Abstract
Radiation therapy (RT) the front-line treatment after surgery for early breast cancer patients is associated with acute skin toxicities in at least 40% of treated patients. Monocyte-derived macrophages are polarized into functionally distinct (M1 or M2) activated phenotypes at injury sites by specific systemic cytokines known to play a key role in the transition between damage and repair in irradiated tissues. The role of M1 and M2 macrophages in RT-induced acute skin toxicities remains to be defined. We investigated the potential value of M1 and M2 macrophages as predictive factors of RT-induced skin toxicities in early breast cancer patients treated with adjuvant RT after lumpectomy. Blood samples collected from patients enrolled in a prospective clinical study (n = 49) were analyzed at baseline and after the first delivered 2Gy RT dose. We designed an ex vivo culture system to differentiate patient blood monocytes into macrophages and treated them with M1 or M2-inducing cytokines before quantitative analysis of their “M1/M2” activation markers, iNOS, Arg1, and TGFß1. Statistical analysis was performed to correlate experimental data to clinical assessment of acute skin toxicity using Common Toxicity Criteria (CTC) grade for objective evaluation of skin reactions. Increased ARG1 mRNA significantly correlated with higher grades of erythema, moist desquamation, and CTC grade. Multivariate analysis revealed that increased ARG1 expression in macrophages after a single RT dose was an independent prognostic factor of erythema (p = 0 .032), moist desquamation (p = 0 .027), and CTC grade (p = 0 .056). Interestingly, multivariate analysis of ARG1 mRNA expression in macrophages stimulated with IL-4 also revealed independent prognostic value for predicting acute RT-induced toxicity factors, erythema (p = 0 .069), moist desquamation (p = 0 .037), and CTC grade (p = 0 .046). To conclude, our findings underline for the first time the biological significance of increased ARG1 mRNA levels as an early independent predictive biomarker of RT-induced acute skin toxicities.
Abbreviations
| 3D-CRT | = | 3D-conformal RT |
| AI | = | aromatase inhibitor |
| ARG1 | = | arginin1 |
| BC | = | breast cancer |
| CI | = | confidence interval |
| CTC | = | Common Toxicity Criteria |
| CTC-AE | = | CTC-adverse events |
| DCIS | = | ductal carcinoma in situ |
| ER | = | estrogen receptor |
| HER-2 | = | Human epidermal receptor-2 |
| HR | = | hormone receptor |
| HT | = | helical tomotherapy |
| iNOS | = | induced nitric oxide synthase |
| LN | = | lymph node |
| LVI | = | lymphovascular invasion |
| M-CSF | = | macrophage colony stimulating factor |
| OR | = | odds ratio |
| PBMCs | = | peripheral blood mononuclear cells |
| PR | = | progesterone receptor |
| RCT | = | randomized controlled trial |
| RT | = | radiation therapy |
| SNP | = | single nucleotide polymorphism |
| TGFb1 | = | transforming growth factor-beta1 |
Introduction
Radiation therapy (RT) is a standard treatment for early and locally advanced breast cancer (BC) as it has been shown to decrease recurrence and improve overall survival.Citation1 The major side effect of RT for breast is skin toxicities, i.e. RT-induced dermatitis.Citation2 Acute skin toxicities arise in greater than 40% of all patients receiving RT; the most common of these include erythema and moist desquamation.Citation2 Severe acute skin toxicities, as scored by Common Toxicity Criteria (CTC) grade (according to the National Cancer Institute CTC v3.0), can result in termination of RT, impairing the adjuvant management of BC.
Intriguingly, the onset of side effects induced by RT varies in cancer patients even when taking patient-related factors (breast size, age, smoking, lymph drainage) and treatment-related factors (total dose, fraction size, type of radiation, volume treated, chemotherapy) into account.Citation3-6 The discrepancy in patient radiosensitivity is attributed then to patient genetic backgrounds and/or predispositions to adverse reactions. Previously, others group have attempted to correlate radiosensitivity in patient populations with in vitro assessments of patient initial DNA damage and repair efficacies, single nucleotide polymorphism (SNPs) in DNA repair genes, lymphocyte apoptosis efficiencies, fibroblast clonogenic survival, and chromosomal aberrations in response to RT.Citation3-6 Even with large patient cohorts and multiple studies, these reports have failed to validate any potential predictive biomarkers for RT-induced skin toxicities. RT inadvertently affects immune cells and triggers a pro-inflammatory response leading to various immune responses with a different extent for the wound healing response, which may account for the onset of acute skin toxicities following radiation tissue damage. We propose that understanding the cellular immune mechanism underlying RT-induced acute skin toxicities would provide a direct evaluation of a patient's response to RT.
Macrophages are dynamic, long-lived cells with great plasticity and are integral regulators of inflammatory and wound-healing immune responses. Differentiated mostly from bone marrow-derived monocytes, macrophages have been reported to adopt at least 2 polarized states, the most characterized of which, M1 and M2.Citation7,8 M1 macrophages are associated with releasing inflammatory cytokines, recruiting immune cells, and up-regulating inducible nitric oxide synthase (iNOS, encoded by NOS2 gene) to produce nitric oxide.Citation7,8 M2 macrophages are associated with matrix remodelling in wound healing and up-regulate the ARG1 gene and transforming growth factor-beta1 (TGFß1) secretion for stimulation of collagen synthesis.Citation7,8 Thus, macrophages play a key role in the resolution of cell and tissue damage. In recent years, it has become evident that more macrophage activation states exist, and that perhaps M1 and M2 are 2 distinct stages of a spectrum of macrophage profiles.Citation9,10 We hypothesize that characterizing M1 and M2 macrophage polarizations (through expression of NOS2, ARG1 mRNA and secreted TGFß1 levels) using an ex vivo culture system before and after delivery of the first RT dose to BC patients may elucidate the contribution of their macrophages in RT-induced acute skin toxicities. In our ex vivo culture system, we will be stimulating monocyte-induced macrophages with M1 and M2 phenotype-inducing cytokines to detect the response of both macrophage phenotypes to radiation. The purpose of this study is to investigate the potential value of M1 and M2 macrophages as predictive factors of RT-induced skin toxicities in early breast cancer patients treated with adjuvant RT after lumpectomy. We report here the biological significance of elevated ARG1 mRNA levels in patient monocyte-derived macrophages as an early independent predictive marker of radiation-induced acute skin toxicities.
Results
Patient characteristics and treatment modalities
The characteristics of the 49 patients including treatment modalities are presented in . There was no significant difference in patient characteristics with respect to treatment arm: standard RT or tomotherapy. All patients enrolled in our study were early-staged breast cancer patients treated with adjuvant RT after lumpectomy. Median age was 59 y (range, 43 to 76 years) and median breast volume was 894 mm3 (range, 458 to 2313 mm3). Sixteen percent of patients presented with ductal carcinoma in situ (DCIS), 73% of patients had Stage T1 tumors (<2 cm), and 10% of patients had Stage T2 tumors (2–5 cm). Twenty percent of patients had a low-grade tumor (Grade 1) and 80% exhibited high- grade tumor (grade 2 and 3). Median tumor margins were 8 mm (range, 5 to 30 mm). Lymphovascular invasion was reported in 18% of patients and 12% showed lymph node involvement. Immunohistochemistry staining showed 92% of patients were ER+/PR+ and 4% with HER-2-overexpressing tumors. Thirty three percent of patients were treated with adjuvant chemotherapy whereas 69% were treated with Tamoxifen, 4% with aromatase inhibitors, and 27% received no adjuvant hormone therapy.
Table 1. Clinical and treatment characteristics by treatment arm (N = 49)
Circulating monocytes numbers stable after first radiation treatment
We hypothesized that expression of genes involved in differential functional polarization of macrophages may pre-determine patient outcome with respect to RT-induced skin toxicities. To investigate whether RT affects the number of circulating monocytes, which in turn may affect macrophage numbers and the onset of skin toxicities, we surveyed the number of circulating peripheral blood monocytes for each blood sample using the standard automated complete blood counts. T1 and T2 elsewhere in this manuscript refer to the time points of blood sample collection before and after radiation, respectively. We found that the monocyte cell counts were not significantly different between T1 and T2 for our patient population (data not shown). Furthermore, for each individual patient, the difference between monocyte numbers at T1 and T2 did not differ by more than 0.1 × 109/L and in 64% of patients there was no detectable difference in circulating monocyte numbers. This suggests that the first RT dose (2Gy) did not significantly alter the number of circulating monocytes, and thus the potential number of macrophages derived from monocytes was not affected by the first dose of RT treatment.
Patient monocyte-derived macrophages distribution of NOS2, ARG1, and TGF-β expression levels, and response to M1 and M2 polarization
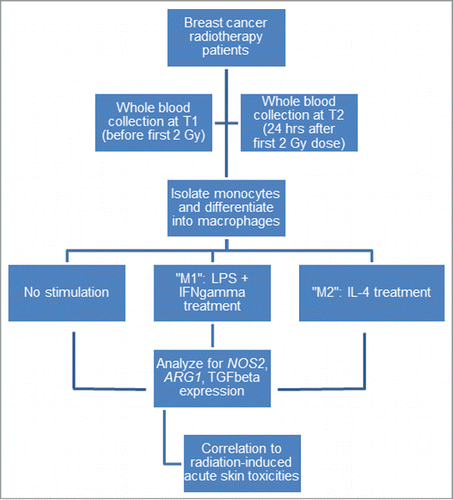
To understand the contribution of macrophages to RT-induced skin toxicities, we optimized an in vitro assay to characterize patient macrophage polarization (). We cultured isolated monocytes from patients before their treatment with RT (T1, n = 49) and differentiated them into macrophages with macrophage colony stimulating factor (M-CSF) using standard protocol. We further activated the macrophages with M1-polarizing cytokines LPS and IFN-γ or M2-polarizing cytokine IL-4 as previously reported. We subsequently analyzed the gene expression of the polarized macrophages. We showed in this patient population at steady state with no cytokine treatment (M0) a very high patient-to-patient variability in mRNA levels of NOS2 (M1 phenotype) and ARG1 (M2 phenotype), and secreted factor TGF-ß1 (M2 phenotype) (). The great variability remained the same when we had stimulated the cells with M1 cytokines or M2 cytokines (). Moreover, contrary to reports using monocytic cell lines showing that their derived macrophages up-regulate NOS2 or ARG1 and TGFß1 following M1 or M2 cytokine exposure respectively, we demonstrate that macrophage response in patients is also widely distributed (). We found that 65% of patients up-regulated NOS2 expression when induced with M1 cytokines, and 51% and 59% of patients upregulated ARG1 expression and TGFß1 secretion respectively when induced with M2 cytokines (). These findings highlight the constitutive heterogeneity in patient genetic backgrounds in macrophage phenotypes in addition to environmental cytokine cues.
Patient macrophages exhibit varied response to treatment with radiation
Next, we probed the effects of RT (2Gy, T2) on macrophage NOS2 and ARG1 gene expression and TGFβ1 secretion compared with their respective baseline levels before treatment (T1). We discovered that patient macrophages exhibit varied response to RT (). With no cytokine stimulation, 55% of patients showed an increase in NOS2 and 55% of patient showed an increase in ARG1 gene expression after the first 2 Gy RT dose (). Interestingly, 74% of patients significantly increased TGFß1 secretion in response to RT alone (p = 0.001) (). Moreover, 63% of patients exhibited increase in NOS2 mRNA levels with M1 cytokine stimulation and RT (), comparable to 65% with M1 cytokine stimulation without RT (). Sixty-one percent of patients exhibited increase in ARG1 gene expression with M2 cytokine stimulation and RT (), which is slightly higher compared to the 51% of patients with M2 cytokine stimulation without RT (). And, 69% of patients increased TGFß1 secretion in response to M2 cytokines with RT (p = 0.007) (), also slightly higher compared to the 59% of patients who showed an increase with M2 cytokine exposure without RT (). Taken together, RT treatment (2 Gy) appears to have increased patient TGFβ1 secretion in response to M2 cytokine stimulation.
Table 2. Univariate logistic regression for toxicity with clinical factors (N= 49)Footnotea
Increased ARG1 mRNA levels detected in patient macrophages after radiation therapy
Univariate logistic regression for skin toxicity outcomes was analyzed with clinical factors: RT treatment (standard RT versus tomotherapy), breast volume, age at diagnosis, tumor size, grade, margins, lymphovascular invasion (LVI), lymph node status (LN), hormone receptor status, adjuvant chemotherapy, and hormone therapy. These factors were not significantly associated with occurrence of moist desquamation (). However, we showed significant association between breast volume and CTC grade (OR:4.60, 95%CI = 1.10–19.22, P = 0.036) as well as RT treatment type and CTC Grade (OR: 0.25, 95%CI = 0.07–0.93, p = 0.038) ().
Univariate analysis showed that patients macrophages derived from patient monocytes collected 24 hours after the first 2 Gy dose exhibited higher M2-associated ARG1 gene transcript expression and correlated with higher grades of erythema (P = 0.032, data not shown), moist desquamation (P = 0.060), and CTC grade (P = 0.048) (). Furthermore, despite stimulation with IL-4, an M2-inducing cytokine in addition to 2 Gy dose, monocyte-derived macrophages still showed ARG1 correlated to higher grades of erythema (P = 0.069, data not shown), moist desquamation (P = 0.037), and CTC grades (P = 0.024) (). In congruence with the M2-like phenotype, univariate logistic regression analysis also showed a correlation between TGFß1 levels at T2 and erythema outcomes (OR = 0.21, 95%CI = 0.05–0.89, P = 0.034) (data not shown). In contrast, higher NOS2 expression detected after RT and the addition of M1 cytokines was also correlated to moist desquamation (P = 0.026) (). Those significant clinical and biological factors (RT treatment, hormone receptors, and breast volume) were included in the subsequent multivariate analysis (). Multivariate analysis also showed that high ARG1 expression in patient monocyte-derived macrophage after a single radiation dose with M2 cytokine induction was an independent prognostic factor of moist desquamation (OR = 6.25, 95%CI = 1.12–34.96, P = 0.037) () and overall CTC grade (OR: 14.88, 95%CI = 1.70–130.49, P = 0.019) (). We also found that RT treatment (OR = 0.015, 95%CI = 0.02–0.66, P = 0.015) and breast volume (OR = 9.63, 95%CI = 1.38–67.26, P = 0.022) are independent prognostic factors significantly associated with CTC grade (). Additionally, we report a significant correlation between increased TGFß1 expression from our patient-derived macrophages after RT and decreased CTC grade using multivariate analysis (OR = 0.08, 95%CI = 0.01–0.65, P = 0.015) (). Interestingly, although not statistically independent predictors, high NOS2 and ARG1 expression with M1 or M2 cytokines after RT trended to be correlated to worse CTC grade outcome ().
Table 3. Univariate logistic regression for toxicity with cutpoint biological parameters (N = 49)Footnotea
Table 4. Multivariate logistical analysis with stepwise inclusion for moist desquamation with biological and clinical factors (N=49)Footnotea
Table 5. Multivariate logistical analyisis with stepwise inclusion for CTC grade with biological and clinical factors (N= 49)Footnotea
Discussion
We and other groups have previously used clinical parameters such as breast size to predict patient toxicity to RT Citation7,8,11 In this study, we have investigated whether a unique cell type, monocyte-derived macrophages, and corresponding biological factors may contribute to the onset of RT-induced skin toxicities. In contrast with previous studies typically using ex vivo irradiation in lymphocytes from hyper-radiosensitive and non-radiosensitive patients, we used blood samples from patients receiving RT treatment to whole breast as an integral component of their adjuvant treatment after breast conserving surgery. To our knowledge, our study is the first to optimize and establish a new protocol to detect macrophage mRNA expression levels of women diagnosed with BC prospectively enrolled within a clinical trial with a primary objective to evaluate radiation induced-skin toxicities. Our study showed that standard RT treatment, large breast volume, and patient monocyte-derived macrophage ARG1 gene expression levels 24 hours after the first RT treatment are independent prognostic factors associated with increased risk of acute skin toxicities. These findings are in agreement with the current knowledge as standard RT treatment exposes the same breast tissue to more radiation compared to tomotherapy treatment. Similarly, increased breast volume results in more irradiated tissue mass. Importantly, we showed that M1 and M2 macrophages are not the only 2 distinct macrophage phenotypes with up or down-regulated corresponding M1 or M2 markers as others have postulated and shown 2 possible profiles on a spectrum of macrophage states. Further, we demonstrate that patient monocyte-derived macrophage ARG1 gene expression levels 24 hours after a patient's first radiation dose are associated with higher grades of acute skin toxicity. This novel finding implicates for the first time macrophages as an early predictor for response to RT in the process of radiation-induced acute skin toxicities.
Previously, macrophages have been thought to adopt 2 main polarized states, M1 and M2, corresponding to the Th1 and Th2-associated phenotypes with control of infection and wound resolution. In recent years, these profiles have been expanded to include an increased number of putative polarization states, arbitrarily named M2a, M2b, and M2c, as well as states labeled M1 through M5.Citation9,10 While in vitro data involving human macrophage-like cell lines have classified specific markers that correspond to the specific macrophage polarization phenotypes, another study profiling the induced M1 and M2 macrophages in a large sampling of normal, healthy patients indicated that the same markers/genes are not necessarily always bound to the M1 and M2 profiles.Citation12 We currently show data from early BC patient samples, which also reflect similar findings, as not every patient monocyte-derived macrophage population stimulated with M1 or M2 inducing cytokines up or downregulated NOS2, ARG1, and TGFß1 levels respectively. The macrophage profiles are indeed a spectrum of polarization states as shown by the NOS2, ARG1, and TGFß1 expression levels. Alternatively, it has also been proposed that heterogeneity lies within the monocytes, whereby distinct subsets of circulating monocytes may differentially contribute inflammatory and wound-healing functions upon injury.Citation13
The correlation between IL4-induced ARG1 and acute skin reactions in response to RT is intriguing as NOS2 has been generally associated with M1 macrophages and the inflammatory response. Thus, it is possible that macrophages exhibiting high NOS2 and/or high ARG1 can produce a strong inflammation response. While not much is known about the mechanisms of Arginase-1 in inflammation, it is known to play a key role in the regulation of immune responses and tissue reconstruction.Citation7,8 Additional studies are warranted to assess changes in expression of matrix metalloproteinases, such as MMP-9 and MMP-2, as this would provide evidence that ARG1-expressing macrophages were involved in extracellular matrix degradation-related inflammation. Interestingly we also showed that M2 cytokines stimulation combined with single RT dose also increased the percentage of patients with increased ARG1 expression compared to those stimulated with M2 cytokines alone without RT.
In addition, our study showed that TGFß1 secretion (an M2 phenotype) from patient monocyte-derived macrophages after the first RT dose with no cytokine stimulation was significantly associated with decreased acute skin toxicity. As a strictly M2 phenotype with ARG1 and TGFβ1 expression would be anti-inflammatory, perhaps an ARG1-expressing phenotype that is pro-inflammatory is conferring RT-induced acute skin toxicities. As extracellular matrix degradation is linked to inflammation, an increase in M2-like, ARG1-expressing macrophages could produce inflammation through a matrix degradation mechanism in response to RT. Further, as TGFß1 can stimulate the differentiation of fibroblasts to myofibroblasts, which can lead to skin fibrosis, it would be interesting to inquire the long-term effects of RT in these patients with early high expression of ARG1 and lower TGFß1 after RT.
One possible extension of our ex vivo culture system and assays is to provide a novel tool to understanding the underlying mechanisms of the contribution of macrophages to acute RT-induced skin toxicities, allowing physicians to identify the patients at risk of developing severe skin toxicities early in the course of RT treatment. Thus, this subset of patients could be better clinically managed at the beginning of treatment before symptoms worsen with increased total RT dosage. Moreover, as macrophages are such a multi-functional part of the breast tumor microenvironment, their plasticity could contribute in various ways. “M2-like” macrophages are also known as tumor associated macrophage and promote tumor growth. Our assay will enable probing for NOS2/ARG1 and other genes that could be important for understanding other macrophage-related molecular mechanisms for RT-induced effects including cancer progression and recurrence.
In conclusion, we showed that standard RT treatment, large breast volume, and patient monocyte-derived macrophage ARG1 gene expression levels 24 hours after first RT treatment are independent prognostic factors associated with increased RT-induced acute skin toxicities. Our study reveals new insights into the differential role of macrophage activation and polarization in the normal tissue response to RT. We also have established a novel tool to identify the patients at risk of developing severe acute skin toxicities early in the course of RT treatment with potential development of adaptive treatment.
Patients, Materials, and Methods
Patient eligibility and blood samples
Women diagnosed with early-stage breast cancer with a confirmed histologic diagnosis of invasive carcinoma or ductal carcinomas in situ were enrolled in this study at a single cancer center between May 2008 and November 2010. Patients were enrolled in a randomized controlled trial (RCT) that compared delivery of adjuvant breast RT with 3D-conformal RT (3D-CRT) compared to helical tomotherapy (HT), with skin toxicity as the primary endpoint (ClinicalTrials.gov Identifier: NCT00563407). Eligibility criteria were tumor size <3 cm, or tumor stage T1 and stage T2, N0-N1 carcinoma of the breast treated with lumpectomy with clear margins and referred for adjuvant RT to the breast alone. Patients were excluded if they had bilateral breast cancer, a postoperative wound infection, a connective tissue disorder, or were pregnant. This study was approved by our institution review board and all patients provided written informed consent. Patients were prospectively assessed on a weekly basis up to 8 weeks after completion of RT for acute skin toxicity using the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0 (CTC-AE v3). All patients received 50 Gy in 25 fractions over 5 weeks. Tumor boost to the surgical cavity was not allowed. In this RCT, we collected blood samples from the first 49 patients enrolled in the study before their first RT dose (T1) and 24 hours after the first 2 Gy fraction delivered either with 3D-CRT or HT (T2). All blood samples were collected in heparin blood collection tubes (Becton Dickinson Canada, Mississauga, ON, CA) and processed within 2 to 3 hours in the same cancer center. This study was designed and the results reported according to the REMARK guidelines.Citation14
Monocyte/macrophage cell cultures
Peripheral blood mononuclear cells (PBMCs) were separated from patient peripheral blood samples by loading blood samples mixed 1:1 with PBS a top of density gradient centrifugation medium Ficoll-Paque PLUS (GE Healthcare Biosciences, Uppsala, Sweden) and centrifuging at 1800 rpm for 25 minutes. Blood samples were depleted of their plasma component by centrifugation at 1100 rpm for 15 minutes prior to submission to the Ficoll gradient. Isolated PBMCs were collected from the gradient and washed 3 times with 20 mL of PBS before they were counted and frozen in liquid nitrogen.
Patient monocytes were isolated from thawed PBMCs using the negative selection magnetic columns in the Monocyte Isolation Kit II (Miltenyi Biotec, Auburn, CA, USA) as described in the manufacturer's protocol. Purified monocytes were divided into 3 aliquots and cultured in 1 mL of RPMI 1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 20% Fetal Bovine Serum (FBS) (Invitrogen) and Penicillin Streptomycin (Invitrogen) for 2 d in 24-well tissue culture plates (Nalgene Nunc International, Rochester, NY, USA) coated with 100% FBS for at least 2 hours at 37°C.
To differentiate the monocytes into macrophages, 100 ng/mL of macrophage colony stimulating factor (M-CSF, Miltenyi Biotec) was added with 1 mL of fresh 20% FBS RPMI 1640 medium and incubated for 5 d This protocol is standard for generation of macrophages from whole blood derived monocytes.Citation15 Macrophage polarization was achieved by treating the cultured monocyte-derived macrophages with 20 ng/mL IFN-γ and 100 ng/mL of LPS (to induce M1 phenotype), 20 ng/mL IL-4 (to induce M2 phenotype), or no cytokines (subsequently referred to as M0), for 2 d.
RNA extraction, cDNA synthesis, quantitative-RT-PCR
Total RNA was extracted from the cultured macrophages directly on the tissue culture plates with the RNeasy Mini Kit (Qiagen Canada, Mississauga, ON, Canada) as described in the manufacturer's protocol. Patient cDNA was prepared with the QuantiTect Reverse Transcription Kit (Qiagen) according to the manufacturer's protocol. SYBR Green PCR Master Mix (Applied Biosystems, Life Technologies, Carlsbad, CA USA) was used to measure NOS2 and ARG1 transcript levels in quantitative real-time PCR assays. An annealing temperature and time of 60°C for 2 minutes was used for 45 cycles. Quantitative real-time primer sequences are as follows: human NOS2 (F): 5′-CAGCGGGATGACTTTCCAAG-3′, NOS2 (R): 5′-AGGCAAGATTTGGACCTGCA-3′; human ARG1 (F): 5′-GGCAAGGTGATGGAAGAAAC-3′, ARG1 (R): 5′-AGTCCGAAACAAGCCAAGGT-3′; human GAPDH (F): 5′-GTGAAGGTCGGTGTCAACGGATTT-3′, GAPDH (R): 5′-CACAGTCTTCTGAGTGGCAGTGAT-3′. Relative gene expression was calculated by the 2−ΔΔCT method as previously described.Citation16 Data were calculated as the fold change in expression of the target gene relative to housekeeping gene (GAPDH).
ELISA assays
Patient macrophage supernatants were collected after 7 d of incubation with or without cytokines, and immediately aliquoted and stored at −20°C. Secreted TGFβ1 in the cultured macrophage supernatants was quantified using the Human TGFβ1 Duoset ELISA Kit (R&D Systems, Minneapolis, MN USA) as described by the manufacturer's protocol. Values of secreted TGFβ1 from patient monocyte-derived macrophages were calculated by subtracting TGFβ1 measurements using the corresponding media and cytokines alone.
Statistical analyses
Descriptive data analysis was conducted with clinical and biological data. The primary endpoints were moist desquamation, erythema and CTC grade and the variables of interest were the biological parameters (NOS2, ARG1, and TGF-β expression, induced to adopt “M0,” M1, or M2 phenotypes at time points T1 (before RT) and T2 (24 hours after the delivery of first 2 Gy fraction). The cofactors included in the analysis were breast volume, age at diagnosis, tumor size, grade, lymphovascular invasion, lymph node status, hormone receptor status, adjuvant chemotherapy, hormone therapy, and type of RT treatment (standard RT or tomotherapy). The comparison of categorical patient characteristics and biological endpoints with treatment arm and the toxicities was done using chi-square tests for association and the Wilcoxon rank sum test was used for continuous variables. A comparison was considered significant at the 5% level of significance for 2-sided tests. A dichotomous cutpoint was obtained for each continuous biological parameter. A series of chi-square tests were performed on each parameter to determine the optimum cutpoint (low vs. high) identified as the one that maximize association with toxicity. The biological parameters are the primary prognostic factors of interest in the univariate and multivariate logistic regression models for toxicity. Univariate logistic regression analysis was performed on moist desquamation and CTC grade toxicity for each biological parameter. Stepwise multivariate logistic regression analysis performed adjusting for the cofactors in the significant univariate regressions. Statistical analysis was performed using the SAS software (SAS Institute Inc., Cary, NC).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Tables S1 and S2
Download MS Word (14.4 KB)Acknowledgments
We thank Elaine Kurtz-Hardowa, MLT and the Hematology/Flow Cytometry Group at the Cross Cancer Institute for their role in patient blood collection.
Funding
This work was financially supported by grants from the Alberta Cancer Foundation awarded to BA. KJ is a recipient of the Izaak Walton Killam Memorial Scholarship, CIHR Vanier Canada Graduate Scholarships, and the University of Alberta's President's Doctoral Prize of Distinction.
Suuplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366(9503):2087-2106; PMID:16360786; http://dx.doi.org/10.1016/S0140-6736(05)67887-7
- McQuestion M. Evidence-based skin care management in radiation therapy: Clinical update. Semin Oncol Nurs. 2011;27(2):e1-17; PMID:21514477; http://dx.doi.org/10.1016/j.soncn.2011.02.009
- Barber JB, Burrill W, Spreadborough AR, Levine E, Warren C, Kiltie AE, Roberts SA, Scott D. Relationship between in vitro chromosomal radiosensitivity of peripheral blood lymphocytes and the expression of normal tissue damage following radiotherapy for breast cancer. Radiother Oncol. 2000;55(2):179-186; PMID:10799730; http://dx.doi.org/10.1016/S0167-8140(99)00158-9
- Fernando IN, Ford HT, Powles TJ, Ashley S, Glees JP, Torr M, Grafton D, Harmer CL. Factors affecting acute skin toxicity in patients having breast irradiation after conservative surgery: A prospective study of treatment practice at the royal marsden hospital. Clin Oncol (R Coll Radiol). 1996;8(4):226-233; PMID:8871000
- Turesson I, Nyman J, Holmberg E, Oden A. Prognostic factors for acute and late skin reactions in radiotherapy patients. Int J Radiat Oncol Biol Phys. 1996;36(5):1065-1075; PMID:8985028; http://dx.doi.org/10.1016/S0360-3016(96)00426-9
- Twardella D, Popanda O, Helmbold I, et al. Personal characteristics, therapy modalities and individual DNA repair capacity as predictive factors of acute skin toxicity in an unselected cohort of breast cancer patients receiving radiotherapy. Radiother Oncol. 2003;69(2):145-153; PMID:14643951; http://dx.doi.org/10.1016/S0167-8140(03)00166-X
- Giorgio S. Macrophages: Plastic solutions to environmental heterogeneity. Inflamm Res. 2013;62(9):835-843; PMID:23872927; http://dx.doi.org/10.1007/s00011-013-0647-7
- Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, Li J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2013;26(2):192-197; PMID:24219909; http://dx.doi.org/10.1016/j.cellsig.2013.11.004
- Gleissner CA. Macrophage phenotype modulation by CXCL4 in atherosclerosis. Front Physiol. 2012; 3:1; PMID:22275902; http://dx.doi.org/10.3389/fphys.2012.00001
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677-686; PMID:15530839; http://dx.doi.org/10.1016/j.it.2004.09.015
- Capelle L, Warkentin H, Mackenzie M, Joseph K, Gabos Z, Pervez N, Tankel K, Chafe S, Amanie J, Ghosh S, et al. Skin-sparing helical tomotherapy vs 3D-conformal radiotherapy for adjuvant breast radiotherapy: In vivo skin dosimetry study. Int J Radiat Oncol Biol Phys. 2012;83(5):e583-90; PMID:22580119; http://dx.doi.org/10.1016/j.ijrobp.2012.01.086
- Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J Immunol. 2006;177(10):7303-7311; PMID:17082649; http://dx.doi.org/10.4049/jimmunol.177.10.7303
- Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204(12):3037-3047; PMID:18025128; http://dx.doi.org/10.1084/jem.20070885
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM; Statistics Subcommittee of NCI-EORTC Working Group on Cancer Diagnostics. REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat. 2006;100(2):229-235; PMID:16932852; http://dx.doi.org/10.1007/s10549-006-9242-8
- Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao YH, Chu CY, Tsai TF, Chiu HC, Dai YS, Inoue H, et al. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J Invest Dermatol. 2009;129(4):1016-1025; PMID:18843292; http://dx.doi.org/10.1038/jid.2008.310
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402-408; PMID:11846609; http://dx.doi.org/10.1006/meth.2001.1262