MET inhibitor, mTOR inhibitor, a PARP inhibitor, and a Hedgehog inhibitor.
OS analyses
Three trials were excluded from OS analysis because they did not report data for this end-point.Citation33,51 In our analysis targeted therapy plus conventional therapy showed a benefit in terms of OS in aGC patients compared to conventional therapy alone (pooled HR 0.823; 95%CI 0.743–0.912; p ≤ 0.001; ). We reported meta-analysis results on each single target-therapy pathway. In particular, in our analyses, the most significant benefit occurred for antiangiogenic agents in terms of OS (HR 0.759; 95%CI 0.655–0.880; p < 0.001; ). Regarding angiogenesis pathway, we reported the advantage in subgroup analysis for second-line treatment (pooled HR 0.808; 95%CI 0.685–0.953; p = 0.011) and subsequent lines of treatment (pooled HR 0.633; 95%CI 0.474–0.846; p = 0.002), but not for first-line treatment. No statistically significance difference was found about subgroup analysis for class of drug (data not shown). Conversely by meta-analysis conducted for EGFR pathway, no benefit was found (HR 1.077; 95%CI 0.847–1.370; p = 0.543; ). No advantage for line of treatment was demonstrated by subgroup analysis for this pathway.
Figure 2. Comparison of OS according to involved pathway and treatment line ((A) all pathways), ((B) anti-angiogenic drugs), ((C) anti-EGFR drugs) and ((D) anti-HER2 drugs), between patients treated with a targeted therapy-containing regimen versus conventional schedule. Abbreviation: overall survival, OS; hazard ratio, HR; chemotherapy, CHT; best supportive care, BSC.
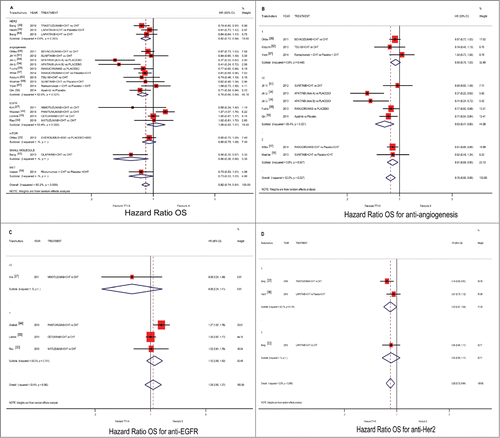
Our meta-analysis performed on HER2 pathway confirmed the improvement in terms of OS outcome known for this class of drugs (HR 0.823; 95%CI 0.722–0.939; p = 0.004; ). No advantage for line of treatment was demonstrated by subgroup analysis for this pathway.
PFS analyses
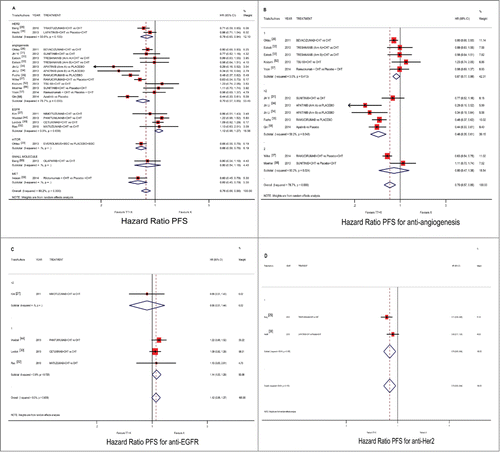
PFS data were not provided in one trial that was therefore excluded from PFS analysis.Citation51 Targeted therapy-based treatment was overall found to be significantly associated with improved PFS (pooled HR 0.762; 95%CI 0.664–0.875; p < 0.001; ). The analysis for single pathway demonstrated a significant PFS benefit in the anti-angiogenic pathway (pooled HR 0.695; 95%CI 0.567–0.853; p < 0.001; ). For this subgroup, we demonstrated an advantage both in first-line of treatment and in subsequent lines of treatment (pooled HR 0.873; 95%CI 0.770–0.990; p = 0.034; HR 0.463; 95%CI 0.351–0.610; p < 0.001, respectively). Conversely, there is no benefit in second-line of treatment. No differences about TKI/Ab subgroup analysis for this pathway. Interestingly, the analysis of anti-EGFR subgroup did not reach a statistically significant PFS advantage (pooled HR 1.117; 95%CI 0.985–1.268; p = 0.085; ). No advantage for line of treatment was demonstrated by subgroup analysis for this pathway. Regarding anti-HER2 analyses, we reported a significant benefit in term of PFS (pooled HR 0.780; 95%CI 0.646–0.941; p = 0.009; ). No evaluable subgroup analysis for line of treatment and for class of drug was performed for limited available studies.
Figure 3. Comparison of PFS according to involved pathway and treatment line ((A) all pathways), ((B) anti-angiogenic drugs), ((C) anti-EGFR drugs) and ((D) anti-HER2 drugs), between patients treated with a targeted therapy-containing regimen vs. conventional schedule. Abbreviation: overall survival, OS; hazard ratio, HR; chemotherapy, CHT; best supportive care, BSC.
RR analyses
One trial did not report data in term of RR, and was excluded from this analysis.Citation27 Regarding RR analyses for anti-angiogenic and for anti-EGFR agents, no advantage was reported (OR for RR 1.096; 95%CI 0.724–1.660; p = 0.665; OR for RR 0.913; 95%CI 0.449–1.858; p = 0.802, respectively; Fig. S1A–C). In the anti-HER2 drugs analysis we reported a significant improvement in term of RR (OR for RR 2.013; 95%CI 1.283–3.160; p = 0.002; Fig. S1D).
Toxicity analyses
In , we reported in detail common adverse events for each pathway. We analyzed specifically haematological toxicities, nausea and vomiting finding no difference for each pathway. In anti-HER2 agents group we observed an increase for diarrhea, while in anti-EGFR group, in first-line of treatment, we reported an increase of rash. No relevant toxicity for anti-angiogenic agents was demonstrated.
Table 1. Most common grade 3–4 adverse events analyzed in the meta-analysis
Discussion
With our meta-analysis we investigated the role of targeted therapy in aGC by comparison of targeted therapy-based regimens to any other conventional treatment. Moreover, we performed single meta-analysis stratified for each involved pathway. The major finding from our study is a significant survival benefit (OS: HR 0.823; PFS: HR 0.762) for targeted therapy in its whole, which can be ascribed to the reported advantage of anti-angiogenic and anti-HER2 agents as compared to conventional treatments in aGC, including all endpoints and subgroups. In fact we demonstrated OS improvement for studies on agents targeting anti-angiogenic and anti-HER2 pathways (HR 0.759; HR 0.823, respectively), paralleled by a significant PFS benefit (HR 0.695; HR 0.780, respectively). In term of survival outcomes no benefit was found for anti-EGFR agents. Only meta-analysis investigating anti-HER2 pathway showed a significant benefit in RR (OR 2.013). By evaluation of studies investigating each pathway, our work confirmed a good tolerability profile of targeted therapy, thus underscoring its potential role in management of aGC particularly in lines of treatment subsequent to front-line.
Some points need however to be addressed. The first point is that, while the survival improvement in this meta-analysis is remarkable, our results do not reflect the common clinical practice because only few trials reached their primary endpoint of improved aGC patient survival and therefore led to regulatory agency drug approval and treatment changes. One possible explanation for this finding is the underpowering of single trials both for the limited number of enrolled patients and for the overestimated expected benefit in the trial design. In particular, we observed that the major limit of the trials included in our meta-analysis is the lack of a priori selection or patient stratification. We are now aware that GC is an heterogeneous disease in which differences in anatomical site, race, and histotype characterize distinct diseases in terms of biology, genotype, and prognosis. Indeed, none of the included studies specifically reported the outcome of the adenocarcinoma subtype (intestinal vs. diffuse tumors). Moreover, with the exception of 3 trials in which a stratification for biomarker analysis (HER2 status) was reported, all the other trials were performed on unselected patient populations.Citation25,36,53 This could be an important information on patients survival outcome, because it is known that the expression of HER2 is different in the different histotypes (30% in the intestinal type and 7 % in the diffuse type). Apart from this specific condition there is no evidence about different prognostic and predictive effect of other pathways. For anti-angiogenic agents, this limitation is often due to the lack of validated predictive biomarkers.Citation26,31,33-35,37,39,52,60-64 In other cases, in particular for the anti-EGFR agents, even if the prominent role of RAS/RAF mutational status in colon cancer has been widely recognized so far, it is conceivable that this information was unknown at the time of studies design.Citation27,30,51,65-67 Therefore it is highlighted the need of new trials designed to investigate the relevance of RAS mutation in predicting response to anti-EGFR mAbs, in aGC. In particular, preliminary findings suggest that a worse outcome could be demonstrated in chemotherapy-treated patients in the presence of N-RAS mutations.Citation68 Furthermore, our results showed that, regardless a prior selection of patients, the use of anti-angiogenic drugs is overally statistical significant on all survival endpoints, specifically in subsequent lines of treatment and with a good tolerability profile.Citation30,43 Concerning RR results, we demonstrated a significant advantage for targeting anti-HER2 pathway alone, thus mimicking the results obtained in breast cancer where this pathway is determinant in driving tumor progression and represents a valuable therapeutic target in neoadjuvant treatment.Citation69-72
A second important point is that the most significant results on survival endpoints were achieved on subsequent lines of treatment as compared to first-line treatment. A possible explanation is that the most effective agents evaluated in subsequent lines of treatment, such as ramucirumab, have not yet been investigated in first line setting. Futhermore, combination regimens used in first line chemotherapy are investigated in trials heterogeneous for sample size which is generally small. Taking into account the choice of chemotherapy, trial design didn't consider drug interactions. Indeed, the combination with recognized anti-angiogenetic effect such as paclitaxel intensifies and supports bevacizumab activity regardless of cancer type.Citation73 Similarly, the combination with agents which has a recognized detrimental effect could underestimate the effectiveness of targeted therapy (e.g. capecitabine and cetuximab combination in colorectal cancer).Citation74
In subsequent lines of treatment conventional therapy is often represented by BSC, mainly because there is not, at present, a recognized standard/control arm in third line treatment.Citation29,34,35 Indeed, we think that these not expected findings open avenues for use of these new drugs in the maintenance settings, as already widely demonstrated in other cancers. New research paths are strongly suggested.Citation75-78
A third point regards the interpretation of our findings about subgroup analysis for TKI/Ab. We reported no differences for class of drugs in each pathway analyzed.
Recent studies proposed a possible alternative mechanism of action for mAbs apart intracellular pathway perturbation.Citation79 Indeed, in several tumors such as breast cancer, non-small cell lung cancer (NSCLC), and colorectal cancer, it was suggested an immune-modulating role for Abs. Based on some experimental findings, it appears that an immune modulating effect should be considered even for mAbs used in aGC treatment.Citation80-86 Interestingly, in aGC, while bevacizumab did not demonstrate a significant benefit, indeed, in our analysis, we observed that trials evaluating other agents showed a highly significant efficacy in term of survival benefit in the anti-angiogenesis subgroup in aGC.Citation17,35,37 Several studies suggested that targeting VEGFR2 may elicit and improve the immune response directed toward the tumor and its microenvironment trough different mechanisms including direct activation of CD8+ cells and targeting of the FOXP3high regulatory T cells.Citation87-95 These findings could explain, at least in part, the efficacy of ramucirumab, a mAb IgG1 direct to VEGFR2 and appear of interest taking also into account that GC is a disease in which VEGFR2 expression has not been reported to retain a prognostic weight.Citation96 Moreover, recent evidence underscored the prognostic roles of both VEGFA and VEGFC expression in GC.Citation97 VEGFC, in particular, is reported to induce VEGFR2/VEGFR3 heterodimer formation thus leading to cell proliferation (via RAS signaling) and angiogenic sprouting.Citation98 Of interest, VEGFC has been associated to both innate and acquired resistance to bevacizumab, and should be noted again that in our analysis VEGFR2-targeting ramucirumab appears to perform better than bevacizumab.Citation99 Consistently with these results, TKIs that mainly target VEGFR2 signaling, such as apatinib, showed the better results in term of OS. Considering the role played by the VEGFR2/VEGFR3 axis in inflammation, immunology, and angiogenesis these agents seems to be promising and warrant further investigation in aGC. Another interesting pathway involved in the process of progression and invasion of cancer is PI3K/AKT/mTOR. This pathway seems to play a central role in aGC, for these reasons specific and taking into account the currently available mTORC1 inhibitors, such as everolimus that proved to be effective in other cancer type, these compounds should be investigated in aGC.Citation100,101
Finally it has to be pointed out that our meta-analysis has some limitations: it was performed on literature data and it was not possible to retrieve data about all end-points from all studies; another potential bias is the heterogeneity of agents used in the included trials. An important limitation, as suggested by pre-planned subgroup analyses in AVAGAST on regional differences in the efficacy of anti-angiogenic therapy, is that we could not analyze data for single ethnic population.Citation26 Additionally, the weight assessment for each drug is difficult and it has to be considered that specific polymorphisms in ADME genes among different ethnic groups could modulate response to treatment.Citation102 Taking into account all these points, it is clear that our findings are not intended to identify the new standard treatment for aGC. Indeed, the goal of a meta-analysis is not to change clinical practice but to suggest rational new trials design, and underscore novel areas of investigation. At this aim our data strongly support additional investigation on anti- angiogenic pathways-targeted agents in aGC. On these basis future trials design could foresee enrollment of patients selected on one or more specific targets predefined by new technologies such as NGS that describe a peculiar molecular profile for related disease but not correlated to the site of disease.
In conclusion, at our knowledge, our work provides the first proof-of-concept that targeted therapy is an effective therapeutic approach against aGC. However, at this time, if we exclude trastuzumab in HER2-selected patients and ramucirumab, this important evidence cannot be transferred into clinical practice for other targeted agents. New trials designed on predefined biomarkers and aimed to identify the right drug for the right patient are eagerly awaited. In the absence of good designed biomarker-driven clinical trials, the use of targeted therapy is presently an unmet goal for clinical practice in a prognostically unfavourable disease as aGC.
Methods
Study design
We performed a systematic review and meta-analysis of all published randomized studies focused on each previously reported targeted pathway, to investigate the weight of targeted therapy-based schedules compared to conventional therapies in the management of aGC. The study includes therefore prospective and RCTs on aGC in all treatment lines with OS, PFS, RR, and toxicity, as predefined endpoints.
Comparisons for each one meta-analysis were performed as follows:
Targeted therapy plus conventional therapy versus conventional therapy alone:
Line of treatment subgroup;
Class of drugs subgroup: monoclonal antibody (mAb)/tyrosine kinase inhibitor (TKI);
Endpoints were OS, PFS, RR and toxicity.
Searching
We retrieved the most widely recognized bibliographic sources (PubMed, Embase, and the Central Registry of Controlled Trials of the Cochrane Library), major meeting proceeding databases (ASCO and ESMO), and selected studies presented between January 2005, at the time of consolidation of chemotherapy-based treatment in the management of aGC, and December 2014. Prospective studies only, were allowed in this analysis in order to reduce or minimize the risk of selection or information bias.Citation103-105 The search was performed by the following key-words: “gastric”, “stomach”, “tumor”, “cancer”, “advanced”, “metastatic”, “therapy”, “targeted”, “prospective”, and “randomized” in different combinations: i.e. “advanced gastric cancer, targeted therapy”. The ‘related articles’ function and references retrieved from articles were used to perform the search of all related studies, abstracts and citations. For this search only papers written in English language were considered.
Selection
In the studies included in the present review, patients had to be enrolled according to the characteristics described in .
Table 2. Main characteristics of the randomized trials included in the meta-analysis. Abbreviations: overall survival, OS; progression free survival, PFS; hazard ratio, HR; TT: target therapy; ST: standard therapy; chemotherapy, CHT; best supportive care, BSC
Inclusion criteria
The studies had to report patients with diagnosis of locally aGC or metastatic disease. Randomized controlled trials, with or without blinding were included. We considered abstracts or unpublished data if sufficient information on study design, characteristics of participants, interventions, and outcomes were available. In the experimental arm patients received a targeted therapy-based schedule. In the control arm patients received a conventional schedule for disease stage.
Exclusion criteria
Non-comparative studies; non-prospective studies; languages other than English; non-comparable end-points; way of chemotherapy or targeted agents administration different from systemic, or oral (e.g., Intra-arterial or intra-peritoneal infusion); no radiotherapy.
Data extraction
The studies were examined, independently, by 2 investigators (DC and NS) in order to select homogeneous studies.Citation106 Different variables from selected trials such as number of patients enrolled, year of publication, treatment schedule, and efficacy results were extracted and evaluated. Data regarding the occurrence of toxicities were obtained from the safety profile of each study. Any discrepancy was resolved by an arbiter (P.T.). Primarily, we analyzed OS and PFS, subsequently, RR and Toxicity Rates (TRs), regarding grade 3–4 of adverse events, for all patients. Data extraction was conducted according to the PRISMA statement.Citation107
Validity assessment
The quality assessment of selected studies was evaluated according to the Cochrane reviewers' handbook for 5 requirements: method of randomization, allocation concealment, blindness, withdrawal/dropout, and adequacy of follow-up.Citation108,109 10 trials were scored A (low risk of bias), 6 trials were scored B (intermediate risk of bias), and 6 trials were scored C (high risk of bias) (Table S1).
Quantitative data synthesis
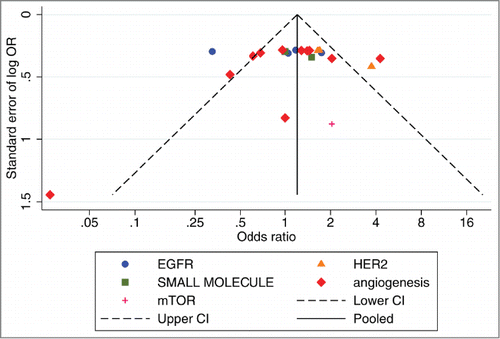
For each targeted-pathway the meta-analyses were carried out in order to evaluate the effects of the targeted-based treatments [biological +/− chemotherapeutic agents] on the pre-specified end-points.Citation110 Regarding the outcome of patients, survival data have been extracted as HRs of OS, and PFS with relative confidence intervals (95%CI). The interaction between survival and experimental treatment was obtained by each study from the HRs logarithm. The overall effect of combined treatments on RR and toxicity rate (TR) was calculated using method for dichotomous data (odds ratio and risk ratio assessment; 95%CI). In each meta-analysis subgroup analyses, based on the class of agents and line of treatment, were performed for all end-points. Cochrane's Q-test and I2 statistics were used to assess heterogeneity between studies and the random-effects model was used for the analysis taking into account the intent of comparing trials based on drugs with different mechanisms of action.Citation111 Pooled data analysis was performed according to the DerSimonian and Laird test.Citation112,113 The presence of publication bias was investigated through Begg's test for single pathway and by visual inspection of funnel plots.Citation114 A 2-tailed p value equal or lower than 0.05 was considered statistically significant. All the statistical analyses were performed by using STATA SE v. 13.1 (STATA_ Corporation, Texas, USA).Citation115
Disclosure of Potential Conflicts of Interest
All authors declare that they have no conflicts of interest.
Contributors
DC, NS, PT, and PT designed the study. DC, NS, FC, and FM did the literature search, and extracted data. DC realized the figures. FC, LF, SC, AMDA and SG performed the tables. All authors collected data. DC, NS, CB, PT, and, PT interpreted and analyzed the data, and wrote the manuscript. All authors read, and approved final version of the manuscript.
Supplemental Figure and Table
Download Zip (1.1 MB)Acknowledgments
This work is supported by PhD program of Magna Graecia University: PhD in molecular oncology and translational and innovative medical and surgical techniques.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893-917; PMID:21351269; http://dx.doi.org/10.1002/ijc.25516
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006; 12:354-62; PMID:16489633
- Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965; 64:31-49; PMID:14320675
- Shah MA, Khanin R, Tang L, Janjigian YY, Klimstra DS, Gerdes H, Kelsen DP. Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res 2011; 17:2693-701; PMID:21430069; http://dx.doi.org/10.1158/1078-0432.CCR-10-2203
- Fiocca R, Luinetti O, Villani L, Mastracci L, Quilici P, Grillo F, Ranzani GN. Molecular mechanisms involved in the pathogenesis of gastric carcinoma: interactions between genetic alterations, cellular phenotype and cancer histotype. Hepatogastroenterology 2001; 48:1523-30; PMID:11813565
- Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Höfler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res 1994; 54:3845-52; PMID:8033105
- Oliveira C, Moreira H, Seruca R, de Oliveira MC, Carneiro F. Role of pathology in the identification of hereditary diffuse gastric cancer: report of a Portuguese family. Virchows Arch 2005; 446:181-4; PMID:15735979; http://dx.doi.org/10.1007/s00428-004-1156-4
- Carneiro F, Huntsman DG, Smyrk TC, Owen DA, Seruca R, Pharoah P, Caldas C, Sobrinho-Simões M. Model of the early development of diffuse gastric cancer in E-cadherin mutation carriers and its implications for patient screening. J Pathol 2004; 203:681-7; PMID:15141383; http://dx.doi.org/10.1002/path.1564
- Cunningham D, Jost LM, Purkalne G, Oliveira J, Force EGT. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of gastric cancer. Ann Oncol 2005; 16(Suppl 1):i22-3; PMID:15888740; http://dx.doi.org/10.1093/annonc/mdi812
- Chen WW, Wang F, Xu RH. Platinum-based versus non-platinum-based chemotherapy as first line treatment of inoperable, advanced gastric adenocarcinoma: a meta-analysis. PloS One 2013; 8:e68974; PMID:23874831; http://dx.doi.org/10.1371/journal.pone.0068974
- Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet 2009; 374:477-90; PMID:19625077; http://dx.doi.org/10.1016/S0140-6736(09)60617-6
- Glimelius B, Hoffman K, Haglund U, Nyren O, Sjoden PO. Initial or delayed chemotherapy with best supportive care in advanced gastric cancer. Ann Oncol 1994; 5:189-90; PMID:8186165
- Scheithauer W, Van Cutsem E. The role of oxaliplatin in the management of upper gastrointestinal tract malignancies. Colorectal Dis 2003; 5(Suppl 3):36-44; PMID:23573559; http://dx.doi.org/10.1046/j.1463-1318.5.s3.5.x
- Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2010:CD004064; PMID:20238327
- Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2010; 362:858-9; PMID:20200397; http://dx.doi.org/10.1056/NEJMc0911925
- Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006; 24:4991-7; PMID:17075117; http://dx.doi.org/10.1200/JCO.2006.06.8429
- Lordick F, Lorenzen S, Yamada Y, Ilson D. Optimal chemotherapy for advanced gastric cancer: is there a global consensus? Gastric Cancer 2013; 17:213-25
- Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008; 9:215-21; PMID:18282805; http://dx.doi.org/10.1016/S1470-2045(08)70035-4
- Iacovelli R, Pietrantonio F, Farcomeni A, Maggi C, Palazzo A, Ricchini F, de Braud F, Di Bartolomeo M. Chemotherapy or targeted therapy as second-line treatment of advanced gastric cancer. A systematic review and meta-analysis of published studies. PloS One 2014; 9:e108940; PMID:25268988; http://dx.doi.org/10.1371/journal.pone.0108940
- Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, Mansoor W, Fyfe D, Madhusudan S, Middleton GW, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014; 15:78-86; PMID:24332238; http://dx.doi.org/10.1016/S1470-2045(13)70549-7
- Cunningham D, Oliveira J, Group EGW. Gastric cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2008; 19(Suppl 2):ii23-4; PMID:18456755
- Wong H, Yau T. Molecular targeted therapies in advanced gastric cancer: does tumor histology matter? Therap Adv Gastroenterol 2013; 6:15-31; PMID:23320047; http://dx.doi.org/10.1177/1756283X12453636
- Cidon EU, Ellis SG, Inam Y, Adeleke S, Zarif S, Geldart T. Molecular targeted agents for gastric cancer: a step forward towards personalized therapy. Cancers 2013; 5:64-91; PMID:24216699; http://dx.doi.org/10.3390/cancers5010064
- Ooi CH, Ivanova T, Wu J, Lee M, Tan IB, Tao J, Ward L, Koo JH, Gopalakrishnan V, Zhu Y, et al. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet 2009; 5:e1000676; PMID:19798449; http://dx.doi.org/10.1371/journal.pgen.1000676
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376:687-97; PMID:20728210; http://dx.doi.org/10.1016/S0140-6736(10)61121-X
- Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011; 29:3968-76; PMID:21844504; http://dx.doi.org/10.1200/JCO.2011.36.2236
- Kim YH, Sasaki Y, Lee KH, Rha SY, Park S, Boku N, Komatsu Y, Kim T, Kim S, Sakata Y. Randomized phase II study of nimotuzumab, an anti-EGFR antibody, plus irinotecan in patients with 5-fluorouracil-based regimen-refractory advanced or recurrent gastric cancer in Korea and Japan: Preliminary results. ASCO Meet Abstr 2011; 29:87
- Okines AF, Ashley SE, Cunningham D, Oates J, Turner A, Webb J, Saffery C, Chua YJ, Chau I. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for advanced esophagogastric cancer: dose-finding study for the prospective multicenter, randomized, phase II/III REAL-3 trial. J Clin Oncol 2010; 28:3945-50; PMID:20679619; http://dx.doi.org/10.1200/JCO.2010.29.2847
- Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol 2013; 31:3935-43; PMID:24043745; http://dx.doi.org/10.1200/JCO.2012.48.3552
- Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013; 14:490-9; PMID:23594786; http://dx.doi.org/10.1016/S1470-2045(13)70102-5
- Yi JH, Lee J, Lee J, Park SH, Park JO, Yim DS, Park YS, Lim HY, Kang WK. Randomised phase II trial of docetaxel and sunitinib in patients with metastatic gastric cancer who were previously treated with fluoropyrimidine and platinum. Br J Cancer 2012; 106:1469-74; PMID:22460270; http://dx.doi.org/10.1038/bjc.2012.100
- Rao S, Starling N, Cunningham D, Sumpter K, Gilligan D, Ruhstaller T, Valladares-Ayerbes M, Wilke H, Archer C, Kurek R, et al. Matuzumab plus epirubicin, cisplatin and capecitabine (ECX) compared with epirubicin, cisplatin and capecitabine alone as first-line treatment in patients with advanced oesophago-gastric cancer: a randomised, multicentre open-label phase II study. Ann Oncol 2010; 21:2213-9; PMID:20497967; http://dx.doi.org/10.1093/annonc/mdq247
- Eatock MM, Tebbutt NC, Bampton CL, Strickland AH, Valladares-Ayerbes M, Swieboda-Sadlej A, Van Cutsem E, Nanayakkara N, Sun YN, Zhong ZD, et al. Phase II randomized, double-blind, placebo-controlled study of AMG 386 (trebananib) in combination with cisplatin and capecitabine in patients with metastatic gastro-oesophageal cancer. Ann Oncol 2013; 24:710-8; PMID:23108953; http://dx.doi.org/10.1093/annonc/mds502
- Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013; 31:3219-25; PMID:23918952; http://dx.doi.org/10.1200/JCO.2013.48.8585
- Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014; 383:31-9; PMID:24094768; http://dx.doi.org/10.1016/S0140-6736(13)61719-5
- Hecht, JR, Bang, Y-J, Qin, S, Chung, H-C, Xu, J-M, Park, JO, Jeziorski, K, Shparyk, Y, Hoff, PM, Sobrero, AF. Lapatinib in combination with capecitabine plus oxaliplatin (CapeOx) in HER2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma (AC): The TRIO-013/LOGiC Trial. ASCO Meet Abstr 2013; 31:LBA4001
- Wilke H, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov ON, Kim T-Y, Cunningham D. RAINBOW: A global, phase III, randomized, double-blind study of ramucirumab plus paclitaxel versus placebo plus paclitaxel in the treatment of metastatic gastroesophageal junction (GEJ) and gastric adenocarcinoma following disease progression on first-line platinum- and fluoropyrimidine-containing combination therapy rainbow IMCL CP12-0922 (I4T-IE-JVBE). ASCO Meet Abstr 2014; 32:LBA7
- Kasper S, Schuler M. Targeted therapies in gastroesophageal cancer. Eur J Cancer 2014; 50:1247-58; PMID:24495747
- Okines AF, Reynolds AR, Cunningham D. Targeting angiogenesis in esophagogastric adenocarcinoma. Oncologist 2011; 16:844-58; PMID:21632459; http://dx.doi.org/10.1634/theoncologist.2010-0387
- Shah MA, Jhawer M, Ilson DH, Lefkowitz RA, Robinson E, Capanu M, Kelsen DP. Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J Clin Oncol 2011; 29:868-74; PMID:21189380; http://dx.doi.org/10.1200/JCO.2010.32.0770
- Shah MA, Ramanathan RK, Ilson DH, Levnor A, D'Adamo D, O'Reilly E, Tse A, Trocola R, Schwartz L, Capanu M, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol 2006; 24:5201-6; PMID:17114652; http://dx.doi.org/10.1200/JCO.2006.08.0887
- Qi WX, Shen Z, Tang LN, Yao Y. The role of anti-VEGF agents in the treatment of advanced gastric cancer: a meta-analysis of randomized controlled trials. Tumour Biol 2014; 35:7675-83; PMID:24801910; http://dx.doi.org/10.1007/s13277-014-2037-3
- Lieto E, Ferraraccio F, Orditura M, Castellano P, Mura AL, Pinto M, Zamboli A, De Vita F, Galizia G. Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann Surg Oncol 2008; 15:69-79; PMID:17896140; http://dx.doi.org/10.1245/s10434-007-9596-0
- Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G, Wadsley J, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 2013; 14:481-9; PMID:23594787; http://dx.doi.org/10.1016/S1470-2045(13)70096-2
- Roth A, Moehler MH, Mauer M, Schad A, Karrasch M, Praet M, Lim, ML, Das-Gupta, A, Lutz, MP. Lapatinib in combination with ECF/x in EGFR1 or HER2-overexpressing first-line metastatic gastric cancer (GC): A phase II randomized placebo controlled trial (EORTC 40071). ASCO Meet Abstr 2010; 28:TPS205
- Breithaupt K, Bichev, D, Lorenz M, Bohnen, D, Dogan, Y, Schlattmann, P, Salah-Eddin Al-Batran, Moehler, MH, Thuss-Patience PC. PaFLO: Pazopanib with 5-fluorouracil, leucovorin, and oxaliplatin (FLO) as first-line treatment in advanced gastric cancer: A randomized phase II study of the Arbeitsgemeinschaft internistische Onkologie (AIO). ASCO Meet Abstr 2012; 30:TPS4138
- Moehler MH, Kim YH, Tan IB, Balogh A, Sanchez TK, Bang Y-J. Sequential ipilimumab (Ipi) versus best supportive care (BSC) following first-line chemotherapy (Ctx) in patients (pts) with unresectable locally advanced or metastatic gastric or gastro-esophageal junction (GEJ) cancer: A randomized, open-label, two-arm, phase II trial (CA184–162) of immunotherapy as a maintenance concept. ASCO Meet Abstr 2013; 31:TPS4151
- Pavlakis N, Goldstein D, Sjoquist KM, Martin A, Tsobanis E, Yip S, Shannon J, Burge ME, Cronk MF, Tebbutt NC. INTEGRATE: A randomized phase II double-blind placebo-controlled study of regorafenib in refractory advanced esophagogastric cancer (AOGC)–A study by the Australasian Gastrointestinal Trials Group (AGITG). ASCO Meet Abstr 2013; 31:TPS4157
- Tabernero J, Hoff PM, Shen L, Ohtsu A, Yu R, Eng-Wong J, Kang Y-K. Pertuzumab (P) with trastuzumab (T) and chemotherapy (CTX) in patients (pts) with HER2-positive metastatic gastric or gastroesophageal junction (GEJ) cancer: An international phase III study (JACOB). ASCO Meet Abstr 2013; 31:TPS4150
- Cunningham D, Bang Y-J, Tabernero J, Shah MA, Lordick F, Hack SP. MetGastric: A randomized phase III study of onartuzumab (MetMAb) in combination with mFOLFOX6 in patients with metastatic HER2-negative and MET-positive adenocarcinoma of the stomach or gastroesophageal junction. ASCO Meet Abstr 2013; 31:TPS4155
- Richards D, Kocs DM, Spira AI, David McCollum A, Diab S, Hecker LI, Cohn A, Zhan F, Asmar L. Results of docetaxel plus oxaliplatin (DOCOX) +/− cetuximab in patients with metastatic gastric and/or gastroesophageal junction adenocarcinoma: results of a randomised Phase 2 study. Eur J Cancer 2013; 49:2823-31; PMID:23747051; http://dx.doi.org/10.1016/j.ejca.2013.04.022
- Koizumi W, Yamaguchi K, Hosaka H, Takinishi Y, Nakayama N, Hara T, Muro K, Baba H, Sasaki Y, Nishina T, et al. Randomised phase II study of S-1/cisplatin plus TSU-68 vs S-1/cisplatin in patients with advanced gastric cancer. Br J Cancer 2013; 109:2079-86; PMID:24045669; http://dx.doi.org/10.1038/bjc.2013.555
- Bang Y-J. A randomized, open-label, phase III study of lapatinib in combination with weekly paclitaxel versus weekly paclitaxel alone in the second-line treatment of HER2 amplified advanced gastric cancer (AGC) in Asian population: Tytan study. ASCO Meet Abstr 2013; 31:11
- Cohen DJ, Christos PJ, Kindler HL, D Catenacci DVT, Bekaii-Saab TB, Tahiri S, Janjigian YY, Gibson MK, Chan E, Rajdev L. Vismodegib (V), a hedgehog (HH) pathway inhibitor, combined with FOLFOX for first-line therapy of patients (pts) with advanced gastric and gastroesophageal junction (GEJ) carcinoma: A New York Cancer Consortium led phase II randomized study. ASCO Meet Abstr 2013; 31:4011
- Bang Y-J, Im S-A, Lee K-W, Cho JY, Song E-K, Kyung Hee Lee KH, Kim YH, Park JO, Chun HG, Zang DY. Olaparib plus paclitaxel in patients with recurrent or metastatic gastric cancer: A randomized, double-blind phase II study. ASCO Meet Abstr 2013; 31:4013
- Moehler MH, Hartmann JT, Lordick F, Al-Batran S, Reimer P, Trarbach T, Ebert MP, Daum S, Weihrauch M, Galle PR. An open-label, multicenter phase II trial of sunitinib for patients with chemorefractory metastatic gastric cancer. ASCO Meet Abstr 2010; 28:e14503
- Yoon HH, Bendell JC, Braiteh FS, Firdaus I, Philip PA, Cohn AL, Lewis N, Anderson DM, Arrowsmith E, Schwartz JD. Ramucirumab (RAM) plus FOLFOX as front-line therapy (Rx) for advanced gastric or esophageal adenocarcinoma (GE-AC): Randomized, double-blind, multicenter phase 2 trial. ASCO Meet Abstr 2014; 32:4004
- Qin S. Phase III study of apatinib in advanced gastric cancer: A randomized, double-blind, placebo-controlled trial. ASCO Meet Abstr 2014; 32:4003
- Iveson T, Donehower RC, Davidenko I, Tjulandin S, Deptala A, Harrison M, Nirni S, Lakshmaiah K, Thomas A, Jiang Y, et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol 2014; 15:1007-18; PMID:24965569; http://dx.doi.org/10.1016/S1470-2045(14)70023-3
- Fonsatti E, Sigalotti L, Arslan P, Altomonte M, Maio M. Emerging role of endoglin (CD105) as a marker of angiogenesis with clinical potential in human malignancies. Curr Cancer Drug Targets 2003; 3:427-32; PMID:14683500; http://dx.doi.org/10.2174/1568009033481741
- Chechlinska M, Kowalska M, Kaminska J. Cytokines as potential tumour markers. Expert Opin Med Diagn 2008; 2:691-711; PMID:23495779; http://dx.doi.org/10.1517/17530059.2.6.691
- Fondevila C, Metges JP, Fuster J, Grau JJ, Palacin A, Castells A, Volant A, Pera M. p53 and VEGF expression are independent predictors of tumour recurrence and survival following curative resection of gastric cancer. Br J Cancer 2004; 90:206-15; PMID:14710231; http://dx.doi.org/10.1038/sj.bjc.6601455
- Takiuchi H, Hirata I, Kawabe S, Egashira Y, Katsu K. Immunohistochemical expression of vascular endothelial growth factor can predict response to 5-fluorouracil and cisplatin in patients with gastric adenocarcinoma. Oncol Rep 2000; 7:841-6; PMID:10854555
- Boku N, Chin K, Hosokawa K, Ohtsu A, Tajiri H, Yoshida S, Yamao T, Kondo H, Shirao K, Shimada Y, et al. Biological markers as a predictor for response and prognosis of unresectable gastric cancer patients treated with 5-fluorouracil and cis-platinum. Clin Cancer Res 1998; 4:1469-74; PMID:9626464
- Brink M, de Goeij AF, Weijenberg MP, Roemen GM, Lentjes MH, Pachen MM, Smits KM, de Bruïne AP, Goldbohm RA, van den Brandt PA. K-ras oncogene mutations in sporadic colorectal cancer in The Netherlands Cohort Study. Carcinogenesis 2003; 24:703-10; PMID:12727799; http://dx.doi.org/10.1093/carcin/bgg009
- Chiang JM. Role of K-ras mutations in colorectal carcinoma. Cancer Lett 1998; 126:179-85; PMID:9585064; http://dx.doi.org/10.1016/S0304-3835(98)00008-1
- Hutchins G, Southward K, Handley K, Magill L, Beaumont C, Stahlschmidt J, Richman S, Chambers P, Seymour M, Kerr D, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol 2011; 29:1261-70; PMID:21383284; http://dx.doi.org/10.1200/JCO.2010.30.1366
- Fukahori M. Analysis of gene mutations in KRAS, NRAS, BRAF, and PIK3CA in patients who received systemic chemotherapy with metastatic gastric cancer. ASCO Meet Abstr 2013; 31:27
- Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol 2012; 9:16-32; PMID:22124364; http://dx.doi.org/10.1038/nrclinonc.2011.177
- Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012; 13:25-32; PMID:22153890; http://dx.doi.org/10.1016/S1470-2045(11)70336-9
- Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, Gómez H, Dinh P, Fauria K, Van Dooren V, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 2012; 379:633-40; PMID:22257673; http://dx.doi.org/10.1016/S0140-6736(11)61847-3
- Pierga JY, Petit T, Delozier T, Ferrero JM, Campone M, Gligorov J, Lerebours F, Roché H, Bachelot T, Charafe-Jauffret E, et al. Neoadjuvant bevacizumab, trastuzumab, and chemotherapy for primary inflammatory HER2-positive breast cancer (BEVERLY-2): an open-label, single-arm phase 2 study. Lancet Oncol 2012; 13:375-84; PMID:22377126; http://dx.doi.org/10.1016/S1470-2045(12)70049-9
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007; 357:2666-76; PMID:18160686; http://dx.doi.org/10.1056/NEJMoa072113
- Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011; 377:2103-14; PMID:21641636; http://dx.doi.org/10.1016/S0140-6736(11)60613-2
- Reguart N, Cardona AF, Rosell R. Role of erlotinib in first-line and maintenance treatment of advanced non-small-cell lung cancer. Cancer Manag Res 2010; 2:143-56; PMID:21188105; http://dx.doi.org/10.2147/CMAR.S5398
- Stinchcombe TE, Socinski MA. Maintenance therapy in advanced non-small cell lung cancer: current status and future implications. J Thorac Oncol 2011; 6:174-82; PMID:21127437; http://dx.doi.org/10.1097/JTO.0b013e318200f9c5
- Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczesna A, Juhasz E, Esteban E, Molinier O, Brugger W, Melezínek I, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010; 11:521-9; PMID:20493771; http://dx.doi.org/10.1016/S1470-2045(10)70112-1
- Mok TS, Ramalingam SS. Maintenance therapy in nonsmall-cell lung cancer: a new treatment paradigm. Cancer 2009; 115:5143-54; PMID:19658185; http://dx.doi.org/10.1002/cncr.24563
- Correale P, Cusi MG, Tagliaferri P. Immunomodulatory properties of anticancer monoclonal antibodies: is the 'magic bullet' still a reliable paradigm? Immunotherapy 2011; 3:1-4; PMID:21174549; http://dx.doi.org/10.2217/imt.10.92
- Hahm KB, Kim WH, Lee SI, Kang JK, Park IS. Comparison of immunomodulative effects of the histamine-2 receptor antagonists cimetidine, ranitidine, and famotidine on peripheral blood mononuclear cells in gastric cancer patients. Scand J Gastroenterol 1995; 30:265-71; PMID:7770717; http://dx.doi.org/10.3109/00365529509093275
- Nio Y, Shiraishi T, Tsubono M, Morimoto H, Tseng CC, Imai S, Tobe T. In vitro immunomodulating effect of protein-bound polysaccharide, PSK on peripheral blood, regional nodes, and spleen lymphocytes in patients with gastric cancer. Cancer Immunol Pmmunother 1991; 32:335-41; PMID:1901030; http://dx.doi.org/10.1007/BF01741328
- Botta C, Barbieri V, Ciliberto D, Rossi A, Rocco D, Addeo R, Staropoli N, Pastina P, Marvaso G, Martellucci I, et al. Systemic inflammatory status at baseline predicts bevacizumab benefit in advanced non-small cell lung cancer patients. Cancer Biol Ther 2013; 14:469-75; PMID:23760488; http://dx.doi.org/10.4161/cbt.24425
- Botta C, Bestoso E, Apollinari S, Cusi MG, Pastina P, Abbruzzese A, Sperlongano P, Misso G, Caraglia M, Tassone P, et al. Immune-modulating effects of the newest cetuximab-based chemoimmunotherapy regimen in advanced colorectal cancer patients. J Immunother 2012; 35:440-7; PMID:22576349; http://dx.doi.org/10.1097/CJI.0b013e31825943aa
- Correale P, Botta C, Basile A, Pagliuchi M, Licchetta A, Martellucci I, Bestoso E, Apollinari S, Addeo R, Misso G, et al. Phase II trial of bevacizumab and dose/dense chemotherapy with cisplatin and metronomic daily oral etoposide in advanced non-small-cell-lung cancer patients. Cancer Biol Ther 2011; 12:112-8; PMID:21525780; http://dx.doi.org/10.4161/cbt.12.2.15722
- Correale P, Rotundo MS, Botta C, Del Vecchio MT, Ginanneschi C, Licchetta A, Conca R, Apollinari S, De Luca F, Tassone P, et al. Tumor infiltration by T lymphocytes expressing chemokine receptor 7 (CCR7) is predictive of favorable outcome in patients with advanced colorectal carcinoma. Clin Cancer Res 2012; 18:850-7; PMID:22142823; http://dx.doi.org/10.1158/1078-0432.CCR-10-3186
- Mortenson ED, Fu YX. Adaptive immune responses and HER2/neu positive breast cancer. Curr Pathobiol Rep 2013; 1:37-42; PMID:23420038; http://dx.doi.org/10.1007/s40139-012-0001-8
- Manning EA, Ullman JG, Leatherman JM, Asquith JM, Hansen TR, Armstrong TD, Hicklin DJ, Jaffee EM, Emens LA. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res 2007; 13:3951-9; PMID:17606729; http://dx.doi.org/10.1158/1078-0432.CCR-07-0374
- Adamcic U, Skowronski K, Peters C, Morrison J, Coomber BL. The effect of bevacizumab on human malignant melanoma cells with functional VEGF/VEGFR2 autocrine and intracrine signaling loops. Neoplasia 2012; 14:612-23; PMID:22904678; http://dx.doi.org/10.1593/neo.11948
- Ren S, Fengyu , Zuo S, Zhao M, Wang X, Wang X, Chen Y, Wu Z, Ren Z. Inhibition of tumor angiogenesis in lung cancer by T4 phage surface displaying mVEGFR2 vaccine. Vaccine 2011; 29:5802-11; PMID:21482223; http://dx.doi.org/10.1016/j.vaccine.2011.03.051
- Block MS, Nevala WK, Leontovich AA, Markovic SN. Differential response of human and mouse dendritic cells to VEGF determines interspecies discrepancies in tumor-mediated TH1/TH2 polarity shift. Clin Cancer Res 2011; 17:1776-83; PMID:21349994; http://dx.doi.org/10.1158/1078-0432.CCR-10-2836
- Yan J, Jia R, Song H, Liu Y, Zhang L, Zhang W, Wang Y, Zhu Y, Yu J. A promising new approach of VEGFR2-based DNA vaccine for tumor immunotherapy. Immunol Lett 2009; 126:60-6; PMID:19665480; http://dx.doi.org/10.1016/j.imlet.2009.07.013
- Seavey MM, Paterson Y. Anti-Angiogenesis immunotherapy induces epitope spreading to Her-2/neu resulting in breast tumor immunoediting. Breast Cancer (Dove Med Press) 2009; 1:19-30; PMID:24367160
- Wada S, Tsunoda T, Baba T, Primus FJ, Kuwano H, Shibuya M, Tahara H. Rationale for antiangiogenic cancer therapy with vaccination using epitope peptides derived from human vascular endothelial growth factor receptor 2. Cancer Res 2005; 65:4939-46; PMID:15930316; http://dx.doi.org/10.1158/0008-5472.CAN-04-3759
- Wang W, Liu H, Song Z, Wang Y, Wang J, Li Y, Li Y, Wang M, Chen J, Zhou Q. [Identification of the offspring of Vegfr2-luc transgenic mouse]. Zhongguo Fei Ai Za Zhi 2011; 14:391-5; PMID:21569642
- Suzuki H, Onishi H, Wada J, Yamasaki A, Tanaka H, Nakano K, Morisaki T, Katano M. VEGFR2 is selectively expressed by FOXP3high CD4+ Treg. Eur J Immunol 2010; 40:197-203; PMID:19902430; http://dx.doi.org/10.1002/eji.200939887
- Ozdemir F, Akdogan R, Aydin F, Reis A, Kavgaci H, Gul S, Akdogan E. The effects of VEGF and VEGFR-2 on survival in patients with gastric cancer. J Exp Clin Cancer Res 2006; 25:83-8; PMID:16761623
- Wang X, Chen X, Fang J, Yang C. Overexpression of both VEGF-A and VEGF-C in gastric cancer correlates with prognosis, and silencing of both is effective to inhibit cancer growth. Int J Clin Exp Pathol 2013; 6:586-97; PMID:23573305
- Feng Y, Hu J, Ma J, Feng K, Zhang X, Yang S, Wang W, Zhang J, Zhang Y. RNAi-mediated silencing of VEGF-C inhibits non-small cell lung cancer progression by simultaneously down-regulating the CXCR4, CCR7, VEGFR-2 and VEGFR-3-dependent axes-induced ERK, p38 and AKT signalling pathways. Eur J Cancer 2011; 47:2353-63; PMID:21680174; http://dx.doi.org/10.1016/j.ejca.2011.05.006
- Lieu CH, Tran H, Jiang ZQ, Mao M, Overman MJ, Lin E, Eng C, Morris J, Ellis L, Heymach JV, et al. The association of alternate VEGF ligands with resistance to anti-VEGF therapy in metastatic colorectal cancer. PloS One 2013; 8:e77117; PMID:24143206; http://dx.doi.org/10.1371/journal.pone.0077117
- Manfredi GI, Dicitore A, Gaudenzi G, Caraglia M, Persani L, Vitale G. PI3K/Akt/mTOR signaling in medullary thyroid cancer: a promising molecular target for cancer therapy. Endocrine 2015; 48:363-70; PMID:25115638; http://dx.doi.org/10.1007/s12020-014-0380-1
- Faggiano A, Ramundo V, Dicitore A, Castiglioni S, Borghi MO, Severino R, Ferolla P, Crin∫ L, Abbruzzese A, Sperlongano P, et al. Everolimus is an active agent in medullary thyroid cancer: a clinical and in vitro study. J Cell Mol Med 2012; 16:1563-72; PMID:21883896; http://dx.doi.org/10.1111/j.1582-4934.2011.01438.x
- Zhong XB, Leeder JS. Epigenetic regulation of ADME-related genes: focus on drug metabolism and transport. Drug Metab Dispos 2013; 41:1721-4; PMID:23935066; http://dx.doi.org/10.1124/dmd.113.053942
- Stewart LA, Parmar MK, Tierney JF. Meta-analyses and large randomized, controlled trials. N Engl J Med 1998; 338:61; author reply −2; PMID:9424569; http://dx.doi.org/10.1056/NEJM199802053380603
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998; 17:2815-34; PMID:9921604; http://dx.doi.org/10.1002/(SICI)1097-0258(19981230)17:24%3c2815::AID-SIM110%3e3.0.CO;2-8
- Parmar MK, Stewart LA, Altman DG. Meta-analyses of randomised trials: when the whole is more than just the sum of the parts. Br J Cancer 1996; 74:496-501; PMID:8761361; http://dx.doi.org/10.1038/bjc.1996.392
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700; PMID:19622552; http://dx.doi.org/10.1136/bmj.b2700
- Moher D, Altman DG, Liberati A, Tetzlaff J. PRISMA statement. Epidemiology 2011; 22:128; author reply; PMID:21150360; http://dx.doi.org/10.1097/EDE.0b013e3181fe7825
- Armijo-Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Assessment of study quality for systematic reviews: a comparison of the cochrane collaboration risk of bias tool and the effective public health practice project quality assessment tool: methodological research. J Eval Clin Pract 2012; 18:12-8; PMID:20698919; http://dx.doi.org/10.1111/j.1365-2753.2010.01516.x
- Detsky AS, Naylor CD, O'Rourke K, McGeer AJ, L'Abbe KA. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol 1992; 45:255-65; PMID:1569422; http://dx.doi.org/10.1016/0895-4356(92)90085-2
- Crowther M, Lim W, Crowther MA. Systematic review and meta-analysis methodology. Blood 2010; 116:3140-6; PMID:20656933; http://dx.doi.org/10.1182/blood-2010-05-280883
- Wang D, Mou ZY, Zhai JX, Zong HX, Zhao XD. [Application of Stata software to test heterogeneity in meta-analysis method]. Zhonghua Liu Xing Bing Xue Za Zhi 2008; 29:726-9; PMID:19031771
- DerSimonian R, Levine RJ. Resolving discrepancies between a meta-analysis and a subsequent large controlled trial. JAMA 1999; 282:664-70; PMID:10517720; http://dx.doi.org/10.1001/jama.282.7.664
- DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007; 28:105-14; PMID:16807131; http://dx.doi.org/10.1016/j.cct.2006.04.004
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343:d4002; PMID:21784880; http://dx.doi.org/10.1136/bmj.d4002
- Boston RC, Sumner AE. STATA: a statistical analysis system for examining biomedical data. Adv Exp Med Biol 2003; 537:353-69; PMID:14995047