Abstract
The Zinc finger X-chromosomal protein (ZFX), a novel member of the Krueppel C2H2-type zinc finger protein family, has been implicated in multiple human cancers. However, the clinical significance of ZFX expression in gallbladder cancer (GBC) remains largely unknown. In this study, we focused on the clinical significance, biological function and mechanism of ZFX in GBC, and found that ZFX protein overexpression was frequently detected in GBC tissues. The expression of ZFX was significantly correlated with histological grade, perineural invasion, and margin status and lead to a significantly poorer prognosis in GBC patients(P <0.001). Furthermore, knockdown of ZFX result in significant inhibition of proliferation, migration, invasion and cause cell cycle arrest in GBC-SD cells, while over-expression of ZFX in NOZ shows the opposite results. Activation of PI3K/AKT pathway maybe the potential mechanism behind these effects. In conclusion, ZFX may serve as a oncogene and could be used as a potential prognostic marker and genetic treatment target for GBC patients.
Introduction
Gallbladder cancer (GBC) is the sixth most common form of digestive tract malignancy, with an incidence rate of 2.5 per 100,000 individuals.Citation1,2 GBC is relatively rare worldwide, but it has higher morbidity and mortality rates in some geographic areas, including China, Thailand, and northern India. There is evidence that the rate of GBC is increasing worldwide.Citation3,4 Due to the early spread of tumors through the lymphatic, perineural, and hematogenous routes as well as direct invasion into the liver, the median survival of GBC patients is less than 1 yCitation5
Therefore, identification of clinical markers that can accurately predict tumor metastasis and prognosis, could allow clinicians to better choose appropriate treatment regimens. Studies have shown that ZFX is highly expressed in malignant tumors, such as laryngeal squamous cell carcinoma, hepatocellular carcinoma, gastric carcinoma and colorectal cancer, suggesting that this protein may have important functions in the malignant transformation of these cell types.Citation6-9 In the previous study, we studied the function of ZFX in GBC and demonstrated by MTT, colony formation, and transwell assays that a suppression of ZFX could inhibit cell growth and migration of GBC-SD and SGC-996 cells.Citation10 This work suggested that ZFX is a key regulator of cell proliferation and migration in GBC. However, the significance of ZFX expression in the prognosis of GBC has not been fully evaluated.
Using immunohistochemistry, here we investigated ZFX expression levels in benign and malignant lesions of the gallbladder and studied the clinicopathological significance of its expression in the prognosis of GBC. Next we examined the effects of ZFX on proliferation, migration, invasion and cell cycle regulation in GBC cells and demonstrated for the first time that the PI3K/AKT pathway activation may be involved in these effects.
Results
ZFX expression in benign and malignant lesions of the gallbladder
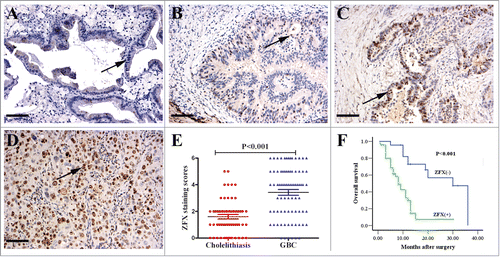
In many types of tumors, ZFX has a critical role in cancer development and progression.Citation11 To determine the potential role of ZFX in GBC progression, we evaluated ZFX expression in GBC and cholelithiasis tissues by IHC (). As shown in , approximately 65.0% (52/80) of the GBC cases had positive nuclear ZFX staining in the tumor cells. In contrast, only 18.3% (11/60) of the cases had positive staining in the cholelithiasis tissues (P < 0.001).
Figure 1. The enhanced expression of ZFX in GBC and negative association with prognosis. ZFX was predominantly localized in the cell nuclei (black arrow, positive staining; scale bar, 100um). (A) Negative staining in normal glandular epithelium. (B) Weak staining in the well differentiated adenocarcinoma. (C) Moderate staining in the partially differentiated adenocarcinoma. (D) Strong staining in the poorly differentiated adenocarcinoma. (E) The average staining scores of cytoplasmic ZFX expression in cholelithiasis and GBC tissues. (F) Kaplan–Meier plots of overall survival in GBC patients with positive and negative ZFX expression scores.
The association of ZFX expression with clinicopathological characteristics of gallbladder adenocarcinoma
As shown in , the ZFX overexpression was found to be significantly correlated with the histological grade (P < 0.001), the perineural invasion (P < 0.001), and the margin status (P = 0.001), indicating a potential role of ZFX expression in promoting aggressive phenotypes in GBC. However, ZFX expression exhibited no significant association with the presence of lymph node metastasis or other clinicopathological characteristics, such as gender, age, the TNM state, or history of gallstones( P > 0.05).
Table 1. Association of ZFX expression with the clinicopathological characteristics of GBC
Correlation between ZFX expression and survival in patients with gallbladder cancer
Among the 80 gallbladder carcinomas, the survival information of 70 patients was obtained through phone calls. Twenty-eight patients survived over 1 y and 42 patients survived less than 1 year, with an average survival time of 11.1 months. The patients that survived less than 1 y were found to have significantly higher ZFX expression than patients that survived over 1 y (). The Kaplan–Meier survival analysis revealed that the histological grade (P = 0.007), the TNM stage (P = 0.049), the presence of lymph node metastasis (P = 0.02), perineural invasion (P = 0.007), and the margin status (P = 0.012) were significantly associated with the average survival time. The average survival time for ZFX-negative patients was significantly higher than that of patients with positive ZFX expression (P < 0.001) (, ). To obtain a more precise estimate about prognosis, the Cox proportional hazard regression model was applied. Results confirmed that the ZFX expression, as well as the presence of lymph node metastasis, was negatively correlated with post-operative survival, suggesting that high ZFX expression is a prognostic factor ().
Table 2. Correlation between patient survival time and the expression of ZFX
Table 3. Univariate analyses showing the overall survival rate for patients with GBC
Table 4. Multivariate analysis the overall survival rate for patients with GBC
Efficiency and specificity of ZFX knockdown/overexpression in GBC cell lines
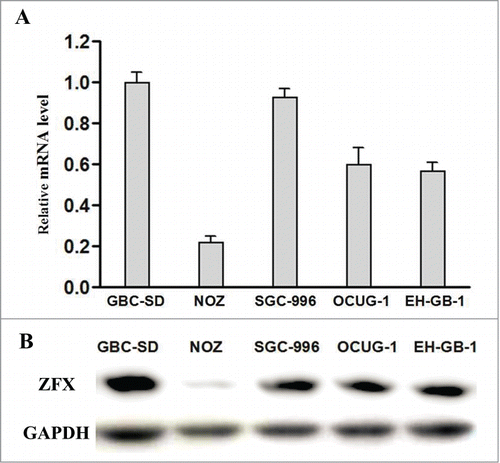
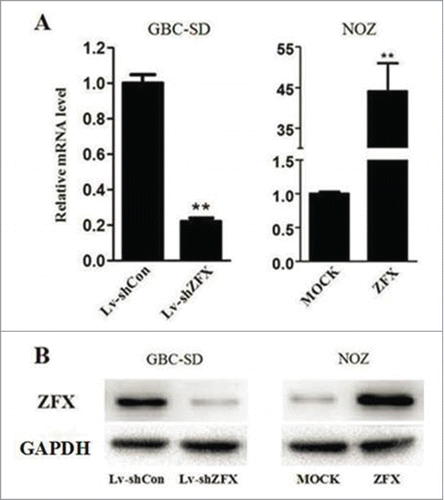
We first detected the endogenous expression of ZFX in 5 GBC cell lines by real-time PCR and western blot analysis. ZFX expression was highest in GBC-SD cells while NOZ cell ZFX expression is the lowest(). Therefore we chose GBC-SD cells for Lentiviral shRNA infection and NOZ cells for ZFX-expression vector transfection. The mRNA and protein expression levels of ZFX were analyzed after treatment. Result showed the levels of ZFX mRNA in GBC-SD cells transduced with Lv-shRNA were significantly decreased by 79% while level of ZFX in NOZ cells elevated 45 times after over-express plasmids transfection. Western blot also showed the consistent results().
Figure 3. Efficiency and specificity of ZFX knockdown/over-express in GBC cell lines. (A) Relative ZFX mRNA expression was determined using real-time PCR. GAPDH was used as an internal control. (B) Total cellular proteins detected by western blot analysis in ZFX-knockdown GBC-SD cells and SPOCK1 over-expressing NOZ cells, GAPDH was used as an internal control. The data represent the mean±SD of 3 independent experiments. Significant differences from the control are indicated by **p < 0.01.
Effect of ZFX on the proliferation of GBC cells in vitro
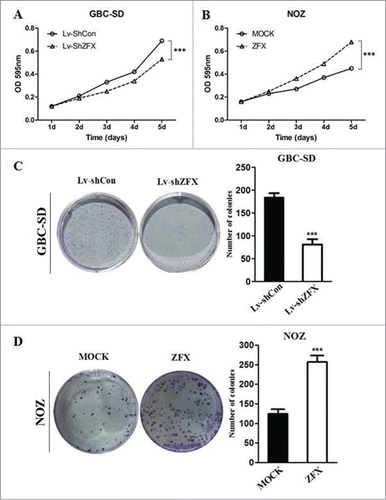
Both MTT and colony formation assays were performed to evaluated the tumorigenic ability of ZFX. As shown in , MTT assay indicated down-regulation of ZFX expression decreased the viability of GBC-SD cells in a time-dependent manner, while over-expression of ZFX showed the opposite effect in NOZ cell. Additionally, the results of the colony formation assay also showed that number of colonies formed by GBC-SD was significantly decreased after ZFX silencing. On the other hand, more colonies were counted after forced expression of ZFX in NOZ cells(). These results suggested that ZFX played a important role in GBC cell growth and proliferation. To further study the molecular mechanism behind this phenomenon, we examined PI3K/AKT phosphorylation states in both ZFX silenced and over-expressed GBC cell models. As shown in , the levels of phosphorylated PI3K and AKT were decreased after ZFX knock-down in GBC-SD cells. Meanwhile, when ZFX was overexpressed in NOZ cells, reversed results were got, indicating PI3K/AKT pathway activation maybe the potential mechanism behind ZFX's oncogenic effect.
Figure 4. Effect of ZFX on the proliferation of GBC cells in vitro. (A and B) Effects of ZFX on cell proliferation were determined using a MTT assay in GBC-SD and NOZ cells. (C and D) Representative photos of colony formation assays. Lv-shCon, Lv-shZFX, MOCK-NOZ, and ZFX-NOZ cells were seeded at 200 cells/well and allowed to form colonies in fresh medium for 14 d The colony numbers were counted and recorded(graph shows mean ± SD. Significant differences from the control are indicated by ***p < 0.001).
Effect of ZFX on the invasion and migration potential of GBC cells in vitro
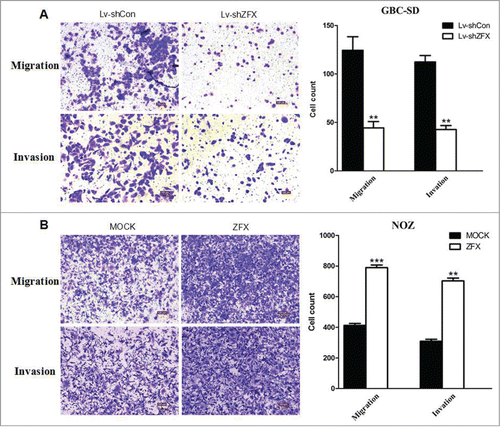
To assess the effect of ZFX on GBC cell migration and invasion in vitro, matrigel/ non-matrigel transwell chamber assays were conducted. Results showed depletion of ZFX displayed a marked increase of migration and invasion ability in GBC-SD cells, while overexpression of ZFX in NOZ cells showed the opposite outcome(). These results strongly indicated that ZFX may be involved in the enhanced metastatic capacity of GBC.
Figure 5. Effect of ZFX on the invasion and migration potential of GBC cells in vitro. (A) Representative photos of crystal violet-stained GBC-SD and NOZ cells that migrated through polycarbonate membranes. (B) Representative photos of crystal violet-stained GBC-SD and NOZ cells that invaded through matrigel-coated membrane. The number of GBC-SD and NOZ cells that migrated through the membranes was calculated and depicted in the bar chart. graph shows mean ± SD. Significant differences from the control are indicated by **P < 0.01 and ***p < 0.001)
Effect of ZFX Downregulation on G1-S phase cell cycle arrest in GBC-SD cells
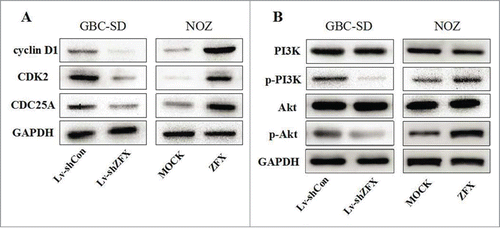
To study the effects of ZFX knockdown on cell cycle progression, flow cytometry analysis was employed. The results showed that proportion of G1 phase cells was significantly increased and the proportion of S phase cells is accordingly lower after ZFX down-regulated in GBC-SD cells(). These results suggest that ZFX induces GBC cell cycle arrest at the G1-S checkpoint. To further understand the mechanism of this phenomenon, several cell cycle related regulators were examined by western blot assays. As shown in , CDK2, CDC25A and cyclinD1 protein levels were significantly inhibited in ZFX silenced GBC-SD cells. ZFX may affects certain regulator's expression to regulate cell cycle progression.
Figure 6. Down-regulation of ZFX induces G1-S phase cell cycle arrest in GBC-SD cells. (A) The cell cycle distribution of ZFX silenced GBC-SD cells was determined using flow cytometry. The data are expressed as the mean ± SD. Significant differences from the control are indicated by **P<0.01. (B) Overexpression of ZFX in NOZ cells shows no cell cycle arrest results.
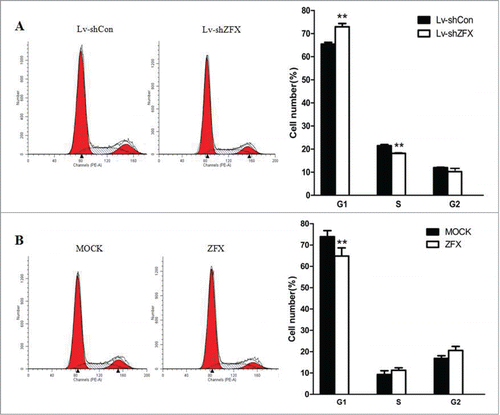
Figure 7. ZFX may affects certain cell cycle regulators and activates PI3k/AKT pathway. (A) The protein levels of cell cycle regulators as CDK2, CDC25A and cyclinD1 are examined by western blot analysis. (B) The levels of phosphorylated PI3K, total PI3K, phosphorylated Akt and total Akt in GBC-SD and NOZ cells are detected.
Discussion
GBC involves a highly invasive, rapidly proliferating tumor. Unfortunately, the most aggressive conventional treatments for this tumor are still associated with poor survival.Citation12 Therefore, it is necessary to identify prognostic biomarkers that are independently correlated with tumor aggressiveness. This study documents ZFX immunostaining in gallbladder carcinomas for the first time, and shows that high ZFX expression is found in approximately 65.0% of cases.
Zinc finger protein X-linked (ZFX) is encoded by the X chromosome, and it contains an acidic transcriptional activation domain (AD), a nuclear localization sequence (NLS), and a DNA binding domain (DBD) consisting of 13 C2H2-type zinc fingers.Citation13 ZFX is highly conserved in vertebrates. Studies have demonstrated that ZFX plays key roles in controlling the self-renewal of embryonic and haematopoietic stem cells.Citation14 Recently, it has been shown that ZFX is involved various physiological functions such as proliferation, differentiation, cell cycle and cell death.Citation15,16
This notion is further confirmed by the study of ZFX function in human carcinomas. ZFX facilitates the development of experimental skin basal cell carcinoma and medulloblastoma in mice initiated by deletion of the Hedgehog (Hh) inhibitory receptor Ptch1, which establish ZFX as a common cell-intrinsic regulator of diverse Hh-induced tumors, with implications for the definition of new therapeutic targets in these malignancies.Citation17 In gastric cancer, ZFX contributed to a poor prognosis by promoting tumor cell growth via the ERK-MAPK pathway.Citation18 Researchers from Japan demonstrated that the regulation of the miR-144-ZFX axis in gastric cancer played an essential role in chemotherapy sensitivity to 5-fluorouracil.Citation19 In this study, our results showed that 65.0% (52/80) of GBC tumors expressed ZFX compared to only 18.3% (11/60) of the paired cholelithiasis samples, which is consistent with the results of JungCitation20 in hepatocellular carcinoma.
In our previous study, a targeted disruption of ZFX resulted in a decelerated growth rate in GBC cells.Citation21 This time we further evaluated the biological significance of ZFX in GBC tumorgenesis by both gene interference and over-expression. According to MTT and colon formation assay results, proliferation ability of GBC-SD cells was significantly impaired with the absence of ZFX. On the other hand, reversed outcome was noticed in NOZ cells when ZFX expression was enhanced, which further verified the role of ZFX in tumor growth. Previous studies have shown activation of the PI3K signaling pathway contributes to cell proliferation, survival and motility as well as angiogenesis. PI3K transduces signals from various growth factors and cytokines into intracellular messages by generating phospholipids, which in turn activate the serine/threonine kinase AKT and other downstream effector pathways.Citation22,23 In the present study, both PI3K and AKT phosphorylation levels were affected by the change of ZFX expression, which suggested activation of PI3K/Akt signaling is involved in ZFX mediated tumor proliferation.
Cell cycle arrest is also a potential reason for tumor growth suppression. Here we used flow cytometry assay to find that ZFX inhibition can cause cell cycle arrest at G0/G1 phase in GBC-SD cells. By further examination of several cell cycle related regulators we found CDK2, CDC25A, and cyclin D1 levels were significantly downregulated. Since progression through the cell cycle depends on the activation of cyclin-dependent kinases (CDKs) and their regulatory subunits, the cyclins,Citation24 we believe ZFX can regulate the expression of these regulators and therefore mediate the cell cycle regulation. It is also noticeable that in the NOZ cells, the original levels of these proteins were lower as compared with GBC cells, which might be explained by cellular heterogeneity.
Invasion and metastasis are the predominant characteristics of malignant diseases. In multiple studies it has been shown that elevated levels of ZFX in different types of tumors correlate with metastatic potential, increased malignancy and thus, an unfavorable prognosis for patients.Citation25 Here we also found that up-regulated ZFX expression was strongly correlated with the degree of tumor differentiation, perineural invasion, and the margin status in GBC. Further survival analysis showed that after surgery, the survival time of cases with ZFX positive expression was significantly lower than that of cases with negative expression. Cox multivariate analysis showed that ZFX expression was negatively correlated with the postoperative survival ratio, and was the evaluation factor for independent poor prognosis. Cytological experiments using tranwell assay also showed migration and invasion capacity of GBC cells was closely correlated with ZFX level. These findings indicate its potential role in promoting aggressive phenotypes of GBC, and a potential utility of ZFX expression levels as a prognostic biomarker of GBC.
In conclusion, ZFX overexpression is present in a substantial proportion of GBC and is significantly correlated with clinical biological behaviors, which represents a significant prognostic for total post-operative survival. ZFX may be closely associated with the occurrence and development of GBC. It is involved in proliferation, cell cycle regulation, migration and invasion process of GBC cells. Thus ZFX may become an effective predictor for the prognosis of GBC and a good biomarker for genetic treatment.
Materials and Methods
Patients and clinicopathological data
The study was approved by the ethics committee of Xinhua Hospital, and all patients provided informed consent. Samples from 80 patients with pathologically confirmed GBC after surgery were collected from 2006 to 2013 at the Department of General Surgery, Xinhua hospital, School of Medicine, Shanghai Jiaotong University, China. None of the patients received either chemotherapy or radiotherapy before surgery. The patients comprised 29 male and 51 female patients with an average age of 67.9 y According to the American Joint Committee on Cancer (AJCC 7th) staging system, 13 (16.3%), 23 (28.8%), 23(28.8%), and 21 (26.3%) patients were diagnosed with stage I, II, III and IV disease, respectively. Additionally, 50 cases were diagnosed with lymph node metastases. In addition, 60 patients with cholelithiasis who underwent simple cholecystectomy were included as controls. All diagnoses of GBC, cholelithiasis, and lymph node metastasis were confirmed by histopathological examination, and all tissue samples were fixed in 4% formalin immediately after removal and embedded in paraffin for immunohistochemical staining.
Immunohistochemical analysis and evaluation of ZFX expression
Immunohistochemical staining was performed using the standard immunoperoxidase staining procedure, and ZFX expression in the specimens was evaluated according to the methods described by Li et al.Citation3,4 The sections were semi-quantitatively scored according to the extent of immunoreactivity as follows: 0, 0% immunoreactive cells; 1, <5% immunoreactive cells; 2, 5-50% immunoreactive cells; and 3, >50% immunoreactive cells. Additionally, the staining intensity was scored semi-quantitatively as follows: 0, negative; 1, weak; 2, intermediate; and 3, strong. The final immunoreaction score was defined as the sum of both parameters (extension and intensity) and the samples were classified as negative (0), weakly stained (1-2), moderately stained (3), and strongly stained (4-6). For statistical purposes, only moderate and strong final immunoreaction scores were considered positive; the other final scores were considered negative.
Cell lines and culture
The human GBC cell lines GBC-SD, NOZ, SGC-996, OCUG and EH-GB-1 were purchased from the Shanghai Cell Institute Country Cell Bank. NOZ and SGC-996 cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium at 37°C in a 5% CO2 incubator, while other cell lines were cultured in high-glucose DMEM (Gibco, USA). Both types of culture medium were supplemented with 10% fetal bovine serum (Gibco, USA), penicillin (100 U/mL) and streptomycin (100 U/mL) (Utah, HyClone, USA).
Quantitative real-time PCR
The expression levels of the ZFX gene were measured using quantitative real-time PCR using Mx3000P(Agilent) as described in our previous study[?]. The following primers were used for detection of ZFX and β-actin (inter-nalcontrol) expression:
ZFX(Sense):GGCAGTCCACAGCAAGAAC;
ZFX(Antisense):TTGGTATCCGAGAAAGTCAGAAG;
β-actin(Sense): GTGGACATCCGCAAAGAC;
β-actin(Antisense):AAAGGGTGTAACGCAACTA.
The relative expression level of the target gene was calculated by 2−ΔCT (ΔCT=CTtarget-CTβ-actin) and normalized to the relative expression detected in the corresponding control cells, which was defined as 1.0.
Lentivirus-mediated RNA interference and over-express plasmids transfection
The shZFX and negative control shRNA lentivirus vectors were synthesized and infected with GBC-SD cells using the same protocol described in our previous study.Citation10 To over-express ZFX, the fragment of human ZFX was amplified from human cDNA by PCR and cloned into the GV143 expression vector (Genechem, Shanghai, China) and transfected into NOZ cells. Both qRT-PCR and western blot assays were used to verify the efficiency of gene silence or over-expression certain period after treatment.
In vitro cell proliferation assay
Both MTT and Colony formation assays were used to measure the effect of ZFX on cell proliferation ability. In MTT assay, 5 d after treatment, GBC-SD and NOZ cells were placed in 96-well culture plates at a density of 2 × 103 cells per well. Ten μl MTT solution at 5mg/ml was added to each well once daily for 5 d After 4 hour of incubation, 100 mL of DMSO was used to dissolve the formazan crystals that formed. Cell proliferation was evaluated by measuring the optical density (OD) at 595 nm using an Automated Microplate Reader (Bio-Tek, USA). In Colony formation assay, cells were trypsinized 5 d after treatment and then seeded in 6-well culture plates at a density of 200 cells/well cultured for 14 d The cells were then fixed with methanol and stained with 5% Giemsa solution. Colonies (>50 cells) were counted under an inverted microscope.
In vitro cell migration and invasion assay
Cell migration and invasion were examined using transwell assay. Briefly GBC-SD (3 × 104) and NOZ (4 × 104) cells in 0.5 μL serum-free DMEM medium or Williams medium, were added to the upper chamber of transwell filters, containing an uncoated or Matrigel (BD Biosciences)-coated membrane. The lower chamber was filled with 500 μl basal medium with 10% fetal bovine serum (FBS). After 24 h of incubation at 37°C in a humidified 5% CO2 incubator, cells remaining on the upper surface of the filter were removed while cells that migrated to the lower compartment were fixed with methanol, stained with crystal violet and counted in 5 randomly chosen fields in each well. Imaging and cell counting were performed at ×10 objective lens under a light microscope.
Cell cycle analysis
The effect of ZFX on cell cycle progression was determined by flow cytometry following staining with PI. The transfected cells were seeded in 6-well plates at 1.5 × 105 cells/well and cultured for 5 days, after that, Then, the cells were collected, fixed with cold 70% ethanol for 4 h at 4°C, then incubated at 37°C for 30 min with 10 mg/mL RNase. Finally, 1 mg/mL propidium iodide (Sigma-Aldrich) was used to stain the cells. DNA content analysis was performed using flow cytometry (San Diego, BD, USA). The percentage of cells present in the different cell cycle phases was determined using Cell Quest acquisition software (BD Biosciences).
Western blot analysis
Proteins of different GBC cell lines were extracted using RIPA buffer according to the method described previously.Citation10 A BCA assay (Shanghai, Beyotime, China) was used to determine cell protein concentrations after extraction. For western blot analysis, equal quantities of total proteins mixed with bromophenol blue (0.01%) were added in each lane and separated by SDS-PAGE, then electrophoretically transferred onto PVDF membranes. After blocking with 5% nonfat milk for 1 h at room temperature, the membranes were incubated with anti-ZFX, anti-CDK2, anti-CDC25A, anti-cyclinD1, anti-PI3K, anti-pPI3K, anti-AKT, anti-pAKT and anti-GAPDH antibodies overnight at 4°C. Sections were washed with PBS for 3 times at room temperature and incubated with secondary antibody. Finally blots were visualized using a chemiluminescent HRP substrate (ECL; GE Healthcare). GAPDH protein levels were used as a control to verify equal protein loading amount.
Statistical analysis
The data were analyzed using SPSS 19.0(Statistical Package for the Social Sciences version 19.0). The inter-relationship of ZFX expression with histology or clinical factors was analyzed using the χ2 independence or Fisher's exact test. The Kaplan-Meier test was used for univariate survival analysis. The Cox proportional hazard model was used for multivariate analysis and for determining the 95% confidence interval. The experiments were performed in triplicate. Each experimental value was expressed as the mean ± standard deviation (SD). P < 0.05 was considered statistically significant.
Disclosure of Potential Conflict of Interests
No potential conflicts of interest were disclosed.
Funding
This work was supported by National Natural Science Foundation of China (No. 81172029, 81272402 and 81402403), National High Technology Rese-arch and Development Program (863 Program) (No. 2012AA022606), Foundation for Interdisciplinary research of Shanghai Jiao Tong University (No. YG2011ZD07), Shanghai science and technology commission medical-guiding project (No. 12401905800), Program for Changjiang Scholars, Natural Science Foundation of Shanxi Province (No. 20110313013-3 and 2014021037-3), and Leading Talent program of Shanghai.
References
- Li M, Zhang Z, Li X, Ye J, Wu X, Tan Z, Shen B, Wang XA, Wu W, Zhou D, et al. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat Genet 2014; 46:872-6; PMID:24997986; http://dx.doi.org/10.1038/ng.3030
- Wistuba, II, Gazdar AF. Gallbladder cancer: lessons from a rare tumour. Nat Rev Cancer 2004; 4:695-706; PMID:15343276; http://dx.doi.org/10.1038/nrc1429.
- Li M, Zhang S, Wang Z, Zhang B, Wu X, Weng H, Ding Q, Tan Z, Zhang N, Mu J, et al. Prognostic significance of nemo-like kinase (NLK) expression in patients with gallbladder cancer. Tumour Biol 2013; 34:3995-4000; PMID:23857283; http://dx.doi.org/10.1007/s13277-013-0988-4.
- Li M, Lu J, Zhang F, Li H, Zhang B, Wu X, Tan Z, Zhang L, Gao G, Mu J, et al. Yes-associated protein 1 (YAP1) promotes human gallbladder tumor growth via activation of the AXL/MAPK pathway. Cancer Lett 2014; 355:201-9; PMID:25218593; http://dx.doi.org/10.1016/j.canlet.2014.08.036.
- Li M, Shen J, Wu X, Zhang B, Zhang R, Weng H, Ding Q, Tan Z, Gao G, Mu J, et al. Downregulated expression of hepatoma-derived growth factor (HDGF) reduces gallbladder cancer cell proliferation and invasion. Med Oncol 2013; 30:587; PMID:23609195; http://dx.doi.org/10.1007/s12032-013-0587-7.
- Kang W, Tong JH, Chan AW, Lee TL, Lung RW, Leung PP, So KK, Wu K, Fan D, Yu J, et al. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res 2011; 17:2130-9; PMID:21346147; http://dx.doi.org/10.1158/1078-0432.CCR-10-2467.
- Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, Ladu S, Singer S, Pinna F, Gretz N, et al. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology 2013; 144:1530-42 e12; PMID:23419361; http://dx.doi.org/10.1053/j.gastro.2013.02.009.
- Wu C, Xu B, Yuan P, Miao X, Liu Y, Guan Y, Yu D, Xu J, Zhang T, Shen H, et al. Genome-wide interrogation identifies YAP1 variants associated with survival of small-cell lung cancer patients. Cancer Res 2010; 70:9721-9; PMID:21118971; http://dx.doi.org/10.1158/0008-5472.CAN-10-1493
- Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X, Schinzel AC, Sood S, Rosenbluh J, Kim JW, et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell 2014; 158:171-84; PMID:24954536; http://dx.doi.org/10.1016/j.cell.2014.06.004.
- Tan Z, Zhang S, Li M, Wu X, Weng H, Ding Q, Cao Y, Bao R, Shu Y, Mu J, et al. Regulation of cell proliferation and migration in gallbladder cancer by zinc finger X-chromosomal protein. Gene 2013; 528:261-6; PMID:23860324; http://dx.doi.org/10.1016/j.gene.2013.06.064.
- Seton-Rogers S. Oncogenes: All eyes on YAP1. Nat Rev Cancer 2014; 14:514-5; PMID:25056702; http://dx.doi.org/10.1038/nrc3791.
- Groot Koerkamp B, Fong Y. Outcomes in biliary malignancy. J Surg Oncol 2014; 110:585-91; PMID:25250887; http://dx.doi.org/10.1002/jso.23762.
- Jiang R, Gao ZL, Sun M, Zhang XY, Wang JC, Wu H. Zinc finger X-chromosomal protein promotes growth and tumorigenesis in human osteosarcoma cells. Pak J Med Sci 2013; 29:997-1002; PMID:24353675.
- Nikpour P, Emadi-Baygi M, Mohammad-Hashem F, Maracy MR, Haghjooy-Javanmard S. Differential expression of ZFX gene in gastric cancer. J Biosci 2012; 37:85-90; PMID:22357206; http://dx.doi.org/10.1007/s12038-011-9174-2.
- Yang H, Lu Y, Zheng Y, Yu X, Xia X, He X, Feng W, Xing L, Ling Z. shRNA-mediated silencing of ZFX attenuated the proliferation of breast cancer cells. Cancer Chemother Pharmacol 2014; 73:569-76; PMID:24448637; http://dx.doi.org/10.1007/s00280-014-2379-y.
- Li K, Zhu ZC, Liu YJ, Liu JW, Wang HT, Xiong ZQ, Shen X, Hu ZL, Zheng J. ZFX knockdown inhibits growth and migration of non-small cell lung carcinoma cell line H1299. Int J Clin Exp Pathol 2013; 6:2460-7; PMID:24228108.
- Palmer CJ, Galan-Caridad JM, Weisberg SP, Lei L, Esquilin JM, Croft GF, Wainwright B, Canoll P, Owens DM, Reizis B. Zfx facilitates tumorigenesis caused by activation of the Hedgehog pathway. Cancer Res 2014; 74:5914-24; PMID:25164012; http://dx.doi.org/10.1158/0008-5472.CAN-14-0834.
- Wu S, Lao XY, Sun TT, Ren LL, Kong X, Wang JL, Wang YC, Du W, Yu YN, Weng YR, et al. Knockdown of ZFX inhibits gastric cancer cell growth in vitro and in vivo via downregulating the ERK-MAPK pathway. Cancer Lett 2013; 337:293-300; PMID:23587796; http://dx.doi.org/10.1016/j.canlet.2013.04.003.
- Akiyoshi S, Fukagawa T, Ueo H, Ishibashi M, Takahashi Y, Fabbri M, Sasako M, Maehara Y, Mimori K, Mori M. Clinical significance of miR-144-ZFX axis in disseminated tumour cells in bone marrow in gastric cancer cases. Br J Cancer 2012; 107:1345-53; PMID:22955854; http://dx.doi.org/10.1038/bjc.2012.326.
- Jung KH, Kim JK, Noh JH, Eun JW, Bae HJ, Xie HJ, Ahn YM, Park WS, Lee JY, Nam SW, et al. Targeted disruption of Nemo-like kinase inhibits tumor cell growth by simultaneous suppression of cyclin D1 and CDK2 in human hepatocellular carcinoma. J Cell Biochem 2010; 110:687-96; PMID:20512928; http://dx.doi.org/10.1002/jcb.22579.
- Tan Z, Li M, Wu W, Zhang L, Ding Q, Wu X, Mu J, Liu Y. NLK is a key regulator of proliferation and migration in gallbladder carcinoma cells. Mol Cell Biochem 2012; 369:27-33; PMID:22733362; http://dx.doi.org/10.1007/s11010-012-1365-0.
- Simpson DR, Mell LK, Cohen EE. Targeting the PI3K/AKT/mTOR pathway in squamous cell carcinoma of the head and neck. Oral Oncol 2015; 51:291-8; PMID:25532816; http://dx.doi.org/10.1016/j.oraloncology.2014.11.012.
- Li D, Zhao LN, Zheng XL, Lin P, Lin F, Li Y, Zou HF, Cui RJ, Chen H, Yu XG. Sox2 is involved in paclitaxel resistance of the prostate cancer cell line PC-3 via the PI3K/Akt pathway. Mol Med Rep 2014; 10:3169-76; PMID:25310235.
- Sherr CJ. Cancer cell cycles. Science 1996; 274:1672-7; PMID:8939849; http://dx.doi.org/10.1126/science.274.5293.1672.
- Vlug EJ, van de Ven RA, Vermeulen JF, Bult P, van Diest PJ, Derksen PW. Nuclear localization of the transcriptional coactivator YAP is associated with invasive lobular breast cancer. Cell Oncol (Dordr) 2013; 36:375-84; PMID:23949920; http://dx.doi.org/10.1007/s13402-013-0143-7.