Abstract
Artesunate, a semi-synthetic derivative of arteminisin originally developed for the treatment of malaria, has recently been shown to possess antitumor properties. One of the cytotoxic effects of artesunate on cancer cells is mediated by induction of oxidative stress and DNA double-strand breaks (DSBs). We report here that in addition to inducing oxidative stress and DSBs, artesunate can also downregulate RAD51 and impair DSB repair in ovarian cancer cells. We observed that the formation of RAD51 foci and homologous recombination repair (HRR) were significantly reduced in artesunate-treated cells. As a consequence, artesunate and cisplatin synergistically induced DSBs and inhibited the clonogenic formation of ovarian cancer cells. Ectopic expression of RAD51 was able to rescue the increased chemosensitivity conferred by artesunate, confirming that the chemosensitizing effect of artesuante is at least partially mediated by the downregulation of RAD51. Our results indicated that artesunatecan compromise the repair of DSBs in ovarian cancer cells, and thus could be employed as a sensitizing agent in chemotherapy.
Abbreviations
| ART | = | artesunate |
| DSBs | = | double-strand breaks |
| HRR | = | homologous recombination repair |
| ROS | = | reactive oxygen species. |
Introduction
Ovarian cancer is relatively uncommon, occurring in 1 of 2,500 postmenopausal women in the US, but it is the most lethal gynecologic malignancy, accounting for 5-6% of all cancer related deaths.Citation1 Most patients present with advanced disease and are usually treated with surgical resection followed by platinum-based chemotherapy.Citation2 However, most patients relapse with platinum-resistant disease and require treatment with a regimen that does not contain platinum. Liposomal doxorubicin, topotecan, gemcitabine, paclitaxel, oral etoposideand vinorelbine are among the alternative agents for platinum-resistant disease, but with response rates in the range of 10 to 20 percent.Citation2 Platinum and all the other drugs tend to also have serious side effects. The limitations with the current therapeutic agents call for development of new drugs or chemosensitizers.
Recent studies showed that artesunate, a derivative of artemisin that was originally developed for the treatment of malaria, possesses antitumor activity.Citation3,4 In particular, it can inhibit the proliferation of cancer cells by inducing oxidative stress and inflicting DNA damage.Citation5-7 In this report, we studied the antitumor effect of artesunate on ovarian cancer in vitro and in tumor xenografts. We found that in addition to inducing oxidative stress and DNA damage, artesunate could effectively downregulate RAD51 and increase the sensitivity of ovarian cancer cells to cisplatin in vitro and in vivo. We also showed that RAD51 foci formation and homologous recombination repair are significantly impaired in artesunate-treated cells. Our findings suggest that artesunate can potentially be used as an adjuvant drug in the therapy of ovarian cancer.
Results
Artesunate induces ROS and DNA double-strand breaks in ovarian cancer cells
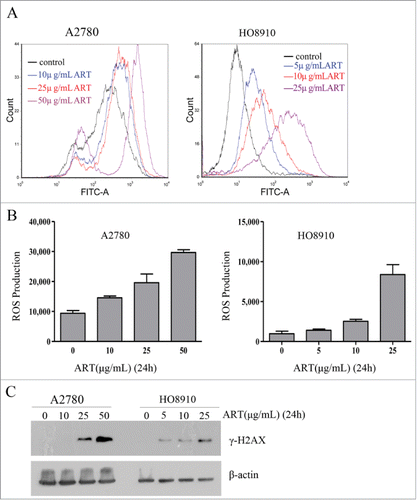
Artesunate has been previously reported to kill cancer cells by inducing oxidative stress and DNA damage.Citation5 To determine whether artesunate can similarly induce oxidative stress and DNA damage in ovarian cancer cells, we measured the levels of reactive oxygen species (ROS) and the level of γ-H2AX, a marker of DNA double-strand breaks, in the cancer cells after they were treated with artesunate. As shown in , artesunate treatment for 24 h caused a significant increase in the levels of ROS in a dose-dependent manner in both cell lines. Moreover, Western blotting showed that the levels of γ-H2AX were significantly elevated when cancer cells were treated with artesunate in the higher dose range for 24 h (). Together, these results indicated that artesunate acts as a genotoxicant in ovarian cancer cells. These findings were consistent with the previous reports.Citation5,6
Figure 1. Artesunate induces ROS and DNA double-strand breaks in ovarian cancer cells. (A and B) ROS induction by artesunate in A2780 and HO8910 cells as measured by FACS. (A) ROS distribution in ovarian cancer cell treated with artesunate for 24h measured by flow cytometry. (B) Data summary, all the data are the means of 3 experiments ±SD. (C) Induction of DSBs in ovarian cancer cells by artesunate. (C) Levels of γ-H2AX in ovarian cancer cells treated with artesunate, determined by Western blot analysis. β-actin served as a loading control.
Artesunate downregulates RAD51 in ovarian cancer cells
The induction of oxidative stress and DSBs by artesunate is reminiscent of the effect of berberine, which also elevates ROS and inflicts DSBs.Citation8-11 We previously showed that berberine can also sensitize cancer cells to ionizing radiation by downregulating RAD51.Citation12 We therefore tested whether artesunate can also downregulate RAD51 in ovarian cancer cells. Western blot analysis showed a dose-dependent decrease in the level of RAD51 in A2780, H8910 and HEY cell lines when cells were treated with artesunate for 24 h (). SKOV3 cells, however, appeared to be unresponsive to artesunate. Artesunate also showed a time-dependent effect on the level of RAD51 in A2780 and HO8910 cells (). In two types of non-malignant cells, normal human fibroblasts and immortalized epithelial cells, FTE-187, the level of RAD51 was not altered by artesunate ()
Figure 2. Artesunate downregulates RAD51 in ovarian cancer cells. (A) Top: Western blot analysis of RAD51 in A2780, HO8910, SKOV3 and HEY cells treated with varying concentrations of artesunate for 24 h. Bottom: Western blot analysis of RAD51 in A2780 and HO8910 cells treated with 5µg/ml of artesunate for varying times. (B) Western blot analysis of RAD51 in NHF and FTE-187 cells treated with artesunate. (C) Transcript level of RAD51 in artesunate-treated A2780 cells. The level of RAD51 mRNA was determined by quantitative real-time PCR. Statistical analysis was performed using unpaired Student's t test. The asterisk denotes statistical significance compared to control values, *p < 0.05, **P < 0.01. (D). Luciferase reporter assay of RAD51 promoter regions that are affected by artesunate in A2780 cells. (E) Level of RAD51 mRNA determined by quantitative real-time PCR in HO8910 cells treated by artesunate. (F) Luciferase reporter assay of RAD51 promoter activity in HO8910 cells.
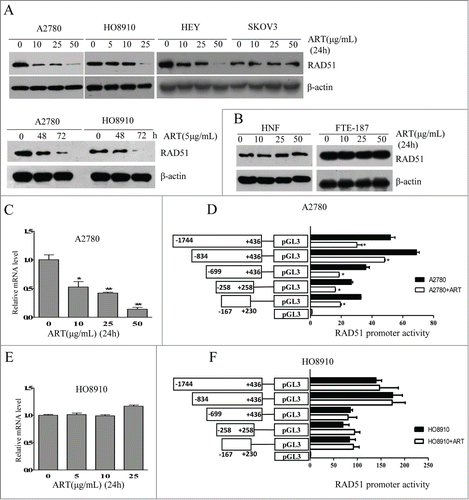
We next determined whether the downregulation of RAD51 by artesunate occurs at transcription level. In A2780 cells, the level of RAD51 mRNA was indeed decreased by the addition of artesunate, in a dose-dependent manner (). Correspondingly, the promoter activity of RAD51 was significantly inhibited by artesunate (). In contrast, the RAD51 mRNA level in H8910 cells was not affected by artesunate (). As expected, the promoter activity showed no response to artesunate (). These results indicate that artesunate can downregulate RAD51 at transcription level and post-transcriptionally. The fact that RAD51 mRNA level in H8910 cells was not reduced by artesunate also implies that the downregulation of RAD51 by artesunate is not due to cell cycle arrest. Consistently, analysis of cell cycle distribution showed that while there was a modest induction of G1 arrest in artestunate-treated A2780 cells, cell cycle distribution was not altered in H8910 cells (Data not shown)
Artesunate inhibits the formation of RAD51 foci and HRR
We next tested whether the downregulation of RAD51 would be translated into a reduced function of HR. Because the formation of RAD51 foci is regarded as critical step in HR, we first determined whether the formation of RAD51 foci is impaired by artesunate. We observed that while the baseline level of RAD51 foci was not different in artesunate-treated ovarian cancer cells when compared to control cells, the induction of RAD51 foci by cisplatin was significantly attenuated by artesunate in both cell lines tested (). This result suggests that with a reduction in the total amount of RAD51, the formation of RAD51 foci in ovarian cancer cells sustaining DNA damage is also significantly reduced by artesunate.
Figure 3. Homologous recombination repair is impaired by artesunate. (A) Artesunate inhibits the formation of RAD51 foci induced by cisplatin. For the combined artesunate and cisplatin treatment, cells were pre-treated with 10μg/ml artesunate for 24h and were then exposed to 30 μM cisplatin for 4h before they were processed for immunofluorescence staining of RAD51 foci. The other groups were untreated, treated with artesunate, and with cisplatin, respectively. Left: Representative examples of immunofluorescence staining of RAD51 foci. Right: Distribution of cells with at least 5 RAD51 foci. For each group 500 cells were counted. Shown are the averages and SD of 3 repeats. (B) Inhibition of HR repair by artesunate. Cells treated with artesunate and control cells were co-transfected with 2 μg linearized GFP HR reporter plasmids and 0.1 μg of the DsRed expression vectors. The DsRed was used to normalize for the differences in transfection efficiency. Cells were analyzed on a green (P2)-versus-red (P4) fluorescence plot. The numbers of GFP+ and DsRed+ cells were determined by flow cytometry. The ratio of GFP+ to DsRed+ cells was used as a measure of repair efficiency. Left: Typical FACS traces, P2 and P4 represent as green and red fluorescence plot, respectively. Right: quantitative summary of HR efficiency in ovarian cancer cells after treatment with artesunate. All experiments were repeated at least 3 times. Error bars, SD.
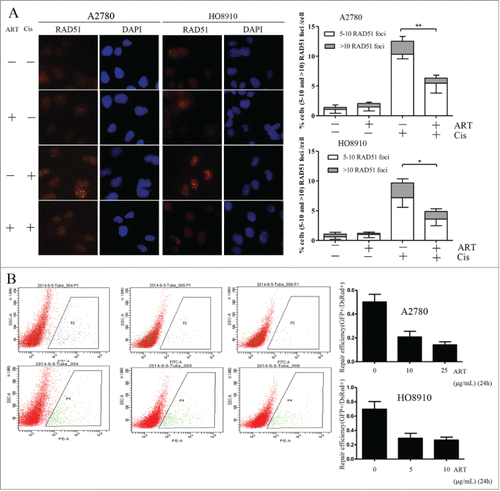
The formation of RAD51 foci as one step of HR only partially reflects the function of HR repair. We then resorted to a GFP (green fluorescence protein) reporter assay that measures the end products of HR repair.Citation13 Linearized GFP reporter plasmids and DsRed plasmids were co-transfected into ovarian cancer cells and the GFP positive cells were analyzed by flow cytometry. As shown in , in cells treated with artesunate, the frequency of GFP positive cells were significantly reduced. This result indicates that HRR function can indeed be reduced by artesunate.
Artesunate sensitizes ovarian cancer cells to cisplatin
Since HRR is essential for the repair of DSBs that arise in S phase, it could be expected that cancer cells treated with artesunate and cisplatin in combination would incur more DNA damage than those treated with artesunate or cisplatin alone. Indeed, while artesunate and cisplatin could each induce DSBs, as reflected by the γ-H2AX foci formed, they acted synergistically in combination (). This result suggests that artesunate may greatly elevate the genotoxic effect of agents that cause DSBs and DNA cross-links.
Figure 4. Artesunate sensitizes ovarian cancer cells to cisplatin. (A) Artesunate and cisplatin act synergistically in inducing double-strand breaks. Cells were treated with artesunate (10µg/ml), cisplatin (30µM), or both for 24 h and then processed for immunofluorescence. Left: representative images of immunofluorescence staining of γ-H2AX. Right: Quantitative summary of γ-H2AX foci. P < 0.001 for interaction between artesunate and cisplatin in both A2780 and HO8910 cells. (B) Left: Representative images of colonies formed by ovarian cancer cells after treatment by artesunate alone or in combination with cisplatin. Right: Quantitative summary of colonogenic assay results. P values for interaction between artesunate and cisplatin are <0.001 and <0.003, respectively, in A2780 and HO8910 cells. Artesunate was applied at 5 μg /ml for 24 h, cisplatin was applied at 0.3 μM for 24 h, the combination group was pre-treated with artesunate at 5 μg /ml for 24 h and then subjected to cisplatin at 0.3 μM for 24 h, and the control group received DMSO only. After treatments, the cells were harvested and plated for colonogenic survival assay.
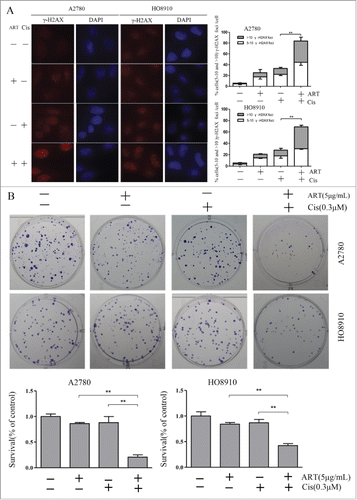
We next tested whether artesunate and cisplatin could act synergistically in inhibiting the clonogenic formation of ovarian cancer cells. As shown in , while cisplatin (0.3 μM) and artesunate (5 μg/ml) each had a very mild inhibitory effect on the colony-forming efficiency of ovarian cancer cells when applied alone, concomitant application of the 2 produced a significant synergistic effect that approaches synthetic lethality
Ectopic expression of RAD51 attenuates the increased chemosensitivity conferred by artesunate
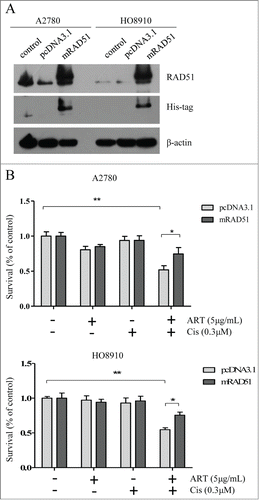
Because artesunate can potentially disrupt many biological processes in the cancer cells, it is possible that the chemosensitizing effect of artesunate may not be caused by the downregulation of RAD51. To demonstrate that the downregulation of RAD51 is primarily responsible for increased sensitivity to cisplatin, we generated A2780 and H8910 cell lines that overexpress RAD51 and subjected them to artesunate and/or cisplatin. The expression of exogenous RAD51 was confirmed by Western blot (). As shown in , ectopic expression of RAD51 rendered the ovarian cancer cells more resistant to the combined treatment when compared to control cells that express empty pcDNA3.1 vectors. This result suggests that the chemosensitizing effect of artesunate is at least partially mediated by the downregulation of RAD51
Figure 5. Ectopic expression of RAD51 attenuates the increased chemosensitivity conferred by artesunate. (A) Overexpression of mRAD51 in A2780 and HO8910 cell. Ovarian cancer cells were stably transfected with pcDNA3.1 or pcDNA3.1B-mRAD51 vector, and subjected to Western blot analysis of RAD51 protein level. (B) RAD51 overexpression attenuates the effect of combination artesunate with cisplatin. The results of 2 groups, which were transfected with pcDNA3.1 or pcDNA3.1B-mRAD51 treated by artesunate combination with cisplatin were compared (P < 0.05).
Artesunate in combination with cisplatin inhibits ovarian cancer xenograft tumor growth
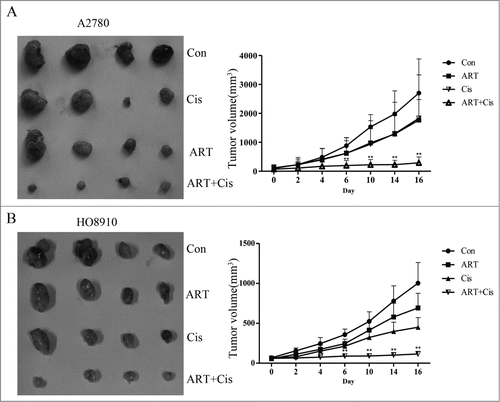
We next tested the effects of artesunate and cisplatin, administered alone or in combination, on the growth of ovarian cancer cells in vivo. A2780 and HO8910 cells, 5×106 cells/ in 0.2 ml PBS, were injected subcutaneously into the left inguinal area of the female nude mice. Tumor-bearing mice were randomly divided into 4 groups and were administered daily via i.p. injection of artesunate (50 mg/kg), cisplatin (2 mg/kg), or in combination of the 2 for 16 days. As shown in , tumor growth was significantly reduced in the group receiving combined treatment of artesunate and cisplatin (P < 0.01). In comparison, artesunate alone had no significant effect on the growth of tumor xenografts for both cell lines. Cisplatin alone appeared to have some inhibitory effect only on HO8910 cells, but not on A2780 cells. These results indicate that artesunate can render ovarian cancer cells sensitive to cisplatin in vivo
Figure 6. Artesunate sensitizes ovarian cancer xenografts to cisplatin Artesunate and cisplatin, administered alone or artesunate in combination, were applied to nude mice bearing A2780 (A) or HO8910 (B) tumor xenografts. Left: photograph of tumor xenografts isolated from nude mice. Right: growth curves of tumor xenografts in nude mice. The data points were the average ±SD in each group. Tumor growth was significantly reduced in the group receiving combined treatment of artesunate and cisplatin (**P < 0.01).
Discussion
We studied the antitumor effect of artesunate on ovarian cancer cells in vitro and in tumor xenografts. We showed that artesunate could indeed exert a significant cytotoxic effect on the cancer cells and could effectively induce oxidative stress and DSBs, which is consistent with previous reports on other types of cancer cells.Citation5,6 Importantly, we observed that artesunate could significantly downregulate RAD51 in 3 of 4 ovarian cancer cell lines tested. Consistent with the reduction in protein amount, formation of RAD51 foci that were induced by cisplatin was greatly impaired. Furthermore, a reporter assay that measures that function of HRR showed that HRR was significantly reduced in artesunate-treated cells. Moreover, the downregulation of RAD51 by artesunate conferred cancer cells an increased sensitivity to cisplatin. Correspondingly, artesunate and cisplatin appeared to act synergistically in inducing DSBs and in inhibiting the proliferation of ovarian cancer cells. Our results thus indicated that artesunate possesses the dual function of inducing DSBs and impairing DSB repair in ovarian cancer cells. Since the DNA damage induced by artesunate particularly requires HHR,Citation5 the downregulation of RAD51 would make the cells more vulnerable to artesunate. As a practical note, platinum-based drugs are most commonly used in ovarian cancer, yet the cancers almost invariably become platinum-resistant after initial response. Thus, with its relatively weak side effect, artesunate especially merits special consideration in the management of ovarian cancer.
In addition to the chemosensitizing effect we described here, artesunate has also been shown to be capable of radiosensitizing glioblastoma cells, reportedly mediated by downregulating survin.Citation14 A recent study showed that cervical cancer cells can also be sensitized by artesunate.Citation15 Because the repair of DSBs induced by ionizing radiation also requires HRR and RAD51,Citation12 it is conceivable that downregulation of RAD51 may have also contributed to the radiosensitizing effect of artesunate in those situations. Because RAD51 is frequently upregulated in malignancies and confers cancer cells with radioresistance,Citation12-16 this property of artesunate offers a new venue for attacking those cells
It should be pointed out that only 3 of the 4 ovarian cancer cell lines tested responded to artesunate treatment by downregulating RAD51, and the downregulation of RAD51 also appeared to be achieved by different mechanisms in different cell lines. This is not surprising considering that RAD51 is regulated at multiple levels, and many factors could lead to RAD51 dysregulation.Citation17-22 Future studies are needed to elucidate the detailed mechanisms whereby RAD51 is downregulated by artesunate
Materials and Methods
Cell culture and drug treatment
Human ovarian cancer cell lines HO8910 and SK-OV-3 were from Shanghai Cell Bank, Chinese Academy of Sciences. Human ovarian carcinoma cell line A2780 was obtained from Sigma-Aldrich. Immortalized fallopian epithelial cell line FTE-187 was as described.Citation23 Normal human fibroblasts (NHF) were generated in this lab.Citation24 A2780 and HO8910 cells were cultured in RPMI 1640, NHF in DMEM, and FTE-187 in M199, supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 100 mg/ml streptomycin. All the cells were incubated in a humidified atmosphere of 95% air and 5% CO2. Artesunate was acquired from Sigma-Aldrichand was dissolved in dimethyl sulfoxide (DMSO)(25 mg/ml) as a stock solution and stored at −20°C in aliquots until use. It was applied to the cultured cells at the concentration of 0, 5, 10, 25, or 50 µg/ml for various periods. Cisplatin, also purchased from Sigma-Aldrich, was dissolved in PBS.
Flow cytometric analysis of ROS
The ROS production following artesunate treatment was determined by membrane permeable CM-H2DCFDA. Briefly, cells were loaded with 5 μM of CM-H2DCFDA and incubated at 37°C for 20 min after treatment with artesunate. Cells were resuspended using preserving fluid and analyzed with a FACSCanto II (Becton Dickinson). The peak excitation wavelength for oxidized CM-H2DCFDA was 490 nm and emission was 530 nm.
Immunofluorescence staining of γ-H2AX and RAD51
Cells grown on coverslips in 6-well plates were treated with artesunate for 24 h before they were processed for determination of the level of γ-H2AX. For the immunofluorescence staining of RAD51, cells were pre-treated with 10 μg/ml artesunate for 24 h, and then 30 μM cisplatin for 4 h to induce RAD51 foci. Cells were fixed with 4% formaldehyde, followed by treatment with 0.2% Triton X-100 in PBS for 5 minutes, then blocked with 5% bovine serum albumin in PBS containing 0.3% Triton X-100 for 30 minutes. Mouse anti-γ-H2AX antibody was from Millipore (Billerica, MA)at a dilution of 1:600 in 5% bovine serum albumin in PBS. Rabbit anti-Rad51 antibody was from Calbiochem (San Diego, CA USA) at a dilution of 1:600. The specimens were incubated overnight at 4°C. Cells were then washed thrice in PBS before incubating in the dark with a Rhodamine-labeled secondary antibody for 60 minutes. After washing with PBS containing 0.3% Triton X-100 for 3 times, cells were then counterstained with 4,6-diamidino-2-phenylindole for 5 minutes. The coverslips were mounted to slides with an antifade solution. Slides were then examined under a fluorescence microscope. At least 500 nuclei were scored for nuclear foci in each of 3 experimental repeats.
Western blot analysis
After treatment with artesunate for 24 h, cells were harvested and lysed in 1×cell lysis buffer (P0013B, Beyotime). Total proteins of 15–25 μg were separated by SDS–PAGE and transferred to polyvinylidenedifluoride (PVDF) membranes. Membranes were blocked with 5% non-fat milk for 1-2 h at room temperature and then probed with primary antibodies and incubated at 4°C overnight. After extensive washing with TBS-T, membranes were incubated with appropriate HRP-conjugated secondary antibody for 1 h at room temperature, and then were detected by Western ECL− enhanced luminol reagent (Thermo scientific).Antibodies against the following proteins were as RAD51(Calbiochem, San Diego, CA USA), β-actin was from (Chemicon, Santa Cruz, CA), and His-tag (Cell signaling Technology, Danvers, MA USA).
Real-time PCR
Total cellular RNA was prepared using TRIzol reagent (Invitrogen). First-strand cDNA was synthesized from 1 μg of total RNA using reverse transcriptase (Thermo Scientific, Beijing, China). The expression levels of RAD51 mRNAs were determined by RT-PCR using SYBR GREEN mix (TOYOBO, Osaka, Japan) and a Light Cycler 480 system (Roche). Human GAPDH gene was used as an internal control. Primers were:
RAD51:5′-CAACCCATTTCACGGTTAGAGC-3′ (forward)
5′-GCTTTGGCTTCACTAATTCCCTT-3′ (reverse)
GAPDH:5′-CAGAACATCATCCCTGCCTCTAC-3′(forward)
5′-TTGAAGTCAGAGGAGACCACCTG-3′ (reverse)
The samples were loaded in quadruple, and the results of each sample were normalized to GAPDH.
Luciferase reporter assay
Cells were transfected with pGL3-basic reporter plasmids carrying a series of truncated RAD51 promoters using Lipofectamine 2000 (Invitrogen). Cells were incubated with/without artesunate (10 μg/ml) for 24 h and were then harvested for luciferase assay using the Dual-Luciferase Reporter Assay system (Promega) with a multilabel counter (Victor 1420; PerkinElmer). The firefly luciferase activity was normalized to Renilla luciferase activity of the pRL-TK reporter (co-transfected internal control). Transfections were performed in 3 independent experiments, and assayed in quadruplicates
Clonogenic survival assay
Ovarian cells were divided into 4 groups, the artesunate group (5 μg /ml for 24 h), the cisplatin group (0.3 μM for 24 h), the combination group (a sequential treatment of artesunate for 24 h and cisplatin for 24 h) and the control group (DMSO). Cells were seeded into 6 cm dishes. 10–12 days later, colonies were fixed with methanol and stained with 1.25% Giemsa for counting. Only those colonies containing at least 50 cells in size were counted
Expression vector of RAD51
The expression vector of pcDNA3.1B-mRAD5 was as described[12]. The mouse Rad51 cDNA was first amplified pCMV-SPORT6- mRAD51 by PCR and was then subcloned into a pcDNA3.1/myc-His B vector
HR reporter assay
Plasmids containing HR reporter cassette were kindly provided by Dr. Gorbunova.Citation13 The reporter cassette consists of 2 mutated copies of GFP. The first copy contains an artificial intron and a deletion of 22 nt and an insertion of 2 I-SceI recognition sites in inverted orientation in the first exon. The second copy contains the first exon but lacks a promoter and a start codon. Upon induction of DSBs by I-SceI, gene conversion events reconstitute a functional GFP gene. The plasmids were linearized by I-SceI restriction enzymes and purified using TIANGEN Universal DNA Purification Kit (Cat.# DP214-03). Exponentially growing A2780 and HO8910 cells were transfected with 2 μg of the HR reporter constructs, and 0.1 μg of pDsRed-N1 as the internal control. Then, cells were treated with artesunate for 24h, replaced with fresh medium, and sequentially cultured for 48h. Cells were harvested, resuspended in 0.4 ml of PBS, pH 7.4, and analyzed by FACS. The DNA repair efficiency expressed as GFP+/DsRed+ ratio, which was independent of the transfection conditions
Tumor xenograft model
Four to 6 weeks old female athymic nude mice (BALB/c, nu/nu) were purchased from Beijing Experimental Animal Center (Beijing, China). A2780 and HO8910 cells were harvested and resuspended in 0.1 ml of PBS, 5 × 106 cells/0.2 ml were injected subcutaneously into the left inguinal area of the mice. Two weeks later, mice bearing tumors (∼70 mm3 for A2780 and HO8910) were randomly divided into 4 groups. Artesunate was administered daily via i.p. injection at doses of 50 mg/kg alone or in combination with cisplatin (2 mg/kg) for 16 days. The tumor growth was monitored every other day. Tumor volume was determined by the formula 1/2a × b2 where a is the long diameter (mm) and b is the short diameter (mm).
Statistics
All values in the present study were reported as mean ±SD from at least 3 independent experiments and the Student's t test was used to evaluate the statistical significance of the result at the 95% confidence level. The synergistic effect of artesunate and cisplatin was analyzed by ANOVA. Significance levels were *, P < 0.05; **, P < 0.01.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author Contributions
Conception and design: B. Wang, C. Shao. Development of methodology: B. Wang, Q. Liu, Z. Liu. Acquisition of data: B. Wang, D. Hou, T. Wu. Analysis and interpretation of data: B. Wang, H. Guo, Y. Gong, C. Shao. Writing, review and/or revision of the manuscript: B. Wang, C. Shao. Administrative, technical, or material support: X. Zhang, Y. Zou, Z. Liu, J. Liu, J. Wei, Y. Gong, C. Shao.
Acknowledgments
We thank Dr. Gorbunova for kindly providing the HRR reporter plasmids.
Funding
This study was supported by National Basic Research Program of China (973 Program) grant (2011CB966200), National Natural Science Foundation Research Grants (81372241, 81171968 and 31371369), International Science & Technology Cooperation Program of China 2013DFG32700 and State Program of National Natural Science Foundation of China for Innovative Research Group (81321061).
Reference
- Kulasingam V, Pavlou MP, Diamandis EP. Integrating high-throughput technologies in the quest for effective biomarkers for ovarian cancer. Nat Rev Cancer 2010; 10:371-8; PMID:20383179; http://dx.doi.org/10.1038/nrc2831
- Cannistra SA. Cancer of the ovary. N Engl J Med 2004; 351:2519-2529; PMID:15590954; http://dx.doi.org/10.1056/NEJMra041842
- Efferth T, Sauerbrey A, Olbrich A, Gebhart E, Rauch P, Weber HO, Hengstler JG, Halatsch ME, Volm M, Tew KD, et al. Molecular modes of action of artesunate in tumor cell lines. Mol Pharmacol 2003; 64:382-94; PMID:12869643
- Hou J, Wang D, Zhang R, Wang H. Experimental therapy of hepatoma with artemisinin and its derivatives: in vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin Cancer Res 2008; 14:5519-30; PMID:18765544; http://dx.doi.org/10.1158/1078-0432.CCR-08-0197
- Li PC, Lam E, Roos WP, Zdzienicka MZ, Kaina B, Efferth T. Artesunate derived from traditional Chinese medicine induces DNA damage and repair. Cancer Res 2008; 68:4347-51; PMID:18519695; http://dx.doi.org/10.1158/0008-5472.CAN-07-2970
- Berdelle N, Nikolova T, Quiros S, Efferth T, Kaina B. Artesunate induces oxidative DNA damage, sustained DNA double-strand breaks, and the ATMATR damage response in cancer cells. Mol Cancer Ther 2011; 10:2224-33; PMID:21998290; http://dx.doi.org/10.1158/1535-7163.MCT-11-0534
- Lisewski AM, Quiros JP, Ng CL, Adikesavan AK, Miura K, Putluri N, Eastman RT, Scanfeld D, Regenbogen SJ, Altenhofen L, et al. Supergenomic network compression and the discovery of EXP1 as a glutathione transferase inhibited by artesunate. Cell 2014; 158:916-28; PMID:25126794; http://dx.doi.org/10.1016/j.cell.2014.07.011
- Hsu WH, Hsieh YS, Kuo HC, Teng CY, Huang HI, Wang CJ, Yang SF, Liou YS, Kuo WH. Berberine induces apoptosis in SW620 human colonic carcinoma cells through generation of reactive oxygen species and activation of JNKp38 MAPK and FasL. Arch Toxicol 2007; 81:719-28; PMID:17673978; http://dx.doi.org/10.1007/s00204-006-0169-y
- Meeran SM, Katiyar S, Katiyar SK. Berberine-induced apoptosis in human prostate cancer cells is initiated by reactive oxygen species generation. Toxicol Appl Pharmacol 2008; 229:33-43; PMID:18275980; http://dx.doi.org/10.1016/j.taap.2007.12.027
- Liu Z, Liu Q, Xu B, Wu J, Guo C, Zhu F, Yang Q, Gao G, Gong Y, Shao C. Berberine induces p53-dependent cell cycle arrest and apoptosis of human osteosarcoma cells by inflicting DNA damage. Mutat Res 2009; 662:75-83; PMID:19159633; http://dx.doi.org/10.1016/j.mrfmmm.2008.12.009
- Wang Y, Liu Q, Liu Z, Li B, Sun Z, Zhou H, Zhang X, Gong Y, Shao C. Berberine, a genotoxic alkaloid, induces ATM-Chk1 mediated G2 arrest in prostate cancer cells. Mutat Res 2012; 734:20-9; PMID:22561209; http://dx.doi.org/10.1016/j.mrfmmm.2012.04.005
- Liu Q, Jiang H, Liu Z, Wang Y, Zhao M, Hao C, Feng S, Guo H, Xu B, Yang Q, et al. Berberine Radiosensitizes Human Esophageal Cancer Cells by Downregulating Homologous Recombination Repair Protein RAD51. PLoS ONE 2011; 6:e23427; PMID:21858113; http://dx.doi.org/10.1371/journal.pone.0023427
- Mao Z, Seluanov A, Jiang Y, Gorbunova V. TRF2 is required for repair of nontelomeric DNA double-strand breaks by homologous recombination. Proc Natl Acad Sci USA 2007; 104:13068-73; PMID:17670947; http://dx.doi.org/10.1073/pnas.0702410104
- Reichert S, Reinboldt V, Hehlgans S, Efferth T, Rödel C, Rödel F. A radiosensitizing effect of artesunate in glioblastoma cells is associated with a diminished expression of the inhibitor of apoptosis protein survivin. Radiother Oncol 2012; 103:394-401; PMID:22560712; http://dx.doi.org/10.1016/j.radonc.2012.03.018
- Luo J, Zhu W, Tang Y, Cao H, Zhou Y, Ji R, Zhou X, Lu Z, Yang H, Zhang S, et al. Artemisinin derivative artesunate induces radiosensitivity in cervical cancer cells in vitro and in vivo. Radiat Oncol 2014; 9:84; PMID:24666614; http://dx.doi.org/10.1186/1748-717X-9-84
- Klein HL. The consequences of Rad51 overexpression for normal and tumor cells. DNA Repair (Amst) 2008; 7:686-93; PMID:18243065; http://dx.doi.org/10.1016/j.dnarep.2007.12.008
- Sørensen CS, Hansen LT, Dziegielewski J, Syljuåsen RG, Lundin C, Bartek J, Helleday T. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat Cell Biol 2005; 7:195-201; PMID:15665856
- Schild D, Wiese C. Overexpression of RAD51 suppresses recombination defects: a possible mechanism to reverse genomic instability. Nucleic Acids Res 2010; 38:1061-70; PMID:19942681; http://dx.doi.org/10.1093/nar/gkp1063
- Tichy ED, Pillai R, Deng L, Tischfield JA, Hexley P, Babcock GF, Stambrook PJ. The abundance of Rad51 protein in mouse embryonic stem cells is regulated at multiple levels. Stem Cell Res 2012; 9:124-34; PMID:22705496; http://dx.doi.org/10.1016/j.scr.2012.05.004
- Simandlova J, Zagelbaum J, Payne MJ, Chu WK, Shevelev I, Hanada K, Chatterjee S, Reid DA, Liu Y, Janscak P, et al. FBH1 helicase disrupts RAD51 filaments in vitro and modulates homologous recombination in mammalian cells. J Biol Chem 2013; 288:34168-80; PMID:24108124; http://dx.doi.org/10.1074/jbc.M113.484493
- Wei N, Shi Y, Truong LN, Fisch KM, Xu T, Gardiner E, Fu G, Hsu YS, Kishi S, Su AI, et al. Oxidative stress diverts tRNA synthetase to nucleus for protection against DNA damage. Mol Cell 2014; 56:323-32; PMID:25284223; http://dx.doi.org/10.1016/j.molcel.2014.09.006
- Huang JW, Wang Y, Dhillon KK, Calses P, Villegas E, Mitchell PS, Tewari M, Kemp CJ, Taniguchi T. Systematic screen identifies miRNAs that target RAD51 and RAD51D to enhance chemosensitivity. Mol Cancer Res. 2013; 11:1564-73; PMID:24088786
- Shan W, Mercado-Uribe I, Zhang J, Rosen D, Zhang S, Wei J, Liu J. Mucinous adenocarcinoma developed from human fallopian tube epithelial cells through defined genetic modifications. Cell Cycle 2012; 11:2107-13; PMID:22592533; http://dx.doi.org/10.4161/cc.20544
- Guo H, Liu Z, Xu B, Hu H, Wei Z, Liu Q, Zhang X, Ding X, Wang Y, Zhao M, et al. Chemokine receptor CXCR2 is transactivated by p53 and induces p38-mediated cellular senescence in response to DNA damage. Aging Cell 2013; 12:1110-21; PMID:23869868; http://dx.doi.org/10.1111/acel.12138
