Abstract
Multiple myeloma (MM) is a common and largely incurable blood cancer for which new treatment options are needed, as resistance to current modalities is an issue. Additionally, because this tumor type often resides in a hypoxic niche of the bone marrow, new therapeutics that remain effective even under hypoxic conditions are sought. Because of the secretory nature of MM cells they are uniquely under proteotoxic stress, and we hypothesized that these tumor cells may alleviate this stress by upregulating the major stress-induced cytosolic form of the chaperone HSP70. In this work we test the efficacy of the HSP70 inhibitor PET-16 for MM. We show that MM cell lines express significant levels of HSP70, and further that inhibition of HSP70 causes decreased viability and apoptosis, along with proteotoxic stress, as assessed by the accumulation of poly-ubiquitylated proteins. Importantly, we show that growth of these tumor cells under hypoxic conditions has no effect on the ability of PET-16 to be cytotoxic. The HSP70 inhibitor PET-16 should thus be considered further for pre-clinical analyses of efficacy in MM.
Abbreviations
| 2-PES | = | 2-Phenylethynesulfonamide |
| 7-AAD | = | 7-Aminoactinomycin D |
| ADP | = | Adenosine Diphosphate |
| ATP | = | Adenosine Triphosphate |
| CHIP | = | C-terminus of Hsc70 Interacting Protein |
| Hsc70 | = | Heat Shock Cognate Protein 70 |
| HSP70 | = | Heat Shock Protein 70 |
| MM | = | Multiple Myeloma |
| PET-16 | = | Triphenyl(phenylethynyl)phosphonium bromide. |
Introduction
Multiple Myeloma (MM) is a hematological cancer that develops in the bone marrow and is formed by the expansion of malignant plasma cells.Citation1 MM is the second most common cancer, and currently represents 1.6% of all new cancer cases.Citation2 The cure rates for this cancer are extremely low, so there is urgent need for new therapeutic approaches for this tumor type. Additionally, MM normally exists under conditions of hypoxia in the body, thus limiting the therapeutic efficacy of many anti-cancer drugs.Citation3 One of the characteristics of MM is that it over-produces monoclonal immunoglobulin (M protein), and hence is believed to be under a great deal of proteotoxic stress.Citation1 To circumvent death by proteotoxic stress, many human tumors induce high levels of the cytosolic, stress-inducible chaperone 70 kDa heat shock protein (HSP70). To prevent protein overload and proteotoxic stress induced by over-production of M protein, MM cells also typically induce the expression of HSP70.Citation4 Maintaining properly functioning HSP70 protein is believed to be crucial for the survival of MM.Citation4,5
The mammalian HSP70 family consists of at least 8 members that are located in various cellular organelles.Citation6 The constitutive form HSC70 is ubiquitously expressed in all cells, whereas the major cytosolic, stress-inducible form is known as HSP70 (also known as HSP72, HSP70-1 or HSPA1A). Stress-induced HSP70 and its co-chaperones are responsible for unfolding misfolded proteins, and in some cases for targeting proteins for degradation via association with the E3 ubiquitin ligase C-terminus of Hsc70 Interacting Protein (CHIP).Citation7 Consistent with the pro-survival role of HSP70, this protein is up-regulated in several cancers, including MM,Citation8-10 but it is not readily detectable in normal tissues. Further, silencing or inhibition of this protein has been found to be cytotoxic for tumor cells, but not normal cells.Citation6 These factors augur well for the potential use of HSP70 inhibitors for MM.
Previously, the compound 2-phenylethynesulfonamide (PES) was described as an HSP70 inhibitor.Citation11 This compound was demonstrated to show cytotoxicity in multiple different tumor cell lines, but not normal, non-transformed cells. Additionally, this compound showed efficacy in a pre-clinical model of Burkitt's lymphoma.Citation11 Subsequent structure-activity-relationship studies revealed a superior derivative of PES called Triphenyl(phenylethynyl)phosphonium (PET-16).Citation12 This compound specifically binds and inhibits HSP70, and was co-crystallized with the bacterial ortholog of HSP70; this analysis revealed that this compound has a unique mechanism of action: by binding to an allosteric pocket of the substrate-binding domain, PET-16 inhibits the ability of HSP70 to cycle between ATP-bound and ADP-bound states.Citation12 However, the pre-clinical efficacy of PET-16 has not been really explored, particularly in MM, where compounds that exacerbate proteotoxic stress might be particularly effective. Further, whether PET-16 causes the accumulation of poly-ubiquitylated proteins, a marker for proteotoxic stress, in MM is not clear. In this study we demonstrate that PET-16 has anti-cancer activity in MM. Further, we show that hypoxic conditions (1% oxygen) have no effect on the efficacy of PET-16 for MM. Taken together, these data suggest that targeting HSP70 with PET-16 could be a potential and viable target for treatment of MM.
Results
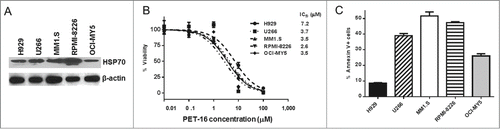
Before testing the efficacy of the HSP70 inhibitor PET-16 on MM cell lines, we first sought to determine whether there was heterogeneity in the level of HSP70 in these cells. To address this question we performed western blot analysis using an antibody specific for the cytosolic stress-induced form of this protein. The steady state levels of HSP70 in 5 different MM cell lines was uniformly high, and showed some variation, with the levels highest in RPMI-8226 cells (). We next opted to determine the IC50 values for PET-16 in MM cell lines. Toward this end, cell viability using Alamar Blue assay was evaluated following 72h treatment of these cells with different doses of compound. All of the MM lines showed cytotoxicity in the presence of PET-16, and the line with the highest steady-state level of HSP70 showed the lowest IC50. In general, the IC50 values were in the low micromolar range, suggesting that this tumor type is generally sensitive to this compound, as the IC50 in normal cells is over 100 µM ().Citation18 To determine whether HSP70 inhibition caused MM cell death, all MM cell lines were treated with a single dose of approximately the IC50 (3 µM) for 24 hours and apoptosis was analyzed by Annexin V/7-AAD staining using flow cytometry. Our data indicated that there was profound apoptosis in treated cells, with up to 50% of cells from the MM1.S and RPMI-8226 lines undergoing apoptosis by 24 hours (). In contrast, the cell line H929, which has lower steady state levels of HSP70 and the highest IC50 of all 5 lines, underwent modest apoptosis after 24 hours (10%, ).
Figure 1. PET-16 is cytotoxic to MM cells. (A) MM cell lines were collected and expression of HSP70 protein was determined by Western blotting. Protein loading was confirmed by re-probing the membrane with antibody against β-actin. (B) MM cells were cultured in the presence of indicated concentrations of PET-16 for 72h. Cell viability was measured in triplicate using Resazurin (alamar blue) dye. Error bars mark standard deviations.(C) MM cells were treated PET-16 (3 μM) for 24h. Cells were then collected and apoptosis was determined by Annexin V binding assay. The combined results of several experiments are shown. Results are presented as mean ± SD.
We next sought to determine whether PET-16 treatment causes increased proteotoxic stress of MM cells. Additionally, because HSP70 is a key player in many different cancer-relevant signaling pathways, we sought to determine whether there was any correlation between the ability of PET-16 to kill MM cells, and the ability of this compound to induce proteotoxic stress. Toward this end, MM cell lines were treated with 3 µM PET-16 for 24 hours. We then fractionated cell extracts for the detergent-soluble and insoluble fractions (see Materials and Methods) and assessed the level of poly-ubiquitylated proteins that accumulate in each fraction. Western blot analysis revealed that there was a significant accumulation of poly-ubiquitylated proteins in MM cell lines after 24h treatment with PET-16. However, there appeared to be no correlation between the ability of this compound to induce MM cell death, and the accumulation of poly-ubiquitylated proteins. For example, the H929 cell line, which was the most resistant to apoptosis and had the highest IC50, accumulated a significant increase in insoluble poly-ubiquitylated proteins. These data indicate that, whereas PET-16 does induce proteotoxic stress in MM, cytotoxicity by HSP70 inhibition may not occur solely due to the accumulated proteotoxic stress, and may occur due to other mechanisms.
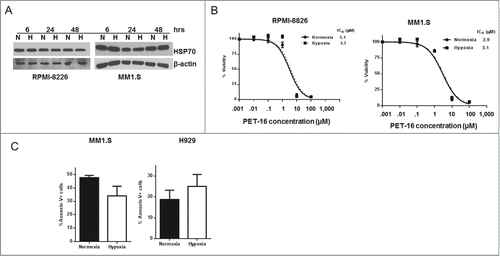
Resistance of MM to chemotherapy remains a persistent obstacle for the treatment of this disease. One of the underlying reasons for the MM chemoresistance is that these tumors exist in hypoxia microenvironment of the bone marrow, and the hypoxia limits the efficacy of some commonly used therapeutic agents, for example such as melphalan.Citation13,14 Therefore, in order to validate HSP70 inhibitors like PET-16 for MM therapy, it became important to assess their efficacy under hypoxic conditions. To address this premise, we first analyzed steady-state HSP70 levels in the MM lines RPMI-8226 and MM1.S under normal and hypoxic conditions. We did not observe marked changes in the level of HSP70 in MM1.S and RPMI-8226 MM cells cultured in normoxic and hypoxic conditions (); this may indicate that the level of this stress-induced protein is already maximally induced by the existent proteotoxic stress. Notably, the IC50 value for PET-16 was indistinguishable in normal and hypoxic conditions, indicating that hypoxia does not inhibit the efficacy of this compound (). We next sought to assess apoptosis in the cell lines MM1.S, which is sensitive to PET-16, and in H929, which is the most resistant line. Annexin V binding assay indicated that programmed cell death induced by PET-16 was not inhibited by hypoxia (). These data support the premise that, unlike the case for melphalan, a hypoxic microenvironment is unlikely to inhibit cell death by PET-16.
Figure 2. PET-16 induces proteotoxic stress in MM cells. MM cells were treated with PET-16 (3 μM) for 24h. Cells were then collected and protein lysates were prepared. Detergent soluble and insoluble fractions were resolved on a 10% acrylamide gel. Ubiquitinated protein levels were detected by Western blotting. The results depicted are representative of multiple independent experiments.
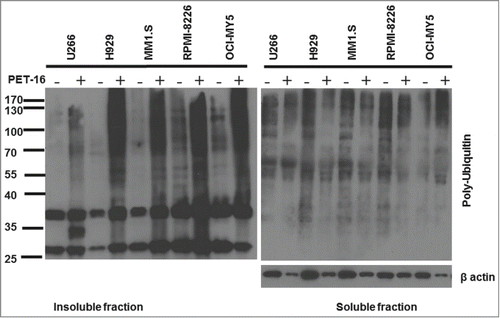
Figure 3. No effect of hypoxia on cytotoxicity by PET-16. (A) MM cells were kept in normoxia (N) or hypoxia (H) for the indicated period of time. Cells were then collected and the level of HSP70 expression was determined by Western blotting. Equal loading was confirmed by re-probing the membrane with antibody against β-actin. The results depicted are representative of multiple independent experiments. (B) MM cells were cultured in the presence of indicated concentrations of PET-16 in normoxia or hypoxia for 72h. Cell viability was measured in triplicate using Resazurin (alamar blue) dye. Error bars mark standard deviations. (C) MM cells were treated with PET-16 (3 μM) for 24 hrs, then collected and apoptosis was determined using Annexin V binding assay. Combined results from 3 independent experiments are shown. Data are presented as mean ± SD.
Discussion
The objective of this study was to perform an initial proof of principal analysis of the efficacy of an HSP70 inhibitor in MM. Whereas other studies have also investigated HSP70 inhibitors for the therapy of MM, these other studies utilized compounds that inhibited several members of the HSP70 family, some of which are essential for the viability of normal cells, like Hsc70.Citation15 In contrast, PET-16 binds to an allosterically regulated domain of HSP70 that is specific for the major, cytosolic stress-induced form of this protein, and treatment with this compound is not associated with normal cell cytotoxicity.Citation12 Our combined data support the further investigational use of this compound, and other HSP70 inhibitors, for this tumor type.
Our data do not support a correlation between the efficacy of PET-16 and the level of proteotoxic stress in the tumor cell lines analyzed. For example, the MM line H929 has been shown to be a high secreter of IgGCitation15; similarly, the line MM1.S has been shown to be characterized by low proteasome activity (high proteotoxic stress), and hence was more susceptible to proteasome inhibitors.Citation16 PET-16 was able to cause accumulation of poly-ubiquitylated proteins, yet there was no correlation between the level of proteotoxic stress with either the IC50 or the level of apoptosis induced. These data suggest 2 things: the first is that HSP70 inhibitors are likely cytotoxic by multiple different pathways, only one of which is proteotoxic stress. The other is that HSP70 inhibitors may impact other pathways in the cell, in addition to apoptosis. One possibility is that these inhibitors may be cytostatic; in support of this premise, treatment of human lung cancer cell lines with the HSP70 inhibitor VER-155008 decreased proliferation by inducing cell cycle arrest in the G0/G1 phase.Citation17 Additionally, the PES derivative PES-Cl was found to inhibit the activity of the Anaphase-Promoting-Complex, and to induce G2/M arrest.Citation18 It will be of interest to determine whether treatment of MM with PET-16 produces the similar effects in cell cycle arrest and proliferation as other HSP70 inhibitors.
One of the functions of HSP70 is to target misfolded proteins for degradation via the ubiquitin-proteasome pathway.Citation4 HSP70 regulates the ubiquitin-proteasome pathway by interacting with its co-chaperone CHIP, thereby targeting proteins for degradation via the 26S proteasome.Citation7 Also, HSP70 maintains protein solubility by binding to the hydrophobic regions of proteins.Citation6 Blocking of this pathway would result in increased ubiquitinated proteins in the detergent insoluble fraction. As expected, our data show that PET-16 induces proteotoxic stress in MM. This observation is evident by an increase in ubiquitinated protein levels in PET-16 treated cells in the insoluble fraction (). Bianchi and colleaguesCitation16 reported that proteasome protein levels and function correlates with cytotoxicity to the proteasome inhibitors bortezomib and carfilzomib. These data prompted us to test the premise that the HSP70 inhibitor PET-16 might synergize with proteasome inhibitors. However, we were unable to find evidence that this was the case (Bailey et al, unpublished data), suggesting that combination therapy with proteasome inhibitors may not be efficacious. However, resistance to proteasome inhibition is a growing issue in MM, and it remains to be determined whether PET-16 will be efficacious in that scenario.
Materials and Methods
Cell culture and reagents
U266, H929, MM1.S, and RPMI-8226 cells were obtained from ATCC and cultured in RPMI-1640 medium supplemented with 1% antibiotic-antimycotic and 10% fetal bovine serum (Life technologies, catalog #10-040-CM). OCI-MY5 cells were kindly provided by Dr. Roger Tiedemann (University of Toronto, Canada) and were cultured in IMDM (Life technologies, catalog# 31980-080) supplemented with 1% antibiotic-antimycotic (Life Technologies, # 15240-062) and 10% fetal bovine serum (Life Technologies, catalog#10082147). MM cells were incubated at 37°C in normoxia (21% oxygen) or hypoxia (1% oxygen). Hypoxia was created using the I-Glove hypoxia system chamber (BioSpherix). Resazurin dye was purchased from Sigma (#R7017-15G) and prepared as 0.5 μM stock solution in water. PET-16 (Phenylethyltrimethyl phosphonium, (Sigma, catalog #S16773) was prepared as 50 mM stock solution in DMSO and stored at −80°C.
Cell viability assay
MM cells (30,000 cells per well) were plated in quadruplicate in 96-well plates and treated with various concentrations of PET-16 for 72 hours. Resazurin dye was then added (10 μL per well) for 4 hours followed by detection of fluorescence using 530/590 nm on Synergy HT Multi-Detection microplate reader (BioTek). The results were calculated as the ratio between fluorescence of treated cells and untreated cells and were presented as % DMSO treated control ±SD.
Flow cytometry
MM cells were treated with 3 μM PET-16 for 24 hours. Cells were then collected, washed twice with ice cold PBS, once with binding buffer followed by addition of Annexin V-PE (BD PharMingen, catalog #556421) and 7-AAD (BD PharMingen, catalog #559925). Analysis was performed using the Guava EasyCyte HT Benchtop Flow Cytometer (EMD Millipore), and data were analyzed using Guava Software (EMD Millipore).
Western blotting
MM cells were harvested and lysed in RIPA buffer (50 mM Tris-HCl pH 8, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) supplemented with protease inhibitor cocktail (Sigma, catalog # P8340). Protein lysates (10-20 µg) were resolved on 10% SDS-PAGE and then transferred to nitrocellulose membrane (0.45μm, Bio Rad, catalog # 162-0115). Membranes were blocked with 5% non-fat milk and incubated with primary antibodies for overnight at 4ºC followed by incubation with secondary antibodies for 2 hours at room temperature. Membranes were developed using Amersham ECL Western Blotting reagent (GE Health Care Life Sciences catalog # RPN 2106,) or Super Signal West Femto Maximum sensitivity substrate (Thermo Scientific, catalog# 34095). The following primary antibodies were used: anti-Ubiquitin (Cell Signaling, catalog #3933), anti-HSP70 (Cell Signaling, catalog #4873), and anti-β-actin (Santa Cruz Biotechnologies, catalog #sc-1616).
Protein fractionation
MM cells were plated in a 6 well plate at a density of 1 × 106cells/mL and treated with 3 μM PET-16 for 24 hours. Cells were then counted using a hematocytometer, and equal numbers of cells were centrifuged at 4°C and lysed in equal volume of Tris/NP40/Triton X buffer (50 mM Tris-HCL, pH 7, 150 mM NaCl, 2 mM EDTA, 0.5% NP40 and 0.5% Triton X-100). The lysates were incubated for 1 hour on ice and centrifuged at 14,000 rpm for 20 minutes at 4°C. The supernatant (soluble fraction) was transferred to a fresh tube. The detergent insoluble pellet was then re-suspended in 30 μl Tris/NP-40/Triton-X buffer. Equal volumes of the lysates were then denatured in the presence of Laemmli buffer and resolved on 10% SDS-PAGE.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 software (GraphPad Software, Inc., La Jolla, CA). Differences between groups were calculated using a 2-tailed paired Student t test. A statistically significant difference was determined at p< 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
CB was supported by T32 CA09171. The research was supported by funding from the NIH (CA139319 to MM).
References
- Davenport EL, Moore HE, Dunlop AS, Sharp SY, Workman P, Morgan GJ, Davies FE. Heat shock protein inhibition is associated with activation of the unfolded protein response pathway in myeloma plasma cells. Blood 2007; 110:2641-9; PMID:17525289; http://dx.doi.org/10.1182/blood-2006-11-053728
- Howlader N, Noone A, Krapcho M, Garshell J, Miller D, Altekruse S, Kosary C, Yu M, Ruhl J, Tatalovich Z, et al. SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Bethesda, MD.
- Asosingh K, De Raeve H, de Ridder M, Storme G, Willems A, Van Riet I, Van Camp B, Vanderkerken K. Role of the hypoxic bone marrow microenvironment in 5T2MM murine myeloma tumor progression. Haematologica 2005; 90:810-7; PMID:15951294
- Zhang L, Fok JJL, Mirabella F, Aronson LI, Fryer RA, Workman P, Morgan GJ, Davies FE. Hsp70 inhibition induces myeloma cell death via the intracellular accumulation of immunoglobulin and the generation of proteotoxic stress. Cancer Lett 2013; 339:49-59; PMID:23887058; http://dx.doi.org/10.1016/j.canlet.2013.07.023
- Zhang L, Fok JHL, Davies FE. Heat shock proteins in multiple myeloma. Oncotarget 2014; 5:1132-48; PMID:24675290
- Murphy M. The HSP70 family and cancer. Carcinogenesis 2013; 34:1181-8; PMID:23563090; http://dx.doi.org/10.1093/carcin/bgt111
- Jiang J, Ballinger CA, Wu Y, Dai Q, Cyr DM, Höhfeld J, Patterson C. CHIP Is a U-box-dependent E3 Ubiquitin Ligase: Identification of Hsc70 as a target for ubiquitylation. J Biol Chem 2001; 276:42938-44; PMID:11557750; http://dx.doi.org/10.1074/jbc.M101968200
- Chatterjee M, Andrulis M, Stühmer T, Müller E, Hofmann C, Steinbrunn T, Heimberger T, Schraud H, Kressmann S, Einsele H, et al. The PI3K/Akt signaling pathway regulates the expression of Hsp70, which critically contributes to Hsp90-chaperone function and tumor cell survival in multiple myeloma. Haematologica 2013; 98:1132-41; PMID:23065523; http://dx.doi.org/10.3324/haematol.2012.066175
- Braga WMT, Zanatta DB, de Carvalho F, de Lourdes Chauffaille M, Strauss BE, Colleoni GWB. Functional studies of Hsp70 gene expression in multiple myeloma cell lines suggest that this gene inhibition can be a potential therapeutic target, independent of 17p/p53 Status. Blood 2013; 122:3771-
- Schmitt E, Maingret L, Puig PE, Rerole AL, Ghiringhelli F, Hammann A, Solary E, Kroemer G, Garrido C. Heat shock protein 70 neutralization exerts potent antitumor effects in animal models of colon cancer and melanoma. Cancer Res 2006; 66:4191-7; PMID:16618741; http://dx.doi.org/10.1158/0008-5472.CAN-05-3778
- Leu JIJ, Pimkina J, Frank A, Murphy ME, George DL. A small molecule inhibitor of inducible heat shock protein 70. Mol Cell 2009; 36:15-27; PMID:19818706; http://dx.doi.org/10.1016/j.molcel.2009.09.023
- Leu JIJ, Zhang P, Murphy ME, Marmorstein R, George DL. Structural basis for the inhibition of HSP70 and DnaK chaperones by small-molecule targeting of a C-Terminal allosteric pocket. ACS Chem Biol 2014; 9:2508-16; PMID:25148104; http://dx.doi.org/10.1021/cb500236y
- Shin D, Choi Y, Park J. SIRT1 and AMPK mediate hypoxia-induced resistance of non-small cell lung cancers to cisplatin and doxorubicin. Cancer Res 2014 74:298-308; PMID:24240701; http://dx.doi.org/10.1158/0008-5472.CAN-13-2620
- Maiso P, Huynh D, Moschetta M, Sacco A, Aljawai Y, Mishima Y, Asara J, Roccaro A, Kimmelman A, Ghobrial I. Metabolic signature identifies novel targets for drug resistance in multiple myeloma. Cancer Res 2015; 75:2071-82; PMID:25769724; http://dx.doi.org/10.1158/0008-5472.CAN-14-3400
- Braunstein MJ, Scott SS, Scott CM, Behrman S, Walter P, Wipf P, Coplan JD, Chrico W, Joseph D, Brodsky JL, et al. Antimyeloma effects of the heat shock protein 70 molecular chaperone inhibitor MAL3-101. J Oncol 2011; 2011:232037; PMID:21977030; http://dx.doi.org/10.1155/2011/232037
- Bianchi G, Oliva L, Cascio P, Pengo N, Fontana F, Cerruti F, Orsi A, Pasqualetto E, Mezghrani A, Calbi V, et al. The proteasome load versus capacity balance determines apoptotic sensitivity of multiple myeloma cells to proteasome inhibition. Blood 2009; 113:3040-9; PMID:19164601; http://dx.doi.org/10.1182/blood-2008-08-172734
- Wen W, Liu W, Shao Y, Chen L. VER-155008, a small molecule inhibitor of HSP70 with potent anti-cancer activity on lung cancer cell lines. Exp Biol Med 2014; 239:638-45; http://dx.doi.org/10.1177/1535370214527899
- Balaburski G, Leu J, Beeharry N, Hayik S, Andrake M, Zhang G, Herlyn M, Villanueva J, Dunbrack RJ, Yen T, et al. A modified HSP70 inhibitor shows broad activity as an anticancer agent. Mol Cancer Res 2013; 11:219-29; PMID:23303345; http://dx.doi.org/10.1158/1541-7786.MCR-12-0547-T
