ABSTRACT
Multiple myeloma (MM), a blood cancer characterized by the uncontrolled proliferation of plasma cells, remains incurable by current therapy. Notch signaling has been implicated in the growth and chemoresistance of various cancer types including MM, and therefore we hypothesized that targeting the Notch pathway could be beneficial for the treatment of this disease. Here, we report an anti-tumor effect of Notch/γ-secretase inhibitor RO4929097 in a pre-clinical model of MM. We demonstrate that this effect was associated with decreased angiogenesis and significant down-regulation of TGF-β1. In addition, we also show that treatment with RO4929097 results in decreased number and functional activity of osteoclasts. Taken together, our data indicate that targeting Notch may be considered as a new strategy to be tested for MM therapy.
Abbreviations
| BM | = | bone marrow |
| Bhlh | = | basic helix-loop-helix |
| CBF-1 | = | C-promoter binding factor |
| DKK1 | = | Dickkopf-related protein 1 |
| EGFR | = | Epidermal growth factor receptor |
| GSIs | = | γ-secretase inhibitors |
| Hes1 | = | hairy and enhancer of split 1 |
| HMVEC-L | = | human lung microvascular endothelial cells |
| MM | = | multiple myeloma |
| M-CSF | = | macrophage colony-stimulating factor |
| MNC | = | mononuclear cells |
| NIC | = | Notch intracellular domain |
| OCL | = | osteoclasts |
| RANKL | = | receptor activator of nuclear factor-κB ligand |
| TGF-β1 | = | transforming growth factor-beta 1 |
| TRAP | = | tartrate-resistant acid phosphatase |
| VEGF | = | vascular endothelial growth factor |
Introduction
Despite the development of several new effective therapies for multiple myeloma (MM) -including proteasome inhibitors, immunomodulatory drugs, histone deacetylase inhibitors, and the continued use of high-dose chemotherapy with autologous stem cell transplant - MM remains a fatal disease. The ultimate drug resistance of all patients with MM warrants studies aimed to identify novel molecular targets and test new agents for the treatment of this disease. Recent data have demonstrated that Notch signaling is activated in MM and could contribute to the pathogenesis of this disease.Citation1-4
Notch signaling is initiated by the binding of the extracellular domain of Notch to a Notch ligand. At present, 2 Notch ligand families, Delta and Jagged have been described.Citation5 The Notch receptor family includes 4 members (Notch-1 to 4). Each member is a large single heterodimeric receptor comprised of noncovalently associated extracellular, transmembrane, and intracellular subunits (NIC). Multiple lines of investigation have demonstrated that NIC translocates to the nucleus in a ligand-dependent fashion.Citation6 Ligand/receptor interactions initiate 2 successive proteolytic cleavages within the transmembrane subunit, the second of which releases NIC. This cleavage requires the function of the proteins presenilin and nicastrin,Citation7 representing 2 components of a multi-subunit complex that possesses γ-secretase activity. NIC translocates to the nucleus where it interacts with the transcriptional repressor CSL (CBF-1, Suppressor of Hairless, Lag-1) thus, turning it into a transcriptional activator. Transcriptional targets of NIC/CBF-1 include genes of the Hairy/Enhancer of Split (Hes) and Hairy/Enhancer of split related with YRPW motif-like protein (Hey) families of basic helix-loop-helix (bHLH) transcription factors, cyclin D1, and c-Myc among others.Citation8-11
RO4929097 is a potent γ-secretase inhibitor (GSI) that also blocks Notch signaling and leads to reduced expression of the Notch transcriptional target gene Hes1.Citation12 This investigational drug has demonstrated evidence of antitumor activity in pre-clinical models of melanoma, colon, pancreatic and lung cancerCitation12,13 and had been tested in phase II clinical trials for solid tumors.Citation14-17 While RO4929097 has been well tolerated, it has insufficient anti-tumor activity as a single agent for solid tumors. However, several studies suggested a potential benefit of using this agent in combination with other treatments.Citation18,19 In addition, the development of other therapeutics targeting various components of the Notch pathway with potentially improved anti-tumor effect is clearly reasonable.
Several studies have implicated Notch in the pathogenesis of MM. Dysregulation of Notch signaling due to overexpression of receptors including Notch1 and Notch2 and/or ligands Jagged1 and Jagged2 has been demonstrated for MM cell lines and primary MM cells.Citation1,3,20,21 Moreover, overexpression of Jagged2 was detected in plasma cells in patients with monoclonal gammopathy of undetermined significance, an early phase of MM disease progression, indicating that the activation of Notch signaling could be an essential event in the pathogenesis of MM.Citation21 Overexpression of Notch receptors and ligands on MM cells resulted in the activation of Notch due to tumor cell-cell interaction. In addition, the activation of Notch in MM cells due to the engagement of their Notch receptors by ligands expressed by surrounding BM cells has been described.Citation1,22 Several reports indicated that activation of Notch promotes survival of MM cells and protects them from chemotherapy-induced apoptosis.Citation1,23 Notch signaling has been involved in the regulation of the expression and function of the CXCR4/SDF1 chemokine axis crucial for MM cell growth, survival, and migration.Citation24 Jagged2 increased the release of interleukin 6, vascular endothelial growth factor (VEGF), and insulin-growth factor 1 from BM stroma, factors known to promote MM cell survival, growth, and BM angiogenesis.Citation21 Notch signaling has been also implicated in the development of MM-associated bone disease, primarily through the stimulation of osteoclastogenesis.Citation25,26
Taken together, these data suggest that targeting the Notch pathway may be therapeutically beneficial for patients with MM. Here, we tested this hypothesis utilizing RO4929097, a pharmacological inhibitor of Notch/γ-secretase.
Results
We investigated whether the inhibition of Notch signaling with RO4929097 would result in an anti-MM effect. Initially, to confirm the ability of RO4929097 to block the Notch pathway in MM cells, the expression of the Notch downstream target gene Hes1 was evaluated. As expected, the treatment of human MM RPMI-8226 cells with RO4929097 resulted in significant downregulation of Hes1 gene expression (). In agreement with previous dataCitation12,27 inhibition of Notch with RO4929097 in MM cells did not affect their viability as evaluated by MTT assay (), and induced only minimal apoptosis in human MM cell line () and primary MM cells isolated from BM of patients with MM (). These data are in line with our previous results showing that the cytotoxic effect of some Notch/γ-secretase inhibitors relies mainly on their ability to inhibit proteasome activity.Citation28 However, when the anti-MM effect of RO4929097 was tested in vivo, using a xenograft mouse model of human RPMI-8226 cells injected into the flank of SCID-beige mice, this compound significantly reduced tumor growth ().
Figure 1. Effect of RO4929097 on MM cells in vitro. (A-C) Human MM RPMI-8226 cells were cultured on Jagged-1 coated plates and treated with the indicated concentrations of RO4929097. (A) Hes1 gene expression was determined by qPCR after 24 hr of treatment. The experiment was repeated at least 3 times with similar results. ** - p<0.001. (B) 8226 cells were cultured for 72 hr with or without 1 µM RO4929097 followed by the MTT assay. Two experiments were performed. The results of one representative experiment performed in triplicate are shown. (C) 8226 cells were treated with RO4929097 for 48 hr followed by detection of apoptosis. Shown are the combined results of 3 independent experiments. (D) Apoptosis of primary CD138+ MM cells treated ex vivo for 48 hr with or without the indicated concentrations of RO4929097.
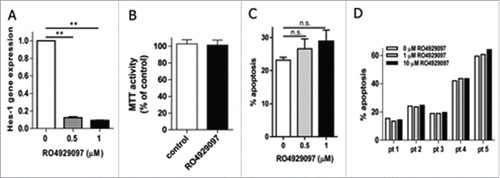
Figure 2. In vivo and ex vivo effect of RO4929097. (A) RPMI-8826-bearing SCID-beige mice were split into 2 groups and treated with 10 mg/kg body weight RO4929097 or vehicle control daily for 14 d (n = 5 per group). Tumor growth was measured with calipers during and after the treatment. Statistical significance between treatment groups was evaluated using 2-way ANOVA. (B,C) RPMI-8826 or H929 tumors were established in SCID-beige mice by subcutaneous inoculation of tumor cells. Mice were split into 2 groups and treated with RO4929097 or vehicle control (VC) for 5 d. Mice were then euthanized; tumor tissues were collected and homogenized. (B) RNA was isolated and subjected to PCR array. Shown are results for H929 tumors (top) and RPMI-8226 tumors (bottom). (C) Lysates were prepared and subjected to Western blotting with an antibody against TGF-β1. Equal loading was confirmed by re-probing the membrane with antibody against β-actin. (D) BM MNCs obtained from 19 patients with MM were cultured with or without 1 µM RO4929097 for 24 hr. Expression of Hes1 and TGF-1β genes was evaluated by qPCR. Shown are combined data on TGF-1β expression in all samples (n = 19), samples where Hes1 was downregulated 2 or more fold (>2 fold, n = 6) and where Hes1 was downregulated less that two fold or unchanged (<2 fold, n = 13).
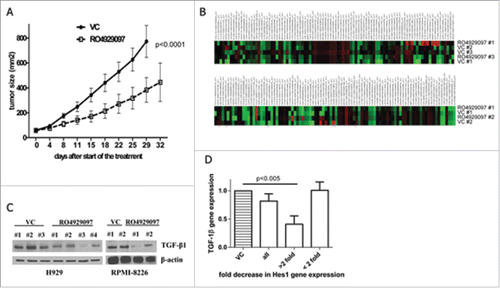
To elucidate the molecular mechanism responsible for the anti-MM effect of RO4929097, tumor tissues were collected from MM-bearing RO4929097-treated or vehicle control-treated mice and subjected to PCR array detecting the expression level of a number of genes encoding soluble factors and adhesion molecules (). The expression of several human genes, including transforming growth factor - β 1 (TGF-β1) was found to be downregulated after treatment with RO4929097. To confirm these data, the protein level of TGF-β1 was evaluated by immunoblotting. A significant decrease in TGF-β1 protein level in MM tumor tissues following treatment with RO4929097 was observed ().
To confirm the relevance of our findings, we next determined the expression of TGF-β1 in BM cells obtained from patients with MM. BM mononuclear cells (MNC) were isolated using Ficoll-Paque gradient centrifugation and were cultured for 24 hr with or without RO4929097. Cells were then collected and the expression of Hes1 was determined by real-time PCR. Thirty two percent (6 out of 19) of samples demonstrated 2 or more fold decrease in Hes1 gene expression. Downregulation of Hes1 in these samples was accompanied by a significant decrease in TGF-β1 expression level (). Hes1 represents only one of the direct target genes of Notch signaling. Our previous data demonstrated that ligand-induced activation of Notch resulted in the upregulation of Hes1, and the treatment with GSIs led to the inhibition of Hes1 expression in MM cells.Citation1,29 Therefore, we used the expression of this gene as a read-out of Notch signaling activation for our experiments. Interestingly, there was no difference in TGF-β1 expression in RO4929097-treated cells that maintained the same level of Hes1 as vehicle control-treated cells (). These data suggest that Hes1 may be involved in the regulation of TGF-β1 expression in MM cells.
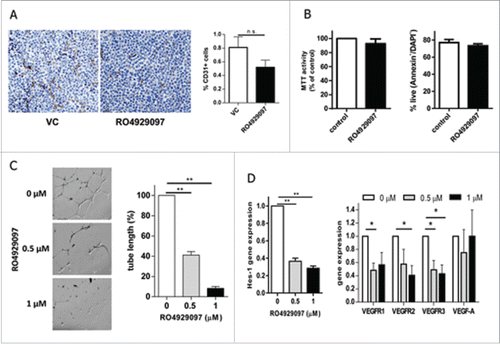
Angiogenesis is known to play a substantial role in MM progression.Citation30,31 To evaluate whether treatment with RO4929097 reduces MM angiogenesis, RPMI-8226-bearing SCID-beige mice were treated with this compound or vehicle control. Tumor tissues were collected and the expression of endothelial marker CD31 was determined by immunohistochemistry and flow cytometry. Blocking Notch resulted in a substantial decrease in CD31 expression (). To confirm these in vivo data we investigated whether RO4929097 could block vascular growth using Matrigel endothelial tube formation assay. Treatment of human lung microvascular endothelial cells (HMVEC-L) with this compound did not affect the viability or apoptosis of these cells (), but resulted in significant inhibition of the vascular structure formation (). These results are in agreement with data reported by Luistro et al showing anti-angiogenic activity of this Notch inhibitor.Citation12 As expected, RO4929097 downregulated Notch target gene Hes1 expression and substantially decreased gene expression of all 3 VEGF receptors, but not VEGF ligand (). Taken together, our data suggest that blocking angiogenesis could be one of the anti-MM mechanisms of the Notch inhibitor RO4929097.
Figure 3. RO4929097 reduces angiogenesis. (A) RPMI-8226-bearing mice were treated with RO4929097 or vehicle control (VC) for 14 d. Expression of CD31 was detected in tumor tissues by immunohistochemistry (left). The proportion of CD31 cells in tumor tissues was evaluated by flow cytometry (right). (B) HMVEC-L were treated with RO4929097 (1 µM) or VC (control) for 72 hr. Cell viability was determined by MTT assay (left panel) and cell apoptosis by Annexin V binding assay (right panel). Shown are combined results from 3 independent experiments. (C,D) HMVEC-L cells were pre-treated with RO4929097 for 48 hr. (C) Cells were then placed on Matrigel. Capillary-like structure formation on Matrigel after 12 hr of incubation with the indicated concentrations of RO4929097 was evaluated. (Left) Micrograph images show the effect of RO4929097 on capillary tube branch formation (magnification, x25). (Right) The bar graph represents quantification of capillary-like tube structures formation in Matrigel after treatment with RO4929097. Data are expressed as the mean ± SD of the length of capillary-like structures. Three experiments with similar results have been performed. Results of one representative experiment are shown. ** - p < 0.005. (D) Expression of indicated genes was determined by qPCR. Two experiments were performed with similar results. The results of one experiment performed in triplicate are shown. * - p < 0.05 and ** - p < 0.005.
Notch signaling has been previously implicated in the bone disease in MM.Citation25,32 Therefore, we investigated whether the inhibition of Notch with RO4929097 could affect osteoclast (OCL) differentiation and function. Peripheral blood MNC were differentiated into OCL by treatment with macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL), with or without 1 µM RO4929097. The ability of RO4929097 to induce peripheral blood MNC death was initially tested. At the dose of 1 µM this compound has no effect on viability and apoptosis of MNC as evaluated by MTT and Annexin V binding assay by flow cytometry (data not shown). After 14 days, tartrate-resistant acid phosphatase (TRAP) activity was evaluated as a marker of OCL differentiation. Treatment with RO4929097 led to a significant decrease in the number of TRAP-positive OCL (). To determine whether decreased OCL differentiation was associated with a reduced bone resorption, we maintained OCL cultures in the presence or absence of RO4929097 on Corning Osteo Assay Surface plates. Inhibition of Notch resulted in the generation of significantly less resorption pits identified by toluidine blue staining (). We also tested whether the inhibition of Notch in mature OCL could affect their function. OCL were initially generated in the presence of M-CSF and RANKL on Corning Osteo Assay Surface plates and were then treated with RO4929097 for 48 hrs. Blocking of Notch in mature OCL resulted in substantial reduction of osteolytic activity (data not shown).
Figure 4. RO4929097 reduces number and activity of OCL. (A,B) Peripheral blood MNC obtained from 5 patients with MM were differentiated toward OCL with or without the continuous presence of 1 µM RO4929097. (A) TRAP staining was performed. Representative micrographs are shown (magnification, x20). (B) Cells were plated on Corning Osteo Assay Surface 24-well plates and differentiated into OCL. Resorption was evaluated 14 d after the start of the cultures. (Left) Representative micrographs are shown (magnification, x2). (Right) Resorption area was then measured and its proportion was calculated. Shown are combined results obtained from 4 different BM samples. (C) Primary CD138+ MM cells (n = 16) isolated from BM of patients with MM were kept with or without 1 µM RO4929097 for 24 hr. The expression of indicated genes was determined by qPCR. Shown are fold changes in expression of indicated genes in treated cells as compared to control untreated cells (presented as a horizontal line).
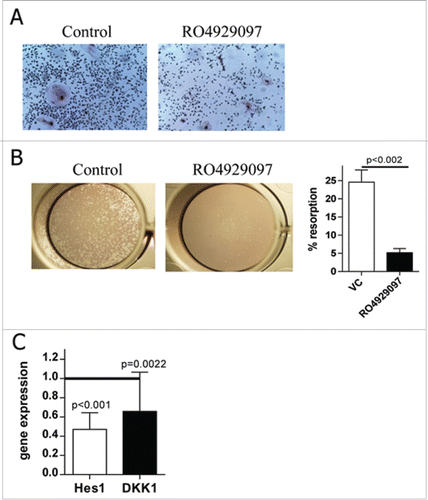
MM cells have been reported to produce the Wnt inhibitor Dickkopf-1 (DKK1) that has been previously implicated in the regulation of osteoblasts differentiation in MM.Citation33 We investigated whether the inhibition of Notch with RO4929097 reduces the DKK1 level and thus, could represent another mechanism by which Notch regulates bone disease in MM. Primary MM cells were isolated from BM of patients with MM and treated overnight with RO4929097. As expected, a significantly reduced Hes1 expression was observed in these cells after treatment with RO4929097 (). This was accompanied by downregulation of DKK-1 expression ().
Discussion
Our study has demonstrated that the pharmacological targeting of Notch signaling with RO4929097 reduces MM growth in vivo in a pre-clinical model of MM and decreases bone disease.
Expression of Notch receptors and ligands has been demonstrated in all MM cell lines and primary MM cells studied.Citation1,3 Members of the Hes family of transcriptional repressors are among known direct targets of Notch signaling. However, the expression pattern of genes encoding these transcriptional factors is cell type specific. The Hes1 member of the Hes family is known to be regulated by Notch. However, MNC isolated from the BM of only 32% patients with MM demonstrated Hes1 inhibition following treatment with RO4929097. Besides the Hes family that includes Hes1-7, a number of genes are known to be direct targets of Notch including members of the Hey family (Hey1, Hey2, and HeyL) among others. There is a possibility that the blocking of Notch receptors cleavage by RO4929097 resulted in the preferential inhibition of other than Hes1 target gene(s). Several reports have indicated Notch-independent regulation of Hes1 expression.Citation34-36 Gamma-secretase complex processes a number of proteins in addition to Notch receptors; some of them could be blocked by RO4929097 and consequently, can contribute to Hes1 inhibition in Notch-independent manner. It is also possible that due to processing BM samples and ex vivo culture conditions, the baseline levels of Notch activation is not sufficient in some patients samples and the presence of additional stimuli is necessary. Therefore, the efficacy of Notch inhibition with RO4929097 when used in the clinic may be significantly higher compared to that observed in vitro.
Our data demonstrate that TGF-β1 is downregulated by RO4929097. TGF-β1 is known to play a significant role in the pathogenesis of solid cancers and has been previously implicated in the progression of MM as well.Citation37,38 Therefore, inhibition of TGF-β1 could be one of the mechanisms by which the Notch/γ-secretase inhibitor RO4929097 mediates its anti-MM effect. A crosstalk between Notch and TGF-β1 has been previously reported in several experimental systems. Thus, TGF-β1 signaling has been shown to affect Notch ligand and receptor levels and induce Hes1 expression.Citation39-44 On the other hand, Notch signaling has been reported to affect TGF-β ligand and receptor levels.Citation45 It is not known, however, whether in MM tumors TGF-β1 is a direct Notch or Hes1 target or regulated via crosstalk of Notch with other pathways. Since γ-secretase processes a number of other proteins in addition to Notch members, there is a possibility that the RO4929097 effect on TGF-β1 expression is mediated through blocking signaling other than Notch. More studies are needed to clarify the mechanism of TGF-β1 downregulation by a Notch/γ-secretase inhibitor in MM cells.
Multiple reports demonstrated that BM angiogenesis is an important hallmark in progression of MM.Citation30,31,46-48 BM microvessel density is significantly higher in MM than in healthy donors and progressively increases with MM progression.Citation49-51 Notch signaling plays a critical role in angiogenesis.Citation52 In line with these data, our study showed an anti-angiogenic effect of RO4929097 in vitro and in vivo suggesting that the inhibition of blood vessel formation may represent one of the mechanisms by which blocking Notch signaling reduces tumor burden.
Destruction of bone is one of the major complications in MM. The main reason for the development of severe osteolytic lesions is the disruption of balance between OCL and osteoblast activity. Thus, increased tumor burden in MM has been associated with the activation of osteoclastogenesis and reduced development of osteoblasts. Notch signaling has been previously implicated in the regulation of osteoclastogenesis; however, the reported findings are controversial, suggesting both the ability of Notch to suppress and enhance OCL differentiation.Citation4,53-55 In MM, induction of Notch in OCL through the interaction with tumor cells has been shown to represent one of the mechanisms driving osteoclastogenesis.Citation2,25 In agreement with these data we found that the pharmacological inhibition of Notch with RO4929097 during OCL differentiation as well as in mature OCL results in a significant reduction in OCL number and function.
DKK-1 protein, a soluble inhibitor of Wnt signaling, has been implicated in the pathogenesis of MM bone disease through the suppression of osteoblast differentiation.Citation56 MM cells are known to secrete DKK-1;Citation56 however, Notch signaling has not been previously shown to be involved in the regulation of DKK-1 expression. Our data demonstrate reduced expression of DKK-1 in primary MM cells after treatment with RO4929097, suggesting that this gene may represent one of the downstream Notch targets and thus one of the mechanisms of Notch-mediated regulation of osteogenic differentiation. Recently published data report that the activation of Notch inhibits osteogenic differentiation, and are in line with our data.Citation57 More studies though are needed to define the role of Notch in osteoblastogenesis in MM.
Thus, our data demonstrate that pharmacological inhibition of Notch with RO4929097 may provide clinical benefit for patients with this disease due to both an anti-tumor effect as well as reduction of bone disease. An approach involving targeting Notch family members or ligands may be considered for further development and integration as a part of an anti-MM therapy.
Materials and methods
Cell cultures and reagents
Human MM RPMI-8226 and H929 cell lines was purchased from ATCC and cultured in RPMI-1640 medium supplemented with 10% FBS and 1% antibiotic-antimycotic (Life Technologies, cat# 15240-062). HMVEC-L cells were kindly provided by Dr. Sophia Ran (SIU School of Medicine, Springfield, IL) and cultured in EGM-2MV BulletKit (Lonza, cat # CC-3202).
Collection of BM and peripheral blood samples from patients with MM was approved by the University of South Florida Institutional Review Board. MNCs were obtained from BM and peripheral blood using Ficoll-Paque (GE Healthcare, cat# 17-1440-03) gradient centrifugation.
For OCL cultures, peripheral blood MNC isolated from patients with MM were plated in α-MEM medium supplemented with 10% FBS at a density of 2 × 106 cells/mL and cultured overnight. After that time, non-adherent cells were removed and adherent cells were cultured in α-MEM medium supplemented with 10% FBS, 50 ng/mL RANKL (Peprotech, cat# 310-08), 25 ng/mL M-CSF (Peprotech, cat# 300-25), and 10 nM dexamethasone (Sigma, cat# D2915) for 14 d.
Notch/γ-secretase inhibitor RO4929097 was provided by Roche (Nutley, NJ) and was dissolved in DMSO.
Isolation of primary MM cells
Primary CD138+ MM cells were isolated from the BM MNC fraction using a magnetic bead cell separation technique (Miltenyi Biotec). Briefly, MNC were incubated with CD138-MicroBeads (Miltenyi Biotec, cat# 130-051-301) followed by magnetic separation of positive cells using the MidiMACS system according to the manufacturer's protocol. Cells were plated in α-MEM medium supplemented with 10% FBS and 1% antibiotic-antimycotic and cultured with or without 1 µM RO4929097.
Animals
SCID-beige (C.B-Igh-1b/GbmsTac-Prkdcscid-Lystbg N7) mice were purchased from Taconic and kept in pathogen-free conditions in the animal facility of the H. Lee Moffitt Cancer Center. All experimental procedures were performed in accordance with the guidelines of the University of South Florida Institutional Animal Care and Use Committee. Female 6-8 week old mice were inoculated subcutaneously in the right flank with 1 × 107 RPMI-8226 cells or 5 × 106 H929 cells. In approximately 3 weeks, when the tumor became measurable, mice were split into 2 groups and treated with either the Notch inhibitor RO4929097 or vehicle control. RO4929097 and the vehicle control were provided by Roche and were given daily for 14 d by oral gavage at a dose of 10 mg per kg of body weight. Tumor size was determined using caliper and was constantly monitored during and after the treatment.
Flow cytometry
Apoptosis of MM cells was detected by Annexin V binding assay using LSR II flow cytometer (BD) as described previously.Citation29 Briefly, MM cells were collected, washed with ice cold PBS and then with binding buffer, and stained with Annexin V conjugated with FITC or APC (BD, cat# 556419 and cat# 550475) and DAPI (Sigma, cat# D9542). Ten thousand events were acquired and analyzed using FlowJo software (Tree Star, Inc.).
For surface staining, cells were labeled with anti-CD31 antibody (BD, cat# 558738) for 30 min on ice, washed twice with PBS, and resuspended in PBS containing DAPI. Ten thousand events were acquired using LSR II flow cytometer and analyzed using FlowJo software (Tree Star, Inc.).
Immunohistochemistry
Tumor tissues were fixed in formaldehyde and paraffin-embedded. Slides were stained using a Ventana Discovery XT automated system (Ventana Medical Systems) as per the manufacturer's protocol with proprietary reagents using anti-CD31 rabbit primary antibody (Abcam, cat# ab28364) at a 1:200 concentration. The detection system used was the Ventana OmniMap kit and slides were then counterstained with Hematoxylin.
Real-rime PCR and PCR array
RNA was extracted from MM cells or MM tumors (H929 and RPMI-8226) and cDNA was synthesized using High-Capacity cDNA Archive Kit (Applied Biosystems, cat# 4374966). PCR array was performed using custom TaqMan Array Cards pre-loaded with selected assays (Applied Biosystems). Expression of DKK1 and TGF-β1 genes was evaluated using CFX96 TouchTM Real-time PCR Detection System (Bio-Rad), Power SYBR Green Master Mix and the following primers: DKK1 FW 5′-AGTACTGCGCTAGTCCCACC-3′ and RV 5′-TCCTCAATTTCTCCTCGGAA-3′; TGF-β1 FW 5′-GTGGAAACCCACAACGAAA-3′ and RV 5′- TAAGGCGAAAGCCCTCAAT-3′; and β-actin FW 5′-CCAAGGCCAACCGCGAGAAGA-3′ and RV 5′-CCGGCCAGCCAGGTCCAGAC-3′. The expression of specific genes was normalized to the expression of the endogenous control gene β-actin. Expression of Hes1 gene was determined by real-time PCR using ABI Prism 7900HT instrument, specific TaqMan Gene Expression assay and reagents from Applied Biosystems or by using CFX96 TouchTM Real-time PCR Detection System (Bio-Rad), Power SYBR Green Master Mix and the following primers: FW 5′-CAAGCTGGAGAAGGCGGACATT-3′ and RV 5′- AGGTGGCCGAGCAGCCGAGTG-3′;.
Western blotting
Tumor tissues were collected from mice, snap frozen and then homogenized using PRO300PC Homogenizer (Pro Scientific).
Homogenized tumor tissues or MM cells were lysed using CelLytic M cell Lysis Reagent (Sigma, cat# C2978). Western blotting was performed according to the standard protocol as previously describedCitation1 using antibodies against TGF-β1 (Cell Signaling Technology, cat# 3709S) and β-actin (Santa Cruz, cat# sc-1616).
MTT assay
MM cells were plated in 96-well plate (0.03 × 106/well) coated with Jagged-1 (R&D systems, cat# 1726-JG-050) and treated with 1 µM RO4929097 for 72 hrs. 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT; Sigma, cat#M5655) dye was added for the last 4h of incubation. Insoluble formazan complexes were solubilized with DMSO and absorbance was measured at 540 nm using a Benchmark Plus microplate spectrophotometer (Bio-Rad). Each experimental condition was done in triplicate and repeated at least once.
TRAP staining
The presence of the OCL specific marker TRAP was determined by using an Acid Phosphatase, Leukocyte kit from Sigma (cat# 387A). Briefly, cells were rinsed with PBS, fixed with ice cold 4% paraformaldehyde and stained for 45 min at 37°C. After that time, cells were rinsed in distilled water and air dried. Cells were imaged with a Leica DMLB upright brightfield microscope with a 20×/0.5NA objective lens (Leica Microsystems, Germany). Images were produced using a SPOT RT Color camera and SPOT advanced software version 4.7 (Diagnostic Instruments Inc.).
Bone resorption assay
OCL cultures were grown on Corning Osteo Assay Surface 24-well plates (Fisher Scientific, cat# 09-762-100). After 14 d of culture medium was aspirated, 100 µL of 10% bleach solution was added per well and incubated for 5 min at room temperature. Wells were then washed twice with distilled water, allowed to air dry, and stained with toluidine blue. Images were captured with an Olympus SZ61 Stereo microscope and DP70 CCD camera (Olympus Corporation, Tokyo, Japan) at 2x magnification. Image Pro Plus version 6.2 (Mediacybernetics Inc.) was used to determine the percent area of resorption via an intensity threshold segmentation.
In vitro angiogenesis assay
Ninety-six well plates were coated with growth factor reduced Matrigel (BD, cat# 356234) and kept at 37°C for 15 min. Thirty thousand HMVEC-L cells pretreated for 48 h with RO4929097 were then added per well and kept overnight at 37°C with or without RO4929097. The micrographs of tube formation were taken using an automated Zeiss Observer Z.1 inverted microscope equipped with a 2.5× /0.075 NA objective. Images were captured using the AxioCam MRm3 CCD camera and Axiovision version 4.7 software suite using the mosaic acquisition tool (Carl Zeiss Inc.). The total tube length was measured using ImageJ software.
Statistics
Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software Inc.). Differences between the treatment groups were calculated using Student's t-test. A statistically significant difference was determined at p < 0.05. Two-way ANOVA was used to evaluate statistical significance in tumor growth between groups of mice.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported in part by NIH/NCI grant R01CA130923 and Bankhead-Coley Cancer Research TSA from the State of Florida. This work has been supported in part by the Flow Cytometry Core Facilities at the H. Lee Moffitt Cancer Center and the Wistar Institute. F.C. was supported by the grant from National Natural Science Foundation of China (No. 81470139).
References
- Nefedova Y, Cheng P, Alsina M, Dalton W, Gabrilovich D. Involvement of Notch-1 signaling in bone marrow stroma-mediated de novo drug resistance of myeloma and other malignant lymphoid cell lines. Blood 2004; 103:3503-10; PMID:14670925; http://dx.doi.org/10.1182/blood-2003-07-2340
- Schwarzer R, Kaiser M, Acikgoez O, Heider U, Mathas S, Preissner R, Sezer O, Doerken B, Jundt F. Notch inhibition blocks multiple myeloma cell-induced osteoclast activation. Leukemia 2008; 12:2273-7; PMID:18528422; http://dx.doi.org/10.1038/leu.2008.138
- Jundt F, Probsting KS, Anagnostopoulos I, Muehlinghaus G, Chatterjee M, Mathas S, Bargou RC, Manz R, Stein H, Dorken B. Jagged1-induced Notch signaling drives proliferation of multiple myeloma cells. Blood 2004; 103:3511-5; PMID:14726396; http://dx.doi.org/10.1182/blood-2003-07-2254
- Sethi N, Dai X, Winter C, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 2011; 19:192-205; PMID:21295524; http://dx.doi.org/10.1016/j.ccr.2010.12.022
- Osborne B, Miele L. Notch and the immune system. Immunity 1999; 11:653-63; PMID:10626888; http://dx.doi.org/10.1016/S1074-7613(00)80140-5
- Kopan R, Goate A. A common enzyme connects notch signaling and Alzheimer's disease. Genes Dev 2000; 14:2799-806; PMID:11090127; http://dx.doi.org/10.1101/gad.836900
- Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song YQ, Rogaeva E, Chen F, Kawarai T, et al. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature 2000; 407:48-54; PMID:10993067; http://dx.doi.org/10.1038/35024009
- Maier M, Gessler M. Comparative analysis of the human and mouse Hey1 promoter: hey genes are new Notch target genes. Biochem Biophys Res Commun 2000; 275:652-60; PMID:10964718; http://dx.doi.org/10.1006/bbrc.2000.3354
- Jarriault S, Brou C, Logeat F, Schroeter E, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature 1995; 377:355-8; PMID:7566092; http://dx.doi.org/10.1038/377355a0
- Weng A, Millholland J, Yashiro-Ohtani Y, Arcangeli M, Lau A, Wai C, Del Bianco C, Rodriguez C, Sai H, Tobias J, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev 2006; 20:2096-109; PMID:16847353; http://dx.doi.org/10.1101/gad.1450406
- Ronchini C, Capobianco A. Induction of cyclin D1 transcription and CDK2 activity by Notch(ic): implication for cell cycle disruption in transformation by Notch(ic). Mol Cell Biol 2001; 21:5925-34; PMID:11486031; http://dx.doi.org/10.1128/MCB.21.17.5925-5934.2001
- Luistro L, He W, Smith M, Packman K, Vilenchik M, Carvajal D, Roberts J, Cai J, Berkofsky-Fessler W, Hilton H, et al. Preclinical profile of a potent gamma-secretase inhibitor targeting notch signaling with in vivo efficacy and pharmacodynamic properties. Cancer Res 2009; 69:7672-80; PMID:19773430; http://dx.doi.org/10.1158/0008-5472.CAN-09-1843
- Huynh C, Poliseno L, Segura M, Medicherla R, Haimovic A, Menendez S, Shang S, Pavlick A, Shao Y, Darvishian F, et al. The novel gamma secretase inhibitor RO4929097 reduces the tumor initiating potential of melanoma. PLoS One 2011; 6:e25264; PMID:21980408; http://dx.doi.org/10.1371/journal.pone.0025264
- Diaz-Padilla I, Wilson M, Clarke B, Hirte H, Welch S, Mackay H, Biagi J, Reedijk M, Weberpals J, Fleming G, et al. A phase II study of single-agent RO4929097, a gamma-secretase inhibitor of Notch signaling, in patients with recurrent platinum-resistant epithelial ovarian cancer: a study of the Princess Margaret, Chicago and California phase II consortia. Gynecol Oncol 2015; 137:216-22; PMID:25769658; http://dx.doi.org/10.1016/j.ygyno.2015.03.005
- Lee S, Moon J, Redman B, Chidiac T, Flaherty L, Zha Y, Othus M, Ribas A, Sondak V, Gajewski T, et al. Phase 2 study of RO4929097, a gamma-secretase inhibitor, in metastatic melanoma: SWOG 0933. Cancer 2015; 121:432-40; PMID:25250858; http://dx.doi.org/10.1002/cncr.29055
- De Jesus-Acosta A, Laheru D, Maitra A, Arcaroli J, Rudek M, Dasari A, Blatchford P, Quackenbush K, Messersmith W. A phase II study of the gamma secretase inhibitor RO4929097 in patients with previously treated metastatic pancreatic adenocarcinoma. Invest New Drug 2014; 32:739-45; PMID:24668033; http://dx.doi.org/10.1007/s10637-014-0083-8
- Strosberg J, Yeatman T, Weber J, Coppola D, Schell M, Han G, Almhanna K, Kim R, Valone T, Jump H, et al. A phase II study of RO4929097 in metastatic colorectal cancer. Eur J Cancer 2012; 48:997-1003; PMID:22445247; http://dx.doi.org/10.1016/j.ejca.2012.02.056
- Richter S, Bedard P, Chen E, Clarke B, Tran B, Hotte S, Stathis A, Hirte H, Razak A, Reedijk M, et al. A phase I study of the oral gamma secretase inhibitor R04929097 in combination with gemcitabine in patients with advanced solid tumors (PHL-078/CTEP 8575). Invest New Drugs 2014; 32:243-9; PMID:23645447; http://dx.doi.org/10.1007/s10637-013-9965-4
- Dantas-Barbosa C, Bergthold G, Daudigeos-Dubus E, Blockus H, Boylan J, Ferreira C, Puget S, Abely M, Vassal G, Grill J, et al. Inhibition of the NOTCH pathway using γ-secretase inhibitor RO4929097 has limited antitumor activity in established glial tumors. Anticancer Drugs 2015; 26:272-83; PMID:25486598; http://dx.doi.org/10.1097/CAD.0000000000000190
- van Stralen E, van de Wetering M, Agnelli L, Neri A, Clevers H, Bast B. Identification of primary MAFB target genes in multiple myeloma. Exp Hematol 2009; 37:78-86; PMID:19013005; http://dx.doi.org/10.1016/j.exphem.2008.08.006
- Houde C, Li Y, Song L, Barton K, Zhang Q, Godwin J, Nand S, Toor A, Alkan S, Smadja NV, et al. Overexpression of the NOTCH ligand JAG2 in malignant plasma cells from multiple myeloma patients and cell lines. Blood 2004; 104:3697-704; PMID:15292061; http://dx.doi.org/10.1182/blood-2003-12-4114
- Xu D, Hu J, De Bruyne E, Menu E, Schots R, Vanderkerken K, Van Valckenborgh E. Dll1/Notch activation contributes to bortezomib resistance by upregulating CYP1A1 in multiple myeloma. Biochem Biophys Res Commun 2012; 428:518-24; PMID:23111325; http://dx.doi.org/10.1016/j.bbrc.2012.10.071
- Ding Y, Shen Y. Notch increased vitronection adhesion protects myeloma cells from drug induced apoptosis. Biochem Biophys Res Commun 2015; 467:717-22; PMID:26494298; http://dx.doi.org/10.1016/j.bbrc.2015.10.076
- Mirandola L, Apicella L, Colombo M, Yu Y, Berta D, Platonova N, Lazzari E, Lancellotti M, Bulfamante G, Cobos E, et al. Anti-Notch treatment prevents multiple myeloma cells localization to the bone marrow via the chemokine system CXCR4/SDF-1. Leukemia 2013; 27:1558-66; PMID:23354012; http://dx.doi.org/10.1038/leu.2013.27
- Colombo M, Thümmler K, Mirandola L, Garavelli S, Todoerti K, Apicella L, Lazzari E, Lancellotti M, Platonova N, Akbar M, et al. Notch signaling drives multiple myeloma induced osteoclastogenesis. Oncotarget 2014; 5:10393-406; PMID:25257302; http://dx.doi.org/10.18632/oncotarget.2084
- Schwarzer R, Nickel N, Godau J, Willie B, Duda G, Schwarzer R, Cirovic B, Leutz A, Manz R, Bogen B, et al. Notch pathway inhibition controls myeloma bone disease in the murine MOPC315. BM model. Blood Cancer J 2014; 4:e217; PMID:24927406; http://dx.doi.org/10.1038/bcj.2014.37
- He W, Luistro L, Carvajal D, Smith M, Nevins T, Yin X, Cai J, Higgins B, Kolinsky K, Rizzo C, et al. High tumor levels of IL6 and IL8 abrogate preclinical efficacy of the γ-secretase inhibitor, RO4929097. Mol Oncol 2011; 5:292-301; PMID:21315665; http://dx.doi.org/10.1016/j.molonc.2011.01.001
- Chen F, Pisklakova A, Li M, Baz R, Sullivan D, Nefedova Y. Gamma-secretase inhibitor enhances the cytotoxic effect of bortezomib in multiple myeloma. Cell Oncol 2011; 34:545-51; PMID:21965140; http://dx.doi.org/10.1007/s13402-011-0060-6
- Nefedova Y, Sullivan D, Bolick S, Dalton W, Gabrilovich D. Inhibition of Notch signaling induces apoptosis of myeloma cells and enhances sensitivity to chemotherapy. Blood 2008; 111:2220-9; PMID:18039953; http://dx.doi.org/10.1182/blood-2007-07-102632
- Jakob C, Sterz J, Zavrski I, Heider U, Kleeberg L, Fleissner C, Kaiser M, Sezer O. Angiogenesis in multiple myeloma. Euro J Cancer 2006; 42:1581-90; PMID:16797965; http://dx.doi.org/10.1016/j.ejca.2006.02.017
- Vacca A, Ribatti D. Bone marrow angiogenesis in multiple myeloma. Leukemia 2006; 20:193-9; PMID:16357836; http://dx.doi.org/10.1038/sj.leu.2404067
- Ashley J, Ahn J, Hankenson K. Notch signaling promotes osteoclast maturation and resorptive activity. J Cell Biochem 2015; 116:2598-609; PMID:25914241; http://dx.doi.org/10.1002/jbc.25205
- Kaiser M, Mieth M, Liebisch P, Oberländer R, Rademacher J, Jakob C, Kleeberg L, Fleissner C, Braendle E, Peters M, et al. Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma. Eur J Haematol 2008; 80:490-4; PMID:18331598; http://dx.doi.org/10.1111/j.1600-0609.2008.01065.x
- Curry C, Reed L, Nickoloff B, Miele L, Foreman K. Notch-independent regulation of Hes-1 expression by c-Jun N-terminal kinase signaling in human endothelial cells. Lab Invest 2006; 86:842-52; PMID:16732296; http://dx.doi.org/10.1038/labinvest.3700442
- Doetzlhofer A, Basch M, Ohyama T, Gessler M, Groves A, Segil N. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell 2009; 16:58-69; PMID:19154718; http://dx.doi.org/10.1016/j.devcel.2008.11.008
- Nakazaki H, Reddy A, Mania-Farnell B, Shen Y, Ichi S, McCabe C, George D, McLone D, Tomita T, Mayanil C. Key basic helix-loop-helix transcription factor genes Hes1 and Ngn2 are regulated by Pax3 during mouse embryonic development. Dev Biol 2008; 316:510-23; PMID:18308300; http://dx.doi.org/10.1016/j.ydbio.2008.01.008
- Jurczyszyn A, Czepiel J, Biesiada G, Gdula-Argasińska J, Cibor D, Owczarek D, Perucki W, Skotnicki A. HGF, sIL-6R and TGF-β1 play a significant role in the progression of multiple myeloma. J Cancer 2014; 5:518-24; PMID:24963356; http://dx.doi.org/10.7150/jca.9266
- Urashima M, Ogata A, Chauhan D, Hatziyanni M, Vidriales M, Dedera D, Schlossman R, Anderson KC. Transforming growth factor-beta1: differential effects on multiple myeloma versus normal B cells. Blood 1996; 87:1928-38; PMID:8634441
- Kurpinski K, Lam H, Chu J, Wang A, Kim A, Tsay E, Agrawal S, Schaffer D, Li S. Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells 2010; 28:734-42; PMID:20146266; http://dx.doi.org/10.1002/stem.319
- Kennard S, Liu H, Lilly B. Transforming growth factor-beta (TGF- 1) down-regulates Notch3 in fibroblasts to promote smooth muscle gene expression. J Biol Chem 2008; 283:1324-33; PMID:17981798; http://dx.doi.org/10.1074/jbc.M706651200
- Blokzijl A, Dahlqvist C, Reissmann E, Falk A, Moliner A, Lendahl U, Ibáñez C. Cross-talk between the Notch and TGF-beta signaling pathways mediated by interaction of the Notch intracellular domain with Smad3. J Cell Biol 2003; 163:723-8; PMID:14638857; http://dx.doi.org/10.1083/jcb.200305112
- Nyhan K, Faherty N, Murray G, Cooey L, Godson C, Crean J, Brazil D. Jagged/Notch signalling is required for a subset of TGFβ1 responses in human kidney epithelial cells. Biochim Biophys Acta 2010; 1803:1386-95; PMID:20833210; http://dx.doi.org/10.1016/j.bbamcr.2010.09.001
- Samon J, Champhekar A, Minter L, Telfer J, Miele L, Fauq A, Das P, Golde T, Osborne B. Notch1 and TGFbeta1 cooperatively regulate Foxp3 expression and the maintenance of peripheral regulatory T cells. Blood 2008; 112:1813-21; PMID:18550850; http://dx.doi.org/10.1182/blood-2008-03-144980
- Zhang Z, Wang H, Ikeda S, Fahey F, Bielenberg D, Smits P, Hauschka P. Notch3 in human breast cancer cell lines regulates osteoblast-cancer cell interactions and osteolytic bone metastasis. Am J Pathol 2010; 177:1459-69; PMID:20651241; http://dx.doi.org/10.2353/ajpath.2010.090476
- Timmerman L, Grego-Bessa J, Raya A, Bertrán E, Pérez-Pomares J, Díez J, Aranda S, Palomo S, McCormick F, Izpisúa-Belmonte J, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev 2004; 18:99-115
- Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred E, Moore D, Meli S, Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst 1992; 84:1875-87; PMID:1281237; http://dx.doi.org/10.1093/jnci/84.24.1875
- Kerbel R. Tumor angiogenesis: past, present and the near future. Carcinogenesis 2000; 21:505-15; PMID:10688871; http://dx.doi.org/10.1093/carcin/21.3.505
- Bertolini F, Mancuso P, Gobbi A, Pruneri G. The thin red line: angiogenesis in normal and malignant hematopoiesis. Exp Hematol 2000; 28:993-1000; PMID:11008011; http://dx.doi.org/10.1016/S0301-472X(00)00508-7
- Rajkumar S, Mesa R, Fonseca R, Schroeder G, Plevak M, Dispenzieri A, Lacy M, Lust J, Witzig T, Gertz M, et al. Bone marrow angiogenesis in 400 patients with monoclonal gammopathy of undetermined significance, multiple myeloma, and primary amyloidosis. Clin Cancer Res 2002; 8:2210-6; PMID:12114422
- Pruneri G, Ponzoni M, Ferreri A, Decarli N, Tresoldi M, Raggi F, Baldessari C, Freschi M, Baldini L, Goldaniga M, et al. Microvessel density, a surrogate marker of angiogenesis, is significantly related to survival in multiple myeloma patients. Br J Haematol 2002; 118:817-20; PMID:12181051; http://dx.doi.org/10.1046/j.1365-2141.2002.03654.x
- Alexandrakis M, Pappa C, Kokonozaki M, Boula A, Vyzoukaki R, Staphylaki D, Papadopoulou A, Androulakis N, Tsirakis G, Sfiridaki A. Circulating serum levels of IL-20 in multiple myeloma patients: its significance in angiogenesis and disease activity. Med Oncol 2015; 32:42; PMID:25631632; http://dx.doi.org/10.1007/s12032-015-0488-z
- Kangsamaksin T, Tattersall I, Kitajewski J. Notch functions in developmental and tumour angiogenesis by diverse mechanisms. Biochem Soc Trans 2014; 42:1563-8; PMID:25399571; http://dx.doi.org/10.1042/BST20140233
- Bai S, Kopan R, Zou W, Hilton M, Ong C, Long F, Ross F, Teitelbaum S. NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J Biol Chem 2008; 283:6509-18; PMID:18156632; http://dx.doi.org/10.1074/jbc.M707000200
- Ashley J, Ahn J, Hankenson K. Notch signaling promotes osteoclast maturation and resorptive activity. J Cell Biochem 2015; 116:2598-609; PMID:25914241; http://dx.doi.org/10.1002/jcb.25205
- Yamada T, Yamazaki H, Yamane T, Yoshino M, Okuyama H, Tsuneto M, Kurino T, Hayashi S, Sakano S. Regulation of osteoclast development by Notch signaling directed to osteoclast precursors and through stromal cells. Blood 2003; 101:2227-34; PMID:12411305; http://dx.doi.org/10.1182/blood-2002-06-1740
- Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JJ. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 2003; 349:2483-94; PMID:14695408; http://dx.doi.org/10.1056/NEJMoa030847
- Yang G, Xu Y, Chen H, Wang X. Acute lymphoblastic leukemia cells inhibit the differentiation of bone mesenchymal stem cells into osteoblasts in vitro by activating notch signaling. Stem Cells Int 2015; 2015:162410; PMID:26339248; http://dx.doi.org/10.1155/2015/162410
