Abstract
We present the case of a 62-year-old-man with moderately differentiated adenocarcinoma of the rectum. This patient underwent neoadjuvant chemoradiation and surgical resection followed by adjuvant chemotherapy. After completing therapy, this patient had 2 instances of CEA elevation, both of which preceded the discovery of recurrent disease. While on treatment for these recurrences, CA 19-9 increased rapidly to 4,405. This CA 19-9 elevation persisted for approximately 4 months in the absence of clinical, radiographic or additional serologic evidence of progressive disease before returning to baseline. Shortly after this tumor marker normalized, a small area of locally recurrent disease was discovered. This case highlights the utility and pitfalls of colorectal cancer disease monitoring with CEA and CA 19-9. The differential diagnosis of CA 19-9 elevation is discussed in this report.
Introduction
Colorectal cancer (CRC) is among the most common malignancies in the developed world. Each year in the United States alone, more than 130,000 patients receive a new diagnosis of CRC.Citation1 This disease claims the lives of nearly 50,000 Americans annually, making it the third most common cause of cancer mortality in both men and women.Citation2
Despite these somber statistics, considerable progress has been made toward improving outcomes through early diagnosis, effective treatment, and close surveillance of CRC. One such advance utilizes antigens that are commonly found in the peripheral circulation of patients with active malignancy, but are typically undetectable or less plentiful in healthy patients. Termed tumor markers, these substances are either shed directly by malignant cells or synthesized by normal cells in response to the presence of cancer. When considered in tandem with imaging, tumor markers provide clinicians with a minimally invasive means to approximate disease burden and to evaluate treatment response.
The tumor markers that are most often employed in the surveillance of CRC are carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9). The glycoprotein CEA is ordinarily produced during fetal development, when it participates in the formation of intercellular adhesions and signal transduction.Citation3 These same functions are exploited in CRC to promote malignant cell survival and the formation of metastases.Citation4-6 In a clinical setting, CEA is best utilized as an indicator of active disease burden in patients with known CRC.Citation7 Patients with elevated CEA at the time of diagnosis typically see this level normalize following surgery with curative intent. Persistent elevation of CEA after surgery suggests the presence of residual disease and portends a poor prognosis.Citation8,9 Similarly, repeat elevation of CEA in a patient with an initially positive serologic response to treatment is highly suggestive of recurrent diseaseCitation9-12 and often warrants further investigation if verified to be significantly elevated and/or consistently rising.
Carbohydrate antigen 19-9 (CA 19-9) is the second tumor marker that is often monitored in CRC. Bearing close resemblance to the Lewis antigen which studs the surface of red blood cells,Citation13 this glycoprotein is a normal component of the glandular epithelium that lines the biliary tree, gastrointestinal tract, salivary glands, and other luminal structures.Citation14 Similar to CEA, this antigen is often present within the peripheral circulation of patients with CRC and may indicate the extent of active disease. Unlike its counterpart, however, the role of CA19-9 in CRC surveillance is incompletely defined. Prior studies have established that elevated pre-treatment CA 19-9 is most common in patients with advanced diseaseCitation15-17 and independently predicts impaired survival regardless of disease stage.Citation18 Despite this, research suggests that this tumor marker is of questionable value in the early detection of CRC recurrenceCitation19 and most clinical guidelines do not endorse routine monitoring of CA 19-9.Citation7,20,21 Nonetheless in clinical practice there are situations where CEA is not elevated while the CA 19-9 can be elevated as a marker of CRC disease activity.
The following patient we cared for further illustrates both the benefits and caveats inherent in utilizing CEA and CA 19-9 to monitor patients with known colorectal adenocarcinoma.
Clinical Case Report
A 62-year-old man was initially diagnosed with rectal cancer during a screening colonoscopy in 2008. The CEA level at the time of diagnosis was 2.2 ng/ml. The patient received neoadjuvant chemoradiation with capecitabine followed by low anterior resection of the primary tumor with clear margins. Surgical pathology revealed stage T3N0M0 moderately differentiated adenocarcinoma of the rectum with wild-type KRAS, BRAF, and NRAS.
Postoperatively, the patient received 12 cycles of FOLFOX (leucovorin, 5-FU, oxaliplatin) chemotherapy. Approximately 1½ years after completing therapy, the patient's CEA was noted to increase to 3 ng/ml. Less than 6 months later, a PET scan indicated a small area of FDG-avidity just lateral to the S2 vertebra. Further imaging revealed encasement of vasculature and the S2 nerve root by the presacral mass, rendering it effectively inoperable. The patient was treated with FOLFIRI plus bevacizumab for a total of 12 cycles with normalization of CEA and radiographic resolution of the presumed metastasis.
Shortly after completing therapy, the patient again experienced persistent CEA elevation to 113 ng/ml and transferred his care to our institution. PET-CT demonstrated progression of recurrent disease within the pelvis, but continued to show no evidence of extra-peritoneal metastasis. He was treated with stereotactic body radiotherapy (SBRT) followed by mFOLFIRI plus cetuximab combination chemotherapy. While on therapy, an alarming increase in CA 19-9 occurred without corresponding radiographic abnormalities or CEA elevation. This persisted for approximately 4 months before declining to baseline without specific intervention. PET/CT shortly after normalization of CA 19-9 uncovered a small focus of suspicious rectal thickening at the prior surgical site. Endoscopic biopsy was obtained and confirmed the presence of recurrent CRC.
This patient continued with at least cycle 13 of mFOLFIRI plus cetuximab at the time of writing this report with radiographically stable disease and good serologic response to treatment. He did not receive any additional locoregional therapy to the recurrent rectal tumor.
Discussion
This patient was diagnosed with colorectal adenocarcinoma during a colonoscopy at the age of 62. He received neoadjuvant chemoradiotherapy followed by surgical resection and adjuvant chemotherapy. After completing this treatment, the patient had 2 episodes of CEA elevation, both of which heralded the appearance of recurrent disease. During treatment for these recurrences, the patient also experienced a brief elevation of CA 19-9. The elevated CA 19-9 in this patient persisted in the absence of clinical, radiographic or additional serologic evidence of progressive disease for approximately 4 months before returning to baseline without specific intervention. Shortly thereafter, a small area of locally recurrent disease was discovered. A graphical representation of this patient's fluctuating tumor markers following the first discovery of recurrent disease, which occurred at an outside institution, is provided in .
Figure 1. Relationship between radiographic findings and tumor markers CEA and CA 19-9 in a patient with metastatic colorectal adenocarcinoma.Legend: Patterns of CA 19-9 and CEA elevation in a patient with recurrent metastatic rectal cancer. CA 19-9 (carbohydrate antigen 19-9) Units/ml on the Y-axis above as indicated, CEA (carcinoembryonic antigen) ng/ml on the Y-axis below as indicated, FOLFIRI (leucovorin, 5-fluorouracil, irinotecan), SBRT (stereotactic body radiotherapy).
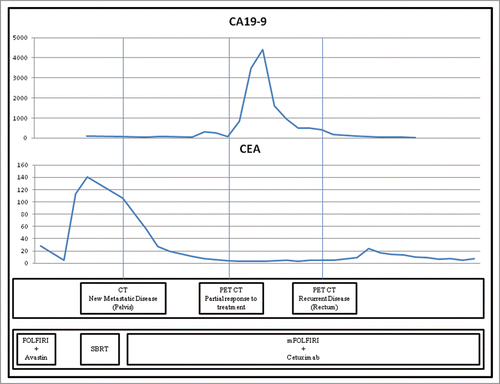
The relationship between CEA, CA 19-9, and disease activity described in this case is illustrative of both the value and caveats inherent in utilizing tumor markers to monitor patients with colorectal cancer. Twice during post-treatment surveillance, this patient demonstrated elevation of CEA and was subsequently found to have recurrent CRC – a pattern that is not uncommon in clinical practice. Among patients who experience normalization of CEA following treatment, a repeat elevation is both sensitive and specific for detecting recurrent disease.Citation19,22 This tumor marker is often the earliest indicator of recurrence, preceding clinical and radiographic evidence in 3-quarters of cases.Citation9-12 As a result, current ASCO guidelines recommend that CEA measurements be routinely obtained at 3 month intervals during postoperative surveillance and at 1–3 month intervals during systemic treatment for metastatic CRC.Citation23
The value of monitoring CA 19-9, on the other hand, is more controversial. While several studies indicate that its sensitivity and specificity rival that of CEA,Citation24,25 most suggest that CA 19-9 is vastly outperformed with respect to these measures.Citation19,26 Furthermore, CA 19-9 elevation trails radiographic detection of recurrent disease in 75% of instances, often by several months.Citation19,25,26 This tumor marker is also of questionable value in evaluating response to treatment. One study of nearly 1000 patients undergoing treatment for CRC found that decreasing CA 19-9 was concordant with radiographic response to treatment in only one-third of patients.Citation27 For these reasons, most clinical guidelines do not recommend the routine monitoring of CA 19-9 for CRC.Citation7,20,21
Despite its exclusion from guidelines, it is common practice at many institutions to trend CA 19-9 in addition to CEA. Consequentially, it is not uncommon for clinicians to face the conundrum of isolated CA 19-9 elevation in the absence of additional clinical, serologic, or radiographic evidence of recurrent disease. In this case, the picture was further clouded by the tumor marker returning to baseline without changing the patient's management. For these reasons, it is important to be cognizant of the sensitivity and specificity of this tumor marker, as well as the breadth of conditions that can cause its elevation.
Release of CA 19-9 into peripheral circulation occurs as a consequence of inflammation affecting glandular epithelium. This induces increased production of mucous glycoproteins such as CA 19-9, which are then extravasated into circulation.Citation28 As such, any condition that causes inflammation of the hepatobiliary system, gastrointestinal tract, or female reproductive system may increase the serum concentration of this tumor marker. Generally, nonmalignant and systemic inflammatory conditions such as hepatitis, gastroenteritis, diabetes, inflammatory myopathies, and idiopathic pulmonary fibrosis are associated with low-grade elevation of CA 19-9.Citation28,29 Scattered evidence also suggests that similar increases may be observed in chronic kidney disease and metabolic bone disease.Citation30 Greater increases, typically in excess of 1,000 U/mL, are generally observed only in cholestasisCitation31 and cancers of the aforementioned glandular epithelia.Citation32
In this patient with known active malignancy and CA 19-9 elevation to 4,405 U/mL, the initial consideration was for progressive disease. Initially, PET/CT was obtained and revealed only stable disease with diminished FDG-uptake, consistent with response to treatment. Three months later, repeat PET/CT revealed a region of intensely FDG-avid rectal thickening that was later biopsy-proven to be recurrent CRC.
While this highly PET-avid tumor is ostensibly a satisfying explanation for this patient's transient CA 19-9 elevation, carefully evaluating the sequence of events casts doubt upon this theory. First, this patient's elevated CA 19-9 normalized rapidly and without any change in management. Had this tumor marker been increased secondary to progressive malignancy, such a decisive and spontaneous correction would be unlikely in the absence of additional treatment. Furthermore, this focus of recurrent disease was not visualized during the ascent of CA 19-9 but instead appeared while the tumor marker trended downward. This again seems unlikely, as the purported “effect” would have been resolving before the “cause” materialized.
Further review of the imaging suggests another possible explanation. Shortly before this patient's CA 19-9 elevation, he had complained intermittently of fatigue and nausea. While these symptoms could reasonably be attributed to his underlying malignancy or chemotherapy, serial CT scans revealed the presence of hepatic steatosis and cholelithiasis. In light of these findings, it is quite possible that this patient suffered transient steatohepatitis or cholestasis secondary to passage of a gallstone. As described earlier, such conditions are known to increase CA 19-9 to heights otherwise observed only in malignancy and would intuitively appear more consistent with the brief, self-limited episode described in this case.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9-29; PMID:24399786; http://dx.doi.org/10.3322/caac.21208
- Jemal A, Simard EP, Dorell C, Noone AM, Markowitz LE, Kohler B, Eheman C, Saraiya M, Bandi P, Saslow D, et al. Annual report to the nation on the status of cancer, 1975–2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst 2013; 105:175-201; PMID:23297039; http://dx.doi.org/10.1093/jnci/djs491
- Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Sem Cancer Biol 1999; 9:67-81; PMID:10202129; http://dx.doi.org/10.1006/scbi.1998.0119
- Paschos KA, Canovas D, Bird NC. The role of cell adhesion molecules in the progression of colorectal cancer and the development of liver metastasis. Cell Signalling 2009; 21:665-74; PMID:19167485; http://dx.doi.org/10.1016/j.cellsig.2009.01.006
- Gangopadhyay A, Lazure D, Thomas P. Adhesion of colorectal carcinoma cells to the endothelium is mediated by cytokines from CEA stimulated Kupffer cells. Clin Exp Metast 1998; 16:703-12; PMID:10211983; http://dx.doi.org/10.1023/A:1006576627429
- Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell 1989; 57:327-34; PMID:2702691; http://dx.doi.org/10.1016/0092-8674(89)90970-7
- Network NCC. Rectal Cancer. NCCN Clin Practice Guidelines in Oncol (NCCN Guidelines ®), 2015
- Boey J, Cheung HC, Lai CK, Wong J. A prospective evaluation of serum carcinoembryonic antigen (CEA) levels in the management of colorectal carcinoma. World J Surg 1984; 8:279-86; PMID:6464483; http://dx.doi.org/10.1007/BF01655052
- Arnaud JP, Koehl C, Adloff M. Carcinoembryonic antigen (CEA) in diagnosis and prognosis of colorectal carcinoma. Dis Colon Rectum 1980; 23:141-4; PMID:7379666; http://dx.doi.org/10.1007/BF02587615
- Minton JP, Hoehn JL, Gerber DM, Horsley JS, Connolly DP, Salwan F, Fletcher WS, Cruz AB Jr, Gatchell FG, Oviedo M, et al. Results of a 400-patient carcinoembryonic antigen second-look colorectal cancer study. Cancer 1985; 55:1284-90; PMID:3971297; http://dx.doi.org/10.1002/1097-0142(19850315)55:6%3c1284::AID-CNCR2820550622%3e3.0.CO;2-B
- McCall JL, Black RB, Rich CA, Harvey JR, Baker RA, Watts JM, Toouli J. The value of serum carcinoembryonic antigen in predicting recurrent disease following curative resection of colorectal cancer. Dis Colon Rectum 1994; 37:875-81; PMID:8076486; http://dx.doi.org/10.1007/BF02052591
- Hine KR, Dykes PW. Serum CEA testing in the post-operative surveillance of colorectal carcinoma. Br J Cancer 1984; 49:689-93; PMID:6733018; http://dx.doi.org/10.1038/bjc.1984.109
- Tempero MA, Uchida E, Takasaki H, Burnett DA, Steplewski Z, Pour PM. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res 1987; 47:5501-3; PMID:3308077
- Malaguarnera G, Giordano M, Paladina I, Rando A, Uccello M, Basile F, Biondi A, Carnazzo S, Alessandria I, Mazzarino C. Markers of bile duct tumors. World J Gastrointest Oncol 2011; 3:49-59; PMID:21528090; http://dx.doi.org/10.4251/wjgo.v3.i4.49
- Ohuchi N, Takahashi K, Matoba N, Sato T, Taira Y, Sakai N, Masuda M, Mori S. Comparison of serum assays for TAG-72, CA19-9 and CEA in gastrointestinal carcinoma patients. Jpn J Clin Oncol 1989; 19:242-8; PMID:2810823
- Yamaguchi A, Kurosaka Y, Ishida T, Nishimura G, Kanno M, Kosaka T, Yonemura Y, Miyazaki I. Clinical significance of tumor marker NCC-ST 439 in large bowel cancers. Dis Colon Rectum 1991; 34:921-4; PMID:1914727; http://dx.doi.org/10.1007/BF02049709
- Iemura K, Moriya Y. A comparative analysis of the serum levels of NCC-ST-439, CEA and CA19-9 in patients with colorectal carcinoma. Eur J Surg Oncol 1993; 19:439-42; PMID:8405479
- Reiter W, Stieber P, Reuter C, Nagel D, Lau-Werner U, Lamerz R. Multivariate analysis of the prognostic value of CEA and CA 19-9 serum levels in colorectal cancer. Anticancer Res 2000; 20:5195-8; PMID:11326694
- Filella X, Molina R, Pique JM, Garcia-Valdecasas JC, Grau JJ, Novell F, Astudillo E, de Lacy A, Daniels M, Ballesta AM. Use of CA 19-9 in the early detection of recurrences in colorectal cancer: comparison with CEA. Tumour Biol 1994; 15:1-6; PMID:8146525; http://dx.doi.org/10.1159/000217867
- Bast RC Jr., Ravdin P, Hayes DF, Bates S, Fritsche H Jr., Jessup JM, Kemeny N, Locker GY, Mennel RG, Somerfield MR, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001; 19:1865-78; PMID:11251019
- Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer. Adopted on May 17, 1996 by the American Society of Clinical Oncology. J Clin Oncol 1996; 14:2843-77; PMID:8874347
- Araujo RL, Gonen M, Allen P, DeMatteo R, Kingham P, Jarnagin W, D'Angelica M, Fong Y. Positive postoperative CEA is a strong predictor of recurrence for patients after resection for colorectal liver metastases. Ann Surg Oncol 2015; Epub ahead of print; PMID:25582745
- Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC Jr, ASCO. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol 2006; 24:5313-27; PMID:17060676; http://dx.doi.org/10.1200/JCO.2006.08.2644
- Yakabe T, Nakafusa Y, Sumi K, Miyoshi A, Kitajima Y, Sato S, Noshiro H, Miyazaki K. Clinical significance of CEA and CA19-9 in postoperative follow-up of colorectal cancer. Ann Surg Oncol 2010; 17:2349-56; PMID:20217258; http://dx.doi.org/10.1245/s10434-010-1004-5
- Kawamura YJ, Tokumitsu A, Mizokami K, Sasaki J, Tsujinaka S, Konishi F. First alert for recurrence during follow-up after potentially curative resection for colorectal carcinoma: CA 19-9 should be included in surveillance programs. Clin Colorectal Cancer 2010; 9:48-51; PMID:20100688; http://dx.doi.org/10.3816/CCC.2010.n.006
- Caglar M, Yener C, Karabulut E. Value of CT, FDG PET-CT and serum tumor markers in staging recurrent colorectal cancer. Int J Comput Assist Radiol Surg 2015; Jul;10(7):993-1002. PMID:25213271; http://dx.doi.org/10.1007/s11548-014-1115-8
- Tampellini M, Ottone A, Alabiso I, Baratelli C, Forti L, Berruti A, Aroasio E, Scagliotti GV. The prognostic role of baseline CEA and CA 19-9 values and their time-dependent variations in advanced colorectal cancer patients submitted to first-line therapy. Tumour Biol 2014; 36:1519-27; PMID:25374062; http://dx.doi.org/10.1007/s13277-014-2693-3
- Hachiya T, Koyama S, Kubo K, Sekiguchi M, Honda T. Elevated serum CA19-9 level and regional lymphadenopathy in a young man with allergic bronchopulmonary aspergillosis. Intern Med 1998; 37:91-3; PMID:9510409; http://dx.doi.org/10.2169/internalmedicine.37.91
- Yamamoto S, Kobayashi S, Tanaka M, Akimoto T, Takasaki Y. [Serum CA 19-9 levels in rheumatic diseases with interstitial pneumonia]. Nihon Rinsho Meneki Gakkai Kaishi 1996; 19:128-35; PMID:8705689; http://dx.doi.org/10.2177/jsci.19.128
- McLaughlin R, O'Hanlon D, Kerin M, Kenny P, Grimes H, Given HF. Are elevated levels of the tumour marker CA19-9 of any clinical significance?–an evaluation. Irish journal of medical science 1999; 168:124-6; PMID:10422394; http://dx.doi.org/10.1007/BF02946481
- Halme L, Karkkainen P, Isoniemi H, Makisalo H, von Bogulawski K, Hockerstedt K. Carbohydrate 19-9 antigen as a marker of non-malignant hepatocytic ductular transformation in patients with acute liver failure. A comparison with alpha-fetoprotein and carcinoembryonic antigen. Scand J Gastroenterol 1999; 34:426-31; PMID:10365905
- Lamerz R. Role of tumour markers, cytogenetics. Ann Oncol 1999; 10 Suppl 4:145-9; PMID:10436809; http://dx.doi.org/10.1093/annonc/10.suppl_4.S145
