Abstract
To develop a potent cancer vaccine, it is important to study how to prepare highly immunogenic antigens and to identify the most appropriate adjuvants for the antigens. Here we show that a tumor lysate works as an effective antigen to prime CD4+ T-cell help when baculovirus is employed as an adjuvant. When immunized intradermally with the combination (BLP) of baculovirus, a CT26 tumor lysate, and a cytotoxic T-cell epitope peptide before a tumor challenge, 60% of mice rejected tumors. In contrast, all mice vaccinated with baculovirus plus a tumor lysate (BL) developed tumors. In addition, flow cytometry showed that tumor-specific, interferon γ-producing CD8+ cytotoxic T lymphocytes (CTLs) were robustly activated by intradermal immunization with BLP. When BLP was administered therapeutically to tumor-bearing mice, antitumor efficacy was better compared to BL. The established tumor was completely eradicated in 50–60% of BLP-treated mice, and induction of tumor-specific CTLs was observed, suggesting that the antitumor efficacy of BLP is mediated by CD8+ T cells. Numerous CD4+ T cells infiltrated the tumors of BLP-treated mice, whereas the antitumor effect of BLP almost disappeared after removal of the tumor lysate from BLP or after depletion of BLP-immunized mice of CD4+ T cells. Thus, the combination of a peptide, lysate, and baculovirus provides stronger antitumor immunity than does a peptide plus baculovirus or a lysate plus baculovirus; effectiveness of BLP is determined by functioning of CD4+ T cells stimulated with a tumor lysate.
Abbreviations
| ANOVA | = | analysis of variance |
| BL | = | a vaccine consisting of baculovirus and a tumor cell lysate |
| BLP | = | a vaccine consisting of baculovirus, a tumor cell lysate, and a CTL epitope peptide |
| BP | = | a vaccine consisting of baculovirus and a CTL epitope peptide |
| CTL | = | CD8+ cytotoxic T lymphocyte |
| DCs | = | dendritic cells |
| FITC | = | fluorescein isothiocyanate |
| IFA | = | incomplete Freund's adjuvant |
| IFNγ | = | interferon gamma |
| IL | = | interleukin |
| mAb | = | monoclonal antibody |
| PBS | = | phosphate-buffered saline |
| PE | = | phycoerythrin |
| pfu | = | plaque-forming units |
| SD | = | standard deviation |
| TLR | = | toll-like receptor |
| Treg | = | regulatory T. |
Introduction
Since the tumor-associated antigen recognized by T cells was first identified in 1991,Citation1 many tumor-associated antigens have been identified, and some of them are used clinically as peptide-based vaccines for the immunotherapy of cancer.Citation2-5 These vaccine strategies focus primarily on effective elicitation of CD8+ cytotoxic T lymphocytes (CTLs), which can kill tumor cells efficiently. When developing cancer vaccines based on a CTL epitope peptide, the peptide has to be combined with an appropriate adjuvant to enhance the immunogenicity and evoke antitumor immunity more efficiently.Citation6
CpG oligodeoxynucleotides, which are a Toll-like receptor (TLR)-9 ligands, are being widely tested as a potent adjuvant for peptide- and protein-based cancer vaccines that stimulate cancer-reactive CTLs, and the effectiveness of a CpG oligodeoxynucleotide appears to increase when it is emulsified with incomplete Freund's adjuvant (IFA).Citation7-10 This effect is considered to be due to the properties of IFA, such as slow antigen release and recruitment of antigen-presenting cells at the vaccination site.
A great deal of effort has been devoted to the development of cancer vaccines, but clinical benefits obtained in vaccine trials have been modest so far.Citation11 It was reported that tumor progression occurs despite increased numbers of circulating tumor antigen-specific CTLs in cancer patients that received peptide vaccines emulsified with IFA.Citation12 These data suggest that the induction of tumor antigen-specific CTLs by immunization with cancer vaccines may not necessarily correlate with antitumor efficacy of the vaccines. Recently, one possible mechanism behind this paradox was reported; CTLs induced by subcutaneous vaccination with a peptide-based vaccine emulsified with IFA are recruited to the vaccination site but do not accumulate within the tumor. This state of affairs results in poor cancer regression.Citation13,14 This finding is suggestive of the necessity to develop novel vaccine modalities not involving IFA for more efficient cancer immunotherapy because almost all of the existing cancer vaccines are IFA-emulsified formulations.
In a previous study, we demonstrated that a wild-type baculovirus (Autographa californica multiple nuclear polyhedrosis virus) possesses an adjuvant effect, and antitumor efficacy is enhanced by intradermal vaccination with a combination of the baculovirus and a tumor cell lysate.Citation15 This vaccine is a saline-based formulation without IFA. In addition, the use of an autologous tumor lysate as a vaccine antigen is expected to be effective against tumor recurrence because the tumor lysate may contain all the relevant epitopes that can stimulate CD4+ helper T cells and CD8+ T cells. In contrast, CTL epitope peptide-based vaccines cannot be expected to stimulate CD4+ T cell functions when priming antitumor immune responses. There is, however, one important concern about the immunoinductivity of tumor lysate vaccines; if the expression level of a tumor-associated antigen on the tumor cells is low, then the lysate derived from such a tumor tissue may not become an effective vaccine antigen because of its weak immunogenicity. To overcome this possible problem, we theorized that a tumor lysate will become highly immunogenic if an appropriate CTL epitope peptide is added, a vaccine using these antigens should evoke a stronger immune response against tumor cells, compared to a peptide or a tumor lysate alone.
In the present study, we hypothesized that a CTL epitope peptide combined with a tumor lysate and baculovirus will be a potent anticancer vaccine. Therefore, we tested whether this saline-based combination vaccine induces enhanced antitumor immunity in a mouse model.
Results
Intradermal immunization with the combination of the peptide, lysate, and baculovirus enhances prophylactic antitumor immunity
To assess the efficacy of prophylactic immunization with BLP, mice were vaccinated intradermally with BLP on days 0, 7, and 14, and then CT 26 tumor cells (4 × 105) were transplanted s.c. on day 21 (). As controls, intradermal (i.d.) inoculation with PBS, the baculovirus alone, the lysate alone, or BL was also performed using the same experimental schedule (). As shown in , 60% of mice receiving BLP did not develop tumors. In contrast, tumorigenesis was observed in all of the mice receiving PBS, lysate alone, baculovirus alone, and BL. As compared with the PBS-treated control group, the antitumor efficacy observed in the groups treated with BLP or BL was statistically significant (p = 0.019 and 0.019, respectively), whereas that in the groups treated with lysate alone or baculovirus alone was not significant (p = 0.073 and 0.237, respectively). Because 40% of mice treated with BLP did not experience a slow tumor growth, the antitumor effect of BLP treatment was not statistically significant as compared with that of the other 3 vaccines. However, treatment with BLP tended to be more effective than that with BL compared to treatment with lysate alone (p = 0.087 vs. 0.954) or baculovirus alone (p = 0.051 vs. 0.035, ). Next, we tested whether the i.d. immunization with BLP elicits tumor-specific CTLs. Seven days after the third prophylactic immunization with the various vaccine formulations, all the mice were euthanized and their splenocytes were harvested. When the cells were stimulated in vitro with the AH1 peptide, the number of IFNγ-producing CD8+ T cells strongly increased in the group treated with BLP (). In contrast, no induction of such CD8+ T cells was seen in the other 4 groups (; p = 0.000132, BLP-treated group vs. the other 4 groups). Thus, immunization with BLP effectively induced tumor-specific CTLs, so that the tumor growth was strongly inhibited. These results indicated that the combination of the CTL epitope peptide with the tumor lysate and baculovirus substantially enhanced antitumor immune responses, compared to the combination of the tumor lysate and baculovirus.
Figure 1. Vaccination with the combination of a peptide, tumor lysate, and baculovirus induces prophylactic tumor resistance. (A) Mice were immunized intradermally with PBS, a tumor lysate alone, baculovirus alone, BL, or BLP once a week for 3 consecutive weeks (n = 5 per group). One week after the final vaccination procedure, CT26 cells (4 × 105) were transplanted subcutaneously. (B) Tumor size measurements. Each line represents an individual mouse. (C) Survival curve. Similar results were obtained in 3 independent experiments.
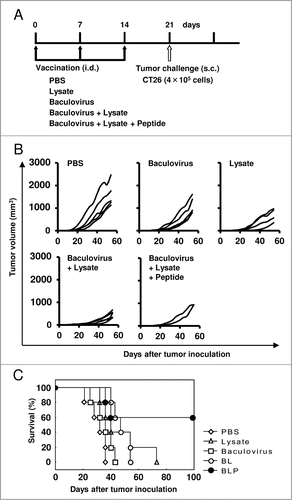
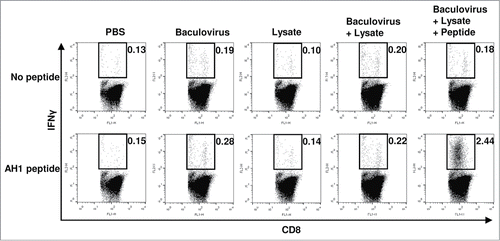
Figure 2. Assessment of CD8+ T cell responses by intracellular staining for cytokines. Seven days after the final immunization procedure with various vaccines indicated (n = 5 per group), splenocytes were collected and cultured with or without the AH1 peptide for 6 h to stimulate peptide-specific CD8+ T cells, and the production of IFNγ by T cells was measured using flow cytometry. Plots are gated on CD8+ cells and representative of data obtained from 5 animals per group. Numbers above each fluorescence-activated cell sorter (FACS) plot are the percentages of IFNγ-producing CD8+ T cells. Similar results were obtained in 2 independent experiments.
Intradermal treatment with BLP generates therapeutic antitumor immunity
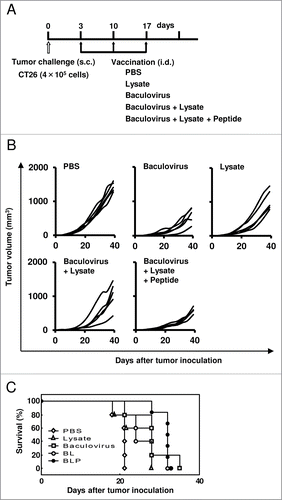
Mice were inoculated s.c. with CT 26 tumor cells (4 × 105) on day 0, so that palpable tumors (1- to 2-mm diameter) were observed in all of the mice inoculated on day 3 after the tumor challenge. To test whether the therapeutic antitumor effect is caused by i.d. vaccination with BLP, these mice were then injected intradermally with various vaccine formulations at a 1- to 2-mm distance from the established tumors on days 3, 10, and 17 (). As depicted in , the established tumors grew more slowly in the group treated with BLP compared to the group treated with PBS (p = 0.009), lysate alone (p = 0.013), or BL (p = 0.049). Little difference in antitumor efficacy between the 2 groups treated with BLP and baculovirus alone was seen, but the treatment with baculovirus alone cannot be expected to induce prophylactic antitumor effect and tumor antigen-specific CTLs, unlike the treatment with BLP (). Based on these combined results, we evaluated BLP formulation as a promising vaccine.
Figure 3. Vaccination with the combination of a peptide, tumor lysate, and baculovirus induces therapeutic antitumor responses. (A) Mice were inoculated subcutaneously with CT26 tumor cells (4 × 105) on day 0, and then treated intradermally with PBS, the tumor lysate alone, baculovirus alone, BL, or BLP at a 1- to 2-mm distance from the established tumors on days 3, 10, and 17 (n = 5 per group). (B) Tumor size measurements. Each line represents an individual mouse. (C) Survival curve. Similar results were obtained in 3 independent experiments.
Intradermal treatment with BLP augments the number of tumor-infiltrating CD4+ T cells
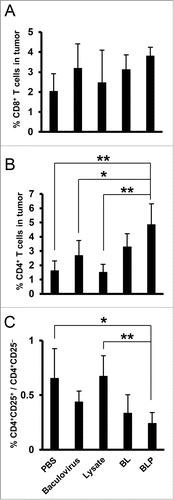
We further tested whether there are differences in the number of tumor-infiltrating CD4+ or CD8+ T cells among the vaccination groups. Seven days after the final treatment procedure, no statistically significant differences in the percentage of CD8+CD3+ T cells were observed among tumors of mice treated with PBS (2.0 ± 0.9%), the baculovirus alone (3.2 ± 1.2%), lysate alone (2.5 ± 1.6%), BL (3.1 ± 0.7%), and BLP (3.8 ± 0.4%; ). In contrast, an increased percentage of tumor-infiltrating CD4+CD3+ T cells was found in mice receiving BLP (4.8 ± 1.5%), followed by BL (3.3 ± 0.9%), and the baculovirus alone (2.7 ± 1.1%), compared to the lysate alone (1.5 ± 0.6%) and PBS (1.6 ± 0.7%; ). Furthermore, when these cells were stained with anti-CD4 and anti-CD25 mAbs, the ratio of CD4+CD25+ cells to CD4+CD25− cells in the group treated with BLP was significantly lower (0.2 ± 0.1) compared to that in the groups receiving PBS (0.7 ± 0.3) and lysate alone (0.7 ± 0.2; ). Thus, a substantial number of CD4+CD25− T cells migrated into the tumor tissue in mice treated with the baculovirus.
Figure 4. Analysis of tumor-infiltrating lymphocytes in mice treated with various vaccines. Mice were inoculated with CT26 tumor cells (4 × 105) on day 0, and then received treatment with PBS, the tumor lysate alone, baculovirus alone, BL, or BLP at a 1- to 2-mm distance from the established tumors on days 3, 10, and 17 (n = 5 per group). The tumors were extirpated on day 24 and a single-cell suspension was prepared and stained with anti-CD4, -CD8, -CD3, and -CD25 monoclonal antibodies. The numbers of CD8+CD3+ T cells (A) and CD4+CD3+ T cells (B) in a tumor and the ratios of CD4+CD25+ cells to CD4+CD25− cells (C) were measured using flow cytometry. The data are presented as mean ± SD, and p values < 0.05 were considered statistically significant. *, p < 0.05; **, p < 0.01. Similar results were obtained in 3 independent experiments.
The tumor lysate is an essential component of the BLP formulation
Mice were inoculated s.c. with the CT 26 tumor cells (4 × 105) on day 0, so that palpable tumors with 1- to 2-mm diameter were observed in all of the mice inoculated on day 3 after the tumor challenge. To examine the immunological role of the tumor lysate in the antitumor effect of BLP, these mice were then immunized with PBS, BP, or BLP 4 times at a 1- to 2-mm distance from the established tumors on days 3, 6, 10, and 17 (). In the BLP-treated group, the established tumors were completely eradicated in 50% of mice compared to 20% of mice treated with BP (p = 0.023; ). In addition, when we compared the tumor size of mice that developed tumors between the 2 groups, the tumor growth in the BLP-treated group was significantly attenuated compared to the BP-treated group (p = 0.030). Thus, the vaccine-induced antitumor effect sharply diminished as a result of removal of the tumor lysate from the BLP vaccine formulation. This result indicated that the tumor lysate was an essential component of BLP for a strong antitumor effect.
Figure 5. The tumor lysate is crucial for the antitumor efficacy of the combination of a peptide, lysate, and baculovirus. (A) Mice were inoculated subcutaneously with CT26 tumor cells (4 × 105) on day 0, and then treated intradermally with PBS, BP, BLP (n = 10 per group), CpG + peptide, or CpG + peptide + lysate (n = 5 per group) at a 1- to 2-mm distance from the established tumors on days 3, 6, 10, and 17. (B) Tumor size measurements. Each line represents an individual mouse. (C) The immune response involving CD8+ T cells was assessed by intracellular staining for cytokines. Forty days after the inoculation of mice with tumor cells, splenocytes from the mice treated with PBS, BP, or BLP (n = 5 per group) were cultured with or without the AH1 peptide for 6 hr to stimulate peptide-specific CD8+ T cells, and the production of IFNγ by T cells was measured using flow cytometry. Plots are gated on CD8+ cells and representative of data obtained from 5 animals per group. Numbers above each FACS plot are the percentages of IFNγ-producing CD8+ T cells. Similar results were obtained in 3 independent experiments.
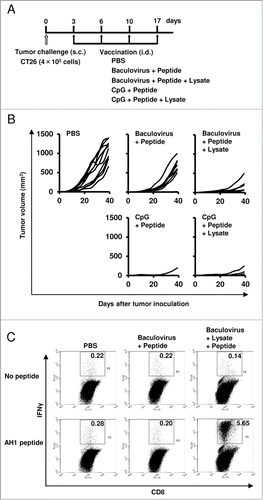
When the magnitude of BLP efficacy was compared with that of CpG oligo + peptide and CpG oligo + peptide + lysate, the difference was not statistically significant (p > 0.05), implying that the efficacy of tumor immunotherapy with BLP was similar to that of CpG oligo + peptide or CpG oligo + peptide + lysate ().
To measure the induction of tumor-specific CTLs, we euthanized these mice on day 40 after the tumor challenge. In spleens from 5 mice in which a preexisting tumor was completely eradicated by the treatment with BLP, IFNγ-producing CD8+ T cells were easily detectable upon in vitro stimulation with the AH1 peptide (3.36 ± 1.58%; ). In contrast, such CD8+ T cell responses were weak in spleens from the mice that received BLP but failed to suppress tumor growth (0.74 ± 0.35%, data not shown). In contrast, when we randomly selected 5 out of the 8 BP-treated mice that showed unimpeded tumor growth, and analyzed their splenocytes, no IFNγ response of CD8+ T cells was detected (0.20 ± 0.02%; ). By contrast, the response was seen in the other 2 mice that received BP and successfully suppressed tumor growth (2.10% and 3.51%, data not shown). In addition, in spleens from 5 randomly selected mice treated with PBS, all the animals exhibited unimpeded tumor growth, and the induction of tumor-specific CTLs was not observed (0.27 ± 0.05%; ). Taken together, these results showed that immunization with BLP eradicated established tumors in 50% of mice, and the antitumor effect correlated with tumor antigen-specific IFNγ response of CD8+ T cells.
CD4+ T cells play a critical role in the induction of antitumor immunity by treatment with BLP
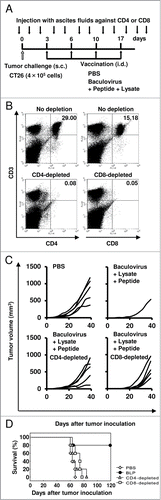
Finally, by conducting the in vivo T cell depletion experiment, we evaluated the association between the efficacy of BLP-induced antitumor immunity and the CD4+ or CD8+ T cells stimulated by the immunization with BLP (). CD4+ or CD8+ T cells were successfully removed by intraperitoneal injection of mice with the ascites fluids containing an anti-CD4 or -CD8 antibody (>99%) (). In 60% of the BLP-treated mice without T-cell depletion, the established tumors were completely eradicated. Furthermore, in 20% of mice, the size of the preexisting tumor remained small during the period of observation, whereas the other mice failed to suppress tumor growth (). Nonetheless, these antitumor effects induced by BLP treatment were severely attenuated by the removal of CD4+ or CD8+ T cells from the mice (p = 0.019 and 0.033, respectively; ). These results demonstrated that BLP-induced antitumor responses were mediated by CD4+ T cells and by CD8+ T cells.
Figure 6. Assessment of the role of T cells in the induction of antitumor immunity by treatment with the combination of a peptide, lysate, and baculovirus. (A) To deplete mice of CD4+ or CD8+ T cells in vivo, we initiated intraperitoneal injection of mice with the ascites fluids (100 µl per mouse) one day before inoculation with CT26 tumor cells and then performed the depletion procedure 2 times a week during the study period. At the same time, these mice were inoculated subcutaneously with CT26 tumor cells (4 × 105) on day 0, and then treated intradermally with PBS or BLP at a 1- to 2-mm distance from the established tumors on days 3, 6, 10, and 17 (n = 5 per group). (B) Flow cytometric analysis confirmed that the depletion of CD4+ or CD8+ T cells exceeded 99% each. Numbers in the upper right quadrant are the percentages of the target cells. (C) Tumor size measurements. Each line represents an individual mouse. (D) Survival curve. Similar results were obtained in 3 independent experiments.
Discussion
In our previous study, the prophylactic i.d. vaccination of mice with a BL vaccine formulation effectively suppressed tumorigenesis after inoculation with 5 × 104 CT26 tumor cells.Citation15 However, in the present study, treatment with the BL vaccine was not able to inhibit tumor growth after the transplantation of 4 × 105 CT26 cells (). To further enhance the efficacy of BL, a CTL epitope peptide was added to the BL vaccine formulation (resulting in BLP), so that the induced antitumor immunity was more potent. Namely, the induction of tumor-specific, IFNγ-producing CTLs is markedly augmented in the present work, and the established tumors regress substantially as a result of therapeutic vaccination with BLP, compared to immunization with either a BL or BP vaccine formulation.
The advantage of a tumor lysate over a peptide is that the tumor lysate should contain all relevant major histocompatibility complex class I and class II epitopes capable of stimulating CD8+ and CD4+ T cells respectively. Several studies have shown that CD4+ T cells perform critical functions in the induction of effective antitumor immune responsesCitation16-20 and in the generation of CD8+ T memory cells.Citation21,22 In the present study, we also demonstrate the essential role of CD4+ helper T cells in the priming of antitumor immunity. The BLP-induced antitumor effect is severely attenuated if we remove the tumor lysate from BLP () or if we deplete BLP-immunized mice of CD4+ T cells ().
Growth of an established tumor is effectively contained for up to 25 days after the tumor challenge even in the BLP-treated mice with CD8+ T cell-depletion. This finding is suggestive of the induction of the cytotoxic activity of natural killer cells toward the tumor cells; natural killer cells may be activated by CD4+ T cells and DCs or we may be witnessing a direct cytotoxic activity of CD4+ T cells toward the tumor as a result of treatment with BLP.Citation23-25 Thus, the tumor lysate derived from CT26 tumor cells appears not to be immunogenic enough to evoke a sufficient number of tumor antigen-specific CTLs but is useful as a vaccine antigen for stimulating CD4+ T cell help.
In mice receiving immunization with BLP, the ratio of CD4+CD25+ cells to CD4+CD25− cells is significantly lower than that in the other groups (). CD4+CD25+ regulatory T (Treg) cells have been shown to control immunological self-tolerance and suppress antitumor immune responses.Citation26-30 In addition, tumor clearance is improved by the removal of CD4+CD25+ T cells in mouse models.Citation31 Therefore, the antitumor efficacy of BLP may be attributed to the presence of a large population of CD4+CD25− cells in tumor tissue relative to CD4+CD25+ cells; this situation probably results in a tumor microenvironment where antitumor immune responses work effectively. It has been shown that CD4+CD25+ cells accumulate abundantly in gastric cancer tissue,Citation32 and that CD4+CD25+ T cells in tumor tissue suppress T cell activation and correlate well with poor survival in patients with ovarian cancer.Citation33
Why did the large number of CD4+ T cells infiltrate the tumor and why did the ratio of CD4+CD25+ cells to CD4+CD25− cells become relatively low in the groups inoculated with the baculovirus? We previously demonstrated that baculovirus-stimulated DCs secrete large amounts of interleukin (IL)-12 and IL-6.Citation15 IL-12 is important for the induction of Th1-type immune responses, and IL-6 has been shown to perform a crucial function in T-cell activation because of its ability to overcome the suppressive effect of Treg cells.Citation34,35 Therefore, these 2 cytokines when produced by baculovirus-stimulated DCs may contribute to the activation of CD4+ helper T cells and downregulation of Treg-cell function, thereby stimulating Th1-type immunity and subsequent effective induction of memory and/or effector CD8+ T cells against the tumor cells. Further research will be needed to understand the relationship between the migration of Treg cells into the tumor and the immunological activity of baculovirus.
It has been shown that vaccination of mice with AH1 peptide without proper adjuvants is not able to induce effective antitumor immunity against CT26 tumor.Citation6 In addition, the results of our study indicate that antitumor efficacy is elicited by immunization with CpG oligo + peptide but not observed after vaccination with baculovirus + peptide, pointing to the importance of the addition of an appropriate adjuvant to antigens like peptides, proteins, or tumor lysates for the effective immunotherapy of cancer. With regard to the baculovirus as an adjuvant, the antitumor effect can be considerably augmented when a mixture of a CTL epitope peptide and a tumor lysate is employed as vaccine antigens rather than a peptide alone or a tumor lysate alone. Because the CpG-based vaccine did not necessarily require the tumor lysate in the present study, the mechanism for priming antigen-specific immunity may differ between baculovirus and CpG oligo. CpG oligo is considered as an extremely potent adjuvant, and a CpG oligo + peptide combination vaccine is now being tested in clinical settings. Therefore, we consider that our finding that treatment using BLP was as effective as that using CpG oligo + peptide or CpG oligo + peptide + lysate is significant because CpG oligo is not a perfect adjuvant for cancer immunotherapy. Thus, our present study suggests that baculovirus may become a promising adjuvant comparable to CpG oligo. In the present study, preexisting tumors are eradicated in 50–60% of mice by therapeutic administration of BLP, and this beneficial effect correlates positively with the inductive efficiency of tumor-specific CTLs. Furthermore, the effectiveness of the induction appears to be strongly dependent on the functioning of CD4+ helper T cells. Therefore, a tumor lysate with weak immunogenicity may become highly immunogenic by the addition of a CTL epitope peptide and consequently may effectively stimulate both CD4+ and CD8+ T cell immune responses against the tumor. The major limitation of our study is that one mouse model with CT26 tumor cells was used for all the experiments. Further studies using different types of tumors are needed to demonstrate the antitumor efficacy of the baculovirus-based cancer vaccine more definitively. However, our present study does suggest the potential of using the baculovirus as an adjuvant in the development of new and effective cancer vaccines.
Our study may open up the possibility of a clinically effective cancer vaccine involving baculovirus as an adjuvant. This virus does not replicate in mammalian cells and has been widely used as a biopesticide;Citation36,37 thus, it is expected to be safe for humans. Moreover, unlike synthetic CpG oligonucleotides, mass production of the virus is easily achieved in an insect cell line, thereby ensuring cost effectiveness of such a vaccine. These characteristics are particularly important for the development of a cancer vaccine because such vaccination will be repeated multiple times.
Materials and Methods
Mice and cell lines
Four-week-old female BALB/c mice (Nippon SLC, Shizuoka, Japan) were used in a biosafety level 2 animal facility at Chiba Institute of Technology, Chiba, Japan. The study was conducted in the experimental animal section under the guidance of an institutional committee for biosafety and animal experiments. Spodoptera frugiperda (Sf-9) cells were cultured at 28°C in the Sf-900 II medium (Cat# 10902, Invitrogen Life Technologies, Gaithersburg, MD). The CT26 murine colon carcinoma cell line was purchased from American Type Culture Collection (ATCC; Manassas, VA, USA) and maintained in RPMI-1640 (Cat# R8758, Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal calf serum (Invitrogen), 100 U/ml penicillin, and 100 µg/ml streptomycin (Sigma-Aldrich).
Purification of the wild-type baculovirus
The wild-type baculovirus was purchased from BD Biosciences (San Jose, CA, USA) and propagated in Sf-9 cells. Baculovirus particles were purified as described previously,Citation38 and the virus titer was determined using a plaque assay.
Preparation of vaccines
To prepare a tumor cell lysate, CT26 cells were divided among 1.5-ml tubes (106 per 50 µl phosphate-buffered saline [PBS] per tube), and the tubes were subjected to 5 freeze-thaw cycles using liquid nitrogen and a 37°C water bath. The lysates were stored at −80°C until use. To prepare the vaccine formulation (BLP) consisting of the baculovirus, the tumor cell lysate and a CTL epitope peptide, we added the baculovirus (108 plaque-forming units [pfu]) and 50 μg of the AH1 peptide, as a CTL epitope peptide derived from the envelope protein (gp70) of an endogenous ecotropic murine leukemia provirus expressed by CT26Citation39 (Cat# TS-M521-P, MBL, Aichi, Japan) to the above-mentioned 1.5-ml tube and stored it on ice until administration. We used one tube per mouse (106-cell lysate + 50 µg AH1 peptide + 108 pfu baculovirus per mouse). As controls, the CT26 cell lysate alone (106-cell lysate), the baculovirus alone (108 pfu), a combination (BL) of the baculovirus (108 pfu) and the CT26 cell lysate (106-cell lysate), or a combination (BP) of the baculovirus (108 pfu) and the AH1 peptide (50 µg) were also prepared. In the indicated experiment, we also used CpG oligonucleotide 1826 (Cat# tlrl-1826, InvivoGen, San Diego, CA, USA) as a positive control adjuvant because baculovirus stimulates dendritic cells (DCs) via TLR-9.Citation40
Immunization with vaccines
All the vaccine formulations used in this study were injected intradermally into the right flank of mice. To assess prophylactic antitumor efficacy of the tumor cell lysate alone, baculovirus alone, BL, or BLP, each type of immunization was performed once a week for 3 consecutive weeks. Seven days after the final vaccination, CT26 cells (4 × 105) were transplanted subcutaneously (s.c.) into the right flank of the immunized mice. Therapeutic antitumor efficacy of BLP was evaluated in 2 settings. In the first condition, the CT26 cells (4 × 105) were injected s.c. into the right flank of mice on day 0, and then the treatment with PBS, the tumor cell lysate alone, the baculovirus alone, BL, or BLP was administered intradermally at a 1- to 2-mm distance from the established tumors on days 3, 10, and 17. In the second condition, the CT26 cells (4 × 105) were similarly injected on day 0, and then the treatment with PBS, BP, BLP, CpG (50 µg) + AH1 peptide, or CpG + lysate + AH1 peptide was administered intradermally at a 1- to 2-mm distance from the established tumors on days 3, 6, 10, and 17. Tumor volume was measured using a slide caliper according to the following formula: tumor volume (mm3) = length × width2 ÷ 2. The mice were examined twice weekly for tumor growth.
Assessment of tumor-specific CTL induction
To evaluate induction of tumor-specific CTLs, splenocytes were harvested 7 days after the final prophylactic immunization with PBS, the tumor cell lysate alone, the baculovirus alone, BL, or BLP. The cells were cultured in the presence or absence of stimulation with 1 µM AH1 peptide for 6 h at 37°C. Brefeldin A (10 µg/ml; Cat# B7651, Sigma-Aldrich) was added 2 h after the initiation of the incubation. Similarly, in the indicated experiment, the induction of tumor-specific CTLs was also tested in the spleen of mice treated therapeutically with PBS, BP, or BLP on day 40 after inoculation with tumor cells.
Analysis of tumor-infiltrating lymphocytes
Mice were inoculated with the CT26 tumor cells (4 × 105) on day 0, and then received treatment with PBS, the tumor cell lysate alone, the baculovirus alone, BL, or BLP on days 3, 10, and 17. The tumors were extirpated on day 24, minced, washed with PBS, and then digested with collagenase (0.5 mg/ml, Cat# C5138, Sigma-Aldrich) for 1 h at 37°C. The pieces were passed through a 100-µm cell strainer to obtain a single-cell suspension.
Flow cytometric analysis
For analysis of tumor-specific CTLs, cell surface CD8 and intracellular interferon gamma (IFNγ) staining was performed. The AH1 peptide-pulsed and unstimulated splenocytes were labeled with a fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD8 monoclonal antibody (mAb; Cat# 11-0081, eBioScience, San Diego, CA, USA), permeabilized with cytofix/cytoperm solution (Cat# 554722, BD Biosciences), and then stained with a phycoerythrin (PE)-conjugated anti-mouse IFNγ mAb (Cat# 12-7311, eBioScience).
For analysis of tumor-infiltrating lymphocytes, the cells were stained with FITC- or PE-conjugated anti-mouse CD4 (Cat# 11-0042), CD8, CD3 (Cat# 12-0031), and CD25 mAbs (Cat# 12-0251, all eBioScience).
The cells were analyzed on a FACSCalibur instrument with the CellQuest software (BD Bioscience).
In vivo removal of CD4+ or CD8+ T cells
Hybridomas (clone GK1.5 for CD4; clone 2.43 for CD8) were grown in nude mice (Nippon SLC), and ascites fluids were collected. To remove CD4+ or CD8+ T cells in vivo, the intraperitoneal injection of mice with the ascites fluids (100 µl per mouse) was initiated one day before the CT26 tumor inoculation (4 × 105 cells) and then performed 2 times a week during the study period. Flow cytometric analysis was used to confirm whether the target cells were absent.
Statistical analysis
Data were analyzed using the Statistica program (StatSoft, Tulsa, OK). Statistical significance was determined by one-way analysis of variance (ANOVA) followed by the Tukey's test for pairwise comparison or the Mann–Whitney U test. The survival end-point was defined as a tumor size of 20 mm in diameter (Kaplan–Meier method), and the log–rank test was used to compare the antitumor efficacy of various vaccines. The results are presented as the mean ± standard deviation (SD), and p values <0.05 were considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Dr. Noboru Hagiwara (Japan BCG Laboratory) for his insightful comments during our discussion of this study.
References
- van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991; 254:1643-7; PMID:1840703; http://dx.doi.org/10.1126/science.1840703
- Schlom J, Gulley JL, Arlen PM. Paradigm shifts in cancer vaccine therapy. Exp Biol Med 2008; 233:522-34; PMID:18375829; http://dx.doi.org/10.3181/0708-MR-226
- Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 2009; 15:5323-37; PMID:19723653; http://dx.doi.org/10.1158/1078-0432.CCR-09-0737
- Purcell AW, McCluskey J, Rossjohn J. More than one reason to rethink the use of peptides in vaccine design. Nat Rev Drug Discov 2007; 6:404-14; PMID:17473845; http://dx.doi.org/10.1038/nrd2224
- Itoh K, Yamada A, Mine T, Noguchi M. Recent advances in cancer vaccines: an overview. Jpn J Clin Oncol 2009; 39:73-80; PMID:19015149; http://dx.doi.org/10.1093/jjco/hyn132
- Muraoka D, Kato T, Wang L, Maeda Y, Noguchi T, Harada N, Takeda K, Yagita H, Guillaume P, Luescher I, et al. Peptide vaccine induces enhanced tumor growth associated with apoptosis induction in CD8+ T cells. J Immunol 2010; 185:3768-76; PMID:20733202; http://dx.doi.org/10.4049/jimmunol.0903649
- Speiser DE, Liénard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F, Krieg AM, Cerottini JC, Romero P. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest 2005; 115:739-46; PMID:15696196; http://dx.doi.org/10.1172/JCI23373
- Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O'Neill D, Pavlick A, Escalon JB, Cruz CM, Angiulli A, et al. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci USA 2007; 104:8947-52; PMID:17517626; http://dx.doi.org/10.1073/pnas.0703395104
- Fourcade J, Kudela P, Andrade Filho PA, Janjic B, Land SR, Sander C, Krieg A, Donnenberg A, Shen H, Kirkwood JM, et al. Immunization with analog peptide in combination with CpG and montanide expands tumor antigen-specific CD8+ T cells in melanoma patients. J Immunother 2008; 31:781-91; PMID:18779741; http://dx.doi.org/10.1097/CJI.0b013e318183af0b
- Karbach J, Gnjatic S, Bender A, Neumann A, Weidmann E, Yuan J, Ferrara CA, Hoffmann E, Old LJ, Altorki NK, et al. Tumor-reactive CD8+ T-cell responses after vaccination with NY-ESO-1 peptide, CpG 7909 and Montanide® ISA-51: association with survival. Int J Cancer 2010; 126:909-18; PMID:19728336
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10:909-15; PMID:15340416; http://dx.doi.org/10.1038/nm1100
- Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, Royal RE, Kammula U, Restifo NP, Hughes MS, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol 2005; 175:6169-76; PMID:16237114; http://dx.doi.org/10.4049/jimmunol.175.9.6169
- Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W, et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med 2013; 19:465-72; PMID:23455713; http://dx.doi.org/10.1038/nm.3105
- Hailemichael Y, Overwijk WW. Peptide-based anticancer vaccines: The making and unmaking of a T-cell graveyard. Oncoimmunology 2013; 2:e24743; PMID:24073366; http://dx.doi.org/10.4161/onci.24743
- Kawahara M, Takaku H. Intradermal immunization with combined baculovirus and tumor cell lysate induces effective antitumor immunity in mice. Int J Oncol 2013; 43:2023-30; PMID:24101126; http://dx.doi.org/10.3892/ijo.2013.2125
- Bour H, Horvath C, Lurquin C, Cerottini JC, MacDonald HR. Differential requirement for CD4 help in the development of an antigen-specific CD8+ T cell response depending on the route of immunization. J Immunol 1998; 160:5522-9; PMID:9605156
- Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol 1998; 10:588-94; PMID:9794842; http://dx.doi.org/10.1016/S0952-7915(98)80228-8
- Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses. J Exp Med 1999; 189:753-6; PMID:10049938; http://dx.doi.org/10.1084/jem.189.5.753
- Sharma RK, Yolcu ES, Srivastava AK, Shirwan H. CD4+ T cells play a critical role in the generation of primary and memory antitumor immune responses elicited by SA-4-1BBL and TAA-based vaccines in mouse tumor models. PLoS One 2013; 8:e73145; PMID:24066030; http://dx.doi.org/10.1371/journal.pone.0073145
- Kissick HT, Sanda MG, Dunn LK, Arredouani MS. Immunization with a peptide containing MHC class I and II epitopes derived from the tumor antigen SIM2 induces an effective CD4 and CD8 T-cell response. PLoS One 2014; 9:e93231; PMID:24690990; http://dx.doi.org/10.1371/journal.pone.0093231
- Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 2003; 421:852-6; PMID:12594515; http://dx.doi.org/10.1038/nature01441
- Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 2003; 300:337-9; PMID:12690201; http://dx.doi.org/10.1126/science.1082305
- Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, Easterbrook P, Grey P, Smith D, McMichael AJ, et al. Characterization of CD4+ CTLs ex vivo. J Immunol 2002; 168:5954-8; PMID:12023402; http://dx.doi.org/10.4049/jimmunol.168.11.5954
- Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, et al. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 2010; 207:637-50; PMID:20156971; http://dx.doi.org/10.1084/jem.20091918
- Marshall NB, Swain SL. Cytotoxic CD4 T cells in antiviral immunity. J Biomed Biotechnol 2011; 2011:954602; PMID:22174559; http://dx.doi.org/10.1155/2011/954602
- Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol 2003; 3:199-210; PMID:12658268; http://dx.doi.org/10.1038/nri1027
- von Herrath MG, Harrison LC. Antigen-induced regulatory T cells in autoimmunity. Nat Rev Immunol 2003; 3:223-32; PMID:12658270; http://dx.doi.org/10.1038/nri1029
- Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev 2001; 182:18-32; PMID:11722621; http://dx.doi.org/10.1034/j.1600-065X.2001.1820102.x
- Shevach EM. Regulatory T cells in autoimmmunity*. Annu Rev Immunol 2000; 18:423-49; PMID:10837065; http://dx.doi.org/10.1146/annurev.immunol.18.1.423
- Taylor PA, Noelle RJ, Blazar BR. CD4+CD25+ immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med 2001; 193:1311-8; PMID:11390438; http://dx.doi.org/10.1084/jem.193.11.1311
- Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol 1999; 163:5211-8; PMID:10553041
- Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res 2003; 9:4404-8; PMID:14555512
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10:942-9; PMID:15322536; http://dx.doi.org/10.1038/nm1093
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science 2003; 299:1033-6; PMID:12532024; http://dx.doi.org/10.1126/science.1078231
- Bhanumathy KK, Zhang B, Ahmed KA, Qureshi M, Xie Y, Tao M, Tan X, Xiang J. Transgene IL-6 enhances DC-stimulated CTL responses by counteracting CD4+25+Foxp3+ regulatory T cell suppression via IL-6-induced Foxp3 downregulation. Int J Mol Sci 2014; 15:5508-21; PMID:24690994; http://dx.doi.org/10.3390/ijms15045508
- Stewart LM, Hirst M, López-Ferber M, Merryweather AT, Cayley PJ, Possee RD. Construction of an improved baculovirus insecticide containing an insect-specific toxin gene. Nature 1991; 352:85-8; PMID:2062383; http://dx.doi.org/10.1038/352085a0
- Cory JS, Hirst ML, Williams T, Hails RS, Goulson D, Green BM, Carty TM, Possee RD, Cayley PJ, Bishop DHL. Field trial of a genetically improved baculovirus insecticide. Nature 1994; 370:138-40; http://dx.doi.org/10.1038/370138a0
- Suzuki T, Chang MO, Kitajima M, Takaku H. Baculovirus activates murine dendritic cells and induces non-specific NK cell and T cell immune responses. Cell Immunol 2010; 262:35-43; PMID:20060108; http://dx.doi.org/10.1016/j.cellimm.2009.12.005
- Huang AYC, Gulden PH, Woods AS, Thomas MC, Tong CD, Wang W, Engelhard VH, Pasternack G, Cotter R, Hunt D, et al. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci USA 1996; 93:9730-5; PMID:8790399; http://dx.doi.org/10.1073/pnas.93.18.9730
- Abe T, Hemmi H, Miyamoto H, Moriishi K, Tamura S, Takaku H, Akira S, Matsuura Y. Involvement of the Toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus. J Virol 2005; 79:2847-58; PMID:15709004; http://dx.doi.org/10.1128/JVI.79.5.2847-2858.2005
