Abstract
ATP-binding cassette sub-family G member 2 (ABCG2) is a transporter protein that has been associated with multidrug resistance and poor prognosis in several types of cancers. In colorectal cancers, however, the prognostic value of ABCG2 expression is not yet clear. ABCG2 expression was analyzed by immunohistochemistry using tissue microarrays in 234 consecutive patients who underwent surgical resection. The ABCG2 expression level was defined by the composite score, determined by multiplying intensity and percentage of tumor staining scores. This was dichotomized at the median, and the association of ABCG2 expression with disease severity and patient survival was determined. ABCG2 expression in the cytoplasm and membrane was observed in 77.8% and 61.5% of the samples, respectively. High expression of ABCG2 in both the cytoplasm and membrane was found more frequently in well-differentiated lesions (P < 0.05). High expression of membranous ABCG2 was significantly associated with better overall survival (hazard ratio [HR], 0.624; 95% confidence interval [CI], 0.411–0.948; P = 0.027) and disease-specific survival (HR, 0.499; 95% CI, 0.308 - 0.808; P = 0.005) compared to low expression. However, cytoplasmic expression of ABCG2 was not significantly associated with patient survival. The expression level of membranous ABCG2 in colorectal tumors can predict post-operative patient survival, suggesting the potential for ABCG2 as a prognostic biomarker.
Abbreviations
| ABCG2 | = | ATP-binding cassette sub-family G member 2 |
| CRC | = | colorectal cancer |
| DSS | = | disease-specific survival |
| HR | = | hazard ratio |
| OS | = | overall survival. |
Introduction
ATP-binding cassette transporters are involved in transferring a broad range of substances including drugs, metabolites, and other compounds across the cell membrane.Citation1 ATP-binding cassette sub-family G member 2 (ABCG2), also known as breast cancer resistance protein, belongs to this family of transporters; it dimerizes or oligomerizes to become functionally active, and uses ATP hydrolysis as an energy source.Citation2-6
Physiologically, ABCG2 is prominently detected in the apical membrane of epithelial cells, with maximum expression occurring in the duodenum while gradually decreasing along the gastrointestinal tract toward the rectum. This protein is also abundant in liver canaliculi and placental syncytiotrophoblasts, as well as in the areas adjacent to the blood-brain barrier and maternal-fetal barriers (particularly in brain capillary endothelial cells).Citation7 These findings suggest that ABCG2 proteins may have a physiologically cytoprotective role involving the absorption of xenobiotics and excretion of toxic metabolites.Citation8-11
Many anticancer drugs such as mitoxantrone, topotecan, irinotecan, flavopiridol, and methotrexate are substrates of ABCG2,Citation12,13 which therefore implicates this protein in reducing intracellular drug accumulation. This suggests that ABCG2 plays a major role in multidrug-resistance during cancer chemotherapy.Citation13-15 In vitro and in vivo studies have demonstrated an inverse relationship between the ABCG2 expression level and concentration of chemotherapeutics in tumor cells.Citation16-18 For this reason, several studies have assessed the relationship between ABCG2 expression and clinical outcome after chemotherapy in acute myeloid leukemia,Citation19,20 lung cancers,Citation21,22 and gastric cancers.Citation23 Taken together, these data suggest that the expression level of ABCG2 protein may be an important factor to consider when treating cancer patients.
Aside from its transporter function, ABCG2 protein has also been evaluated as a cancer stemness marker. Human ABCG2 is highly expressed in stem cells, and several previous reports have suggested that it is fundamentally important for the maintenance of these cells. In studies with knock-out mice, ABCG2 was shown to provide a strong survival advantage under hypoxic conditions, as well as protection from cytotoxic substrates in haematopoietic cells.Citation7,24,25 One study assessed a panel of 66 stemness markers and their correlation with stage II and III colorectal cancer (CRC); patients were grouped according to length of time to relapse. The expression of the ABCG2 gene tended to contribute to poor prognosis, although this observation was not statistically significant.Citation26
A few studies have analyzed the expression of ABCG2 and its influence on prognosis in CRC.Citation27-30 However, these studies have several limitations in adequately assessing ABCG2 protein expression in relation to clinical outcomes because of the small number of analyzed patients and the short follow-up duration. Furthermore, the reported clinical outcome conflicted with some earlier studies that reported that high expression of this protein was associated with poor prognosisCitation29,30 but was in agreement with others studies.Citation26,28 Therefore, we investigated the association of ABCG2 expression with disease severity and clinical outcomes to clarify its prognostic value in patients with CRC.
Results
Characteristics of the patient population and tumor
Clinical data of 234 patients with ABCG2 expression were analyzed, and their characteristics are shown in . The mean age and median survival time were 62.2 ± 11.6 years and 84.2 months, respectively. The sigmoid colon and rectum were the most commonly involved sites (74.4%), followed by the ascending colon (15.8%), cecum (5.2%), transverse colon (4.3%), and descending colon (0.9%). The cases comprised of the following by TNM stage: I (18.4%), II (25.6%), III (35.0%), and IV (20.9%) based on 7th AJCC criteria. Lymph node metastasis was observed in 51.7% of patients and distant metastasis was found in 20.9% of cases at initial diagnosis. Low grade (well or moderately differentiated) lesions were observed in 85.5% cases based on pathology, and a mucinous type tumor was found in 10.3% of patients. Regarding surgery, 83.8% of patients received curative resection, of which 88.5% received post-operative chemotherapy.
Table 1. Characteristics of the patient population and tumors
ABCG2 expression and clinicopathologic factors
Cytoplasmic and membranous ABCG2 expression was observed in 77.8% and 61.5% of the tumors, respectively. We classified ABCG2 expression as high or low based on a median composite score of 4. ABCG2 expression in either the cytoplasm or the membrane did not show statistically significant association with age, sex, or tumor site. However, high ABCG2 expression in both the membrane and cytoplasm tended to be associated with more pathologically differentiated lesions (P = 0.017 and P = 0.001, respectively). As shown in , membranous ABCG2 expression did not significantly differ according to demographic, pathological, or clinical findings.
Table 2. ABCG2 expression and clinicopathologic features
Patient survival and ABCG2 expression
Results of membranous and cytoplasmic ABCG2 expression are shown in . Patients with high versus low expression of membranous ABCG2 showed better overall survival (OS) (131.2 ± 7.6 vs. 119.5 ± 8.0 months, P = 0.042) and better disease-specific survival (DSS) (146.2 ± 7.1 vs. 130.5 ± 8.1 months, P = 0.011) (). Neither group showed any statistical difference in age, sex, recurrence rate, rate of curative resection, chemotherapy, tumor site, or pathologic stage. For cytoplasmic expression, patient survival was not significantly different between the 2 groups: OS (132.2 ± 8.0 vs. 124.7 ± 8.6 months, P = 0.704) and DSS (145.3 ± 7.9 vs. 138.0 ± 8.6 months, P = 0.748) ().
Figure 1. Immunohistochemical staining of ABCG2 in human colorectal cancer. Representative tissue sections show low (A) and high (B) expression of ABCG2. (All × 400).
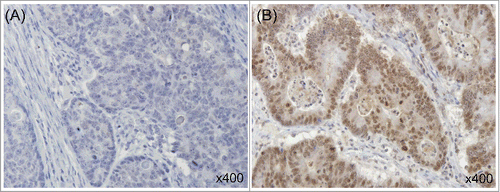
Figure 2. Overall and disease-specific survival according to ABCG2 expression, (A) membranous ABCG2 expression and overall survival, (B) membranous ABCG2 expression and disease-specific survival, (C) Cytoplasmic ABCG2 expression and overall survival, (D) Cytoplasmic ABCG2 expression and disease-specific survival.
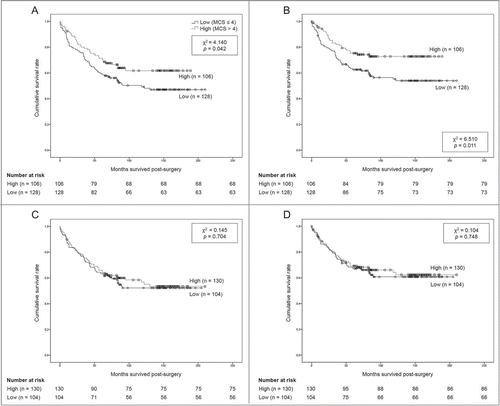
A Cox proportional hazards regression analysis was performed for multiple parameters (). Age, curative resection, chemotherapy, TNM stage, and ABCG2 expression levels were significantly associated with patient survival. High expression of membranous ABCG2 showed better DSS (HR, 0.499; 95% CI, 0.308 - 0.808; P = 0.005) as well as OS (HR, 0.624; 95% CI, 0.411 - 0.948; P = 0.027) than low expression of membranous ABCG2.
Table 3. Multivariate analysis for patient survival
Cancer stage, ABCG2 expression, and patient survival
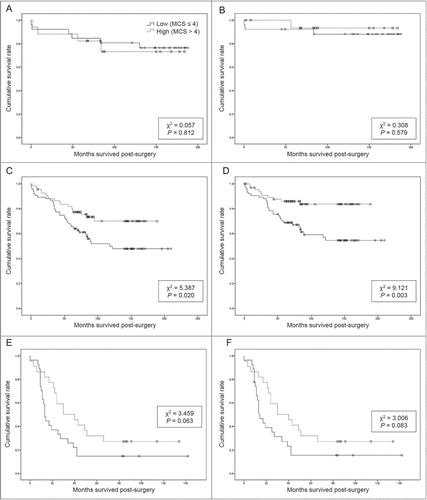
Correlation between patient survival and membranous ABCG2 expression in different cancer stages was investigated. There was no correlation with survival in either the stage I or stage IV groups. () However, patients with stage II and III disease with higher membranous ABCG2 expression tended to show better OS. (146.1 ± 8.7 vs. 126.7 ± 10.1, P = 0.020) and DSS. (163.8 ± 7.3 vs. 137.4 ± 10.2, P = 0.003) () The median composite score of membranous ABCG2 expression was the same in the whole group and in the stage II and III group. A Cox proportional hazards regression analysis was also performed to stage II and III group by same parameters of the whole patient group. The result showed that high expression of membranous ABCG2 was an independent risk factor for better OS and DSS in patients with CRC.
Figure 3. Overall and disease-specific survival from different cancer stages, according to membranous ABCG2 expression. MCS, membranous composite score. (A) Membranous ABCG2 expression and overall survival, stage I, (B) membranous ABCG2 expression and disease-specific survival, stage I, (C) membranous ABCG2 expression and overall survival, stage II and III, (D) membranous ABCG2 expression and disease-specific survival, stage II and III, (E) membranous ABCG2 expression and overall survival, stage IV, (F) membranous ABCG2 expression and disease-specific survival, stage IV.
Discussion
The purpose of this study was to determine the relationship between ABCG2 expression and prognosis in patients with CRC. ABCG2 expression was observed in over 60% of CRC tumors. The expression level of ABCG2 protein was not significantly associated with disease severity. However, high expression of membranous ABCG2 was associated with better patient survival, especially in stage II and III tumors. In contrast, the expression level of cytoplasmic ABCG2 was not associated with patient survival.
We initially hypothesized that increased expression of ABCG2 correlates with poor clinical outcomes in patients with CRC, as this protein has previously been regarded as a marker of cancer stemness and drug resistance. However, the current results showed that higher expression of membranous ABCG2 was associated with significantly better patient survival in CRCs. A previous study showed that high levels of ABCG2 mRNA and protein were observed in normal colonic epithelium but that these levels were markedly diminished in cancer tissues from colectomy specimens.Citation28 Another study showed lower level of ABCG2 in adenomas and carcinomas compared to the level in unaffected tissue from the same individuals and healthy individuals.Citation31 One may thus postulate that decreased ABCG2 expression might be associated with poor prognosis due to diminished ability to prevent accumulation of toxins in cells, and that decreased expression of ABCG2 might play a role in carcinogenesis by allowing the accumulation of carcinogens. In another study, loss of ABCG2 expression was associated with lymph node involvement and advanced histological grade in moderately and poorly differentiated intrahepatic cholangiocarcinoma, resulting in poor prognosis and progression with aggressive tumor behavior.Citation32 A recent genetic study aimed at evaluating the expression levels of several potential stemness markers, including ABCG2, as prognostic indicators in CRC patients showed that higher expression of ABCG2 may be related to better prognosis, even though the findings were not statistically significant.Citation26
On the other hand, there have been studies demonstrating a direct correlation between increased ABCG2 expression and poor prognosis in CRCs. Membranous ABCG2 expression was significantly associated with Dukes' stage, lymph node metastasis, distant metastasis, and shorter survival in CRC patients,Citation30 and high expression of ABCG2 was detected in carcinomatous tissue and in patients with positive lymph node metastasis of CRCs.Citation29 Another study analyzed ABCG2 expression in metastatic colorectal cancer but did not show a statistically significant association between ABCG2 expression and patient survival and clinical response.Citation33 Considering these reports, the role of ABCG2 in colorectal cancers remains ambiguous, and our current study points to a potential role for ABCG2 in predicting the outcome of stage II and III patients after surgery.
Our result showed that the expression level of ABCG2 protein was predictive of survival, especially in patients with stages II and III CRC. These patients are usually treated with adjuvant chemotherapy after surgery, which was 5-FU-based chemotherapy in our study population. Although previous studies reported the efflux of several chemotherapeutic agents by ABCG2, which was thought to be one of the mechanisms of multi-drug resistance,Citation12,13 there has been no report regarding a role for ABCG2 in the efflux of 5-FU in CRC cells. This should be investigated in future studies. In addition, our result revealed the difference between locations of the ABCG2 expression in their effect on CRC prognosis. As ABCG2 is regarded to play roles in transmembrane transportation of various molecules, expression in membrane and cytoplasm might vary in their functions.
Surgery-induced inflammation can be a promoter of tumor recurrence and metastasis in colorectal cancer.Citation34 For diminishing peritoneal tumor recurrence, scavengers has been studied to inhibit the oxidative potential after surgical peritoneal trauma.Citation35 ABCG2 proteins exports toxic materials, which declines reactive oxygen species. Signaling of reactive oxygen species (ROS) acts as a key regulator in tumor cell survival and in the cellular processes required for tumor cells to successful metastatic cascade including invasion, adhesion, angiogenesis and proliferation. Surgical trauma creates a ROS-rich environment, which facilitates redox signaling.Citation36 Therefore, we can postulate the plausible explanation of our current result that high expression of ABCG2 can reduce ROS production, conferring the better OS and DSS.
High grade tumor tissues showed significantly lower expression of ABCG2. Considering this, we can postulate why the lower expression of ABCG2 results in poorer patient survival as follows. Epithelial cells of the gastrointestinal tract physiologically express the ABCG2 protein because these cells have absorptive functions, thus making the ability to transport toxic materials out of the cell necessary.Citation28 The loss of transport functionality may be accompanied by a decreased level of other transporter proteins like ABCG2, which in turn can be linked to carcinomatous change.
Our study has advantages compared to previously published investigations. First, the number of cases that underwent both immunohistochemistry analysis and clinical review is larger than those in previous reports. Others have reported their findings based on analyzing less than 200 immunohistochemistry samples and less than 100 cases of their corresponding clinical histories; we analyzed both molecular data and medical records from over 200 patients. Second, this study evaluated ABCG2 expression in the cytoplasm and membrane separately. This may imply that ABCG2's protective role in carcinogenesis is site-specific. Third, we used multiple statistical methods to estimate survival time.
The current study has some limitations. When reviewing the cases, we found that most patients received post-operative chemotherapy even in the very early stages of the CRC. Their regimens were diverse, including frequent use of oral 5-FU agents, which is different from the current strategy. This makes it difficult to analyze the effect of membranous expression of ABCG2. Thus, further studies are required to explain the mechanism of ABCG2 in the cell membrane and its specific effect on 5-FU. Another limitation is the possibility of intratumoral heterogeneity. For tissue microarray analysis, we obtained the sections from 2 rich viable tumor cell areas in the tumor center and invasive front with a 2 mm-diameter tissue cylinder, which is larger size compared to commonly used 0.6 mm-diameter cylinder used for tissue microarray studies. Although the results from each area showed similar results, the question of heterogeneity of the tumor still arises. Our study population consisted entirely of Asian populations, particularly Koreans. Hence, studies in other ethnic groups are also needed.
In conclusion, our data showed that high expression levels of membranous ABCG2 in tumors was associated with better clinical outcome in patients with CRC after surgery. This finding suggests the potential use of ABCG2 expression as a predictive biomarker for determining prognosis in CRC patients.
Patients & Methods
Patients and tissue specimens
A total of 234 consecutive patients who underwent surgical resection for primary CRC between November 1996 and August 2007 at Seoul St. Mary's Hospital, Seoul, Korea, were enrolled. Medical records of the patients were reviewed retrospectively. Data for patient demographics, clinical findings, pathological diagnosis, and additional treatments such as chemotherapy were collected. Survival time was counted from the day of surgery to the last known date the patient was confirmed to be alive. Data from medical records were used, and the database of the National Cancer Center of Korea was used if data from Seoul St. Mary's Hospital was not available. The patient's cancer stage was defined based on AJCC (American Joint Committee on Cancer) criteria, 7th edition. Tumor histologic grade was determined as defined by the AJCC Prognostic Factors Consensus Conference (grade 1, well differentiated; grade 2, moderately differentiated; grade 3, poorly differentiated; grade 4, undifferentiated).Citation37 This study was performed according to the Declaration of Helsinki and approved by the Institutional Review Board of the Catholic University Medical Center (KC14SISI0728).
Immunohistochemistry
Tissue microarrays were produced from formalin-fixed, paraffin-embedded tumor tissues acquired from primary CRC specimens using a manual tissue arrayer (Quick-Ray Manual Tissue Microarrayer; Unitma Co., Ltd.; Seoul, Korea). The tissues were obtained from the 2 areas with rich viable tumor cells in the tumor center and invasive front, and identified by light microscopy with hematoxylin-eosin-stained sections, by tissue cylinders with a diameter of 2 mm. Those specimens were inserted into a recipient paraffin block, resulting in a 6 × 10 array. The sections were incubated with a rabbit polyclonal anti-ABCG2 monoclonal antibody (Santa Cruz Biotechnology, Inc., Dallas, TX USA). For negative controls, the primary antibodies were substituted with normal rabbit IgG at the same concentrations.
Scoring of immunohistochemistry
Tumors were deemed positive for ABCG2 if more than 5% of tumor cells were stained with an intensity grade of 1 or above. Staining intensity was graded as 0 = not detectable, 1 = weak, 2 = moderate, or 3 = strong. Staining extent was defined as the percentage of positive tumor cells and was scored as follows: 0 (0%), 1 (1-10%), 2 (11-25%), 3 (26-50%), 4 (51-75%), 5 (76-90%), and 6 (>90%). This scoring was done for both cytoplasmic and membranous ABCG2 expression. Repeated measurements were performed by a gastrointestinal pathologist (C.K.J.) in a blinded fashion, and cases with inconsistent results were examined for a third time. The staining extent and intensity scores were multiplied to produce a composite score for each tumor specimen. The composite score was dichotomized at the median for all subsequent analyses ().
Outcome measures
We used the composite score as a degree of ABCG2 expression, which was then assessed for any association with disease severity and patient survival. OS and DSS were estimated.
Statistical Analyses
Continuous data are presented as the mean ± standard deviation, and categorical data are presented as quantities and proportions. To evaluate differences between the groups of patients according to their immunoreactivity of ABCG2 expression, the χ2 test and the 2-sample independent t-test were used for categorical data and continuous variables, respectively. Cumulative patient survival curves according to ABCG2 immunoreactivity were generated using the Kaplan-Meier method, and the differences between the groups were compared using the log-rank test. Multivariate analyses were performed using the Cox proportional hazard regression model to identify factors that were associated with influenced OS and DSS. OS and DSS were calculated as the number of years from random assignment to the date of death or last contact. The distribution of survival was estimated using the Kaplan-Meier methodology. Univariate and multivariate Cox proportional hazards models were used to explore the association between biomarkers or clinical variables and OS. Multivariate models were adjusted for covariates. The score and likelihood ratio test P values were used to test the significance of each covariate in the univariate and multivariate models, respectively.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by program of Global Research and Development Center through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2011-0031644) and by Basic Science Research Program through the NRF funded by the Ministry of Education, Science and Technology (NRF-2013R1A1A2007985).
References
- Higgins CF. ABC transporters: from microorganisms to man. Ann Rev Cell Biol 1992; 8:67-113; PMID:1282354; http://dx.doi.org/10.1146/annurev.cb.08.110192.000435
- Kage K, Tsukahara S, Sugiyama T, Asada S, Ishikawa E, Tsuruo T, Sugimoto Y. Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int J Cancer J Int du Cancer 2002; 97:626-30; PMID:11807788; http://dx.doi.org/10.1002/ijc.10100
- Xu J, Peng H, Chen Q, Liu Y, Dong Z, Zhang JT. Oligomerization domain of the multidrug resistance-associated transporter ABCG2 and its dominant inhibitory activity. Cancer Res 2007; 67:4373-81; PMID:17483351; http://dx.doi.org/10.1158/0008-5472.CAN-06-3169
- Doyle L, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003; 22:7340-58; PMID:14576842; http://dx.doi.org/10.1038/sj.onc.1206938
- Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A 1998; 95:15665-70; PMID:9861027; http://dx.doi.org/10.1073/pnas.95.26.15665
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2002; 2:48-58; PMID:11902585; http://dx.doi.org/10.1038/nrc706
- van Herwaarden AE, Schinkel AH. The function of breast cancer resistance protein in epithelial barriers, stem cells and milk secretion of drugs and xenotoxins. Trends Pharmacol Sci 2006; 27:10-6; PMID:16337280; http://dx.doi.org/10.1016/j.tips.2005.11.007
- Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res 2001; 61:3458-64; PMID:11309308
- Schnepf R, Zolk O. Effect of the ATP-binding cassette transporter ABCG2 on pharmacokinetics: experimental findings and clinical implications. Exp Opin Drug Metab Toxicol 2013; 9:287-306; PMID:23289909; http://dx.doi.org/10.1517/17425255.2013.742063
- Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 2001; 7:1028-34; PMID:11533706; http://dx.doi.org/10.1038/nm0901-1028
- Gutmann H, Hruz P, Zimmermann C, Beglinger C, Drewe J. Distribution of breast cancer resistance protein (BCRP/ABCG2) mRNA expression along the human GI tract. Biochem Pharmacol 2005; 70:695-9; PMID:15998509; http://dx.doi.org/10.1016/j.bcp.2005.05.031
- An Y, Ongkeko WM. ABCG2: the key to chemoresistance in cancer stem cells? Exp Opin Drug Metab Toxicol 2009; 5:1529-42; PMID:19708828; http://dx.doi.org/10.1517/17425250903228834
- Mo W, Zhang JT. Human ABCG2: structure, function, and its role in multidrug resistance. Int J Biochem Mol Biol 2012; 3:1-27; PMID:22509477
- Candeil L, Gourdier I, Peyron D, Vezzio N, Copois V, Bibeau F, Orsetti B, Scheffer GL, Ychou M, Khan QA, et al. ABCG2 overexpression in colon cancer cells resistant to SN38 and in irinotecan-treated metastases. Int J Cancer J Int du Cancer 2004; 109:848-54; PMID:15027118; http://dx.doi.org/10.1002/ijc.20032
- Mazard T, Causse A, Simony J, Leconet W, Vezzio-Vie N, Torro A, Jarlier M, Evrard A, Del Rio M, Assenat E, et al. Sorafenib overcomes irinotecan resistance in colorectal cancer by inhibiting the ABCG2 drug-efflux pump. Mol Cancer Ther 2013; 12:2121-34; PMID:23960095; http://dx.doi.org/10.1158/1535-7163.MCT-12-0966
- Liu L, Zuo LF, Guo JW. ABCG2 gene amplification and expression in esophageal cancer cells with acquired adriamycin resistance. Mol Med Rep 2014; 9:1299-304; PMID:24535197
- Huang WC, Hsieh YL, Hung CM, Chien PH, Chien YF, Chen LC, Tu CY, Chen CH, Hsu SC, Lin YM, et al. BCRP/ABCG2 inhibition sensitizes hepatocellular carcinoma cells to sorafenib. PloS One 2013; 8:e83627; PMID:24391798; http://dx.doi.org/10.1371/journal.pone.0083627
- Suvannasankha A, Minderman H, O'Loughlin KL, Nakanishi T, Ford LA, Greco WR, Wetzler M, Ross DD, Baer MR. Breast cancer resistance protein (BCRP/MXR/ABCG2) in adult acute lymphoblastic leukaemia: frequent expression and possible correlation with shorter disease-free survival. Br J Haematol 2004; 127:392-8; PMID:15521915; http://dx.doi.org/10.1111/j.1365-2141.2004.05211.x
- Benderra Z, Faussat AM, Sayada L, Perrot JY, Chaoui D, Marie JP, Legrand O. Breast cancer resistance protein and P-glycoprotein in 149 adult acute myeloid leukemias. Clin Cancer Res 2004; 10:7896-902; PMID:15585622; http://dx.doi.org/10.1158/1078-0432.CCR-04-0795
- Uggla B, Stahl E, Wagsater D, Paul C, Karlsson MG, Sirsjo A, Tidefelt U. BCRP mRNA expression v. clinical outcome in 40 adult AML patients. Leukemia Res 2005; 29:141-6; PMID:15607361; http://dx.doi.org/10.1016/j.leukres.2004.06.004
- Kim YH, Ishii G, Goto K, Ota S, Kubota K, Murata Y, Mishima M, Saijo N, Nishiwaki Y, Ochiai A. Expression of breast cancer resistance protein is associated with a poor clinical outcome in patients with small-cell lung cancer. Lung Cancer 2009; 65:105-11; PMID:19036469; http://dx.doi.org/10.1016/j.lungcan.2008.10.008
- Yoh K, Ishii G, Yokose T, Minegishi Y, Tsuta K, Goto K, Nishiwaki Y, Kodama T, Suga M, Ochiai A. Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clin Cancer Res 2004; 10:1691-7; PMID:15014021; http://dx.doi.org/10.1158/1078-0432.CCR-0937-3
- Zhang Q, Li K, Xu JH, Zhao CG, Gao Q, Wu B, Liu XY. Role of ABCG2 expression driven by cisplatin in platinum-containing chemotherapy for gastric cancer. World J Gastroenterol 2013; 19:6630-6; PMID:24151392; http://dx.doi.org/10.3748/wjg.v19.i39.6630
- Bunting KD. ABC transporters as phenotypic markers and functional regulators of stem cells. Stem Cells 2002; 20:11-20; PMID:11796918; http://dx.doi.org/10.1002/stem.200011
- Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, Rosing H, et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A 2002; 99:15649-54; PMID:12429862; http://dx.doi.org/10.1073/pnas.202607599
- Giampieri R, Scartozzi M, Loretelli C, Piva F, Mandolesi A, Lezoche G, Del Prete M, Bittoni A, Faloppi L, Bianconi M, et al. Cancer stem cell gene profile as predictor of relapse in high risk stage II and stage III, radically resected colon cancer patients. PloS one 2013; 8:e72843; PMID:24023782; http://dx.doi.org/10.1371/journal.pone.0072843
- Glasgow SC, Yu J, Carvalho LP, Shannon WD, Fleshman JW, McLeod HL. Unfavourable expression of pharmacologic markers in mucinous colorectal cancer. Br J Cancer 2005; 92:259-64; PMID:15655543
- Gupta N, Martin PM, Miyauchi S, Ananth S, Herdman AV, Martindale RG, Podolsky R, Ganapathy V. Down-regulation of BCRP/ABCG2 in colorectal and cervical cancer. Biochem Biophy Res Commun 2006; 343:571-7; PMID:16554028; http://dx.doi.org/10.1016/j.bbrc.2006.02.172
- Liu HG, Pan YF, You J, Wang OC, Huang KT, Zhang XH. Expression of ABCG2 and its significance in colorectal cancer. Asian Pac J Cancer Prev 2010; 11:845-8; PMID:21133588
- Wang X, Xia B, Liang Y, Peng L, Wang Z, Zhuo J, Wang W, Jiang B. Membranous ABCG2 expression in colorectal cancer independently correlates with shortened patient survival. Cancer Biomark 2013; 13:81-8; PMID:23838136
- Andersen V, Vogel LK, Kopp TI, Saebo M, Nonboe AW, Hamfjord J, Kure EH, Vogel U. High ABCC2 and low ABCG2 gene expression are early events in the colorectal adenoma-carcinoma sequence. PloS One 2015; 10:e0119255; PMID:25793771; http://dx.doi.org/10.1371/journal.pone.0119255
- Larbcharoensub N, Sornmayura P, Sirachainan E, Wilasrusmee C, Wanmoung H, Janvilisri T. Prognostic value of ABCG2 in moderately and poorly differentiated intrahepatic cholangiocarcinoma. Histopathology 2011; 59:235-46; PMID:21884202
- Silvestris N, Simone G, Partipilo G, Scarpi E, Lorusso V, Brunetti AE, Maiello E, Paradiso A, Mangia A. CES2, ABCG2, TS and Topo-I primary and synchronous metastasis expression and clinical outcome in metastatic colorectal cancer patients treated with first-line FOLFIRI regimen. Int J Mol Sci 2014; 15:15767-77; PMID:25198900; http://dx.doi.org/10.3390/ijms150915767
- Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg 2011; 253:890-9; PMID:21394013; http://dx.doi.org/10.1097/SLA.0b013e3182128929
- van Rossen ME, Sluiter W, Bonthuis F, Jeekel H, Marquet RL, van Eijck CH. Scavenging of reactive oxygen species leads to diminished peritoneal tumor recurrence. Cancer Res 2000; 60:5625-9; PMID:11059751
- O'Leary DP, Wang JH, Cotter TG, Redmond HP. Less stress, more success? Oncological implications of surgery-induced oxidative stress. Gut 2013; 62:461-70; PMID:22147551; http://dx.doi.org/10.1136/gutjnl-2011-300948
- Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer 2000; 88:1739-57; PMID:10738234; http://dx.doi.org/10.1002/(SICI)1097-0142(20000401)88:7%3c1739::AID-CNCR30%3e3.0.CO;2-T
