ABSTRACT
Perivascular epithelioid cell tumor is a rare tumor. To date, there is no consensus of therapy to be recommended for unresectable disease. For a low incidence and a rarely curable disease, the finding of new therapy is essential.
Here we report the first case of a patient with perivascular epithelioid cell tumor whose disease had a rapid progression after surgery and had a rapid remarkable response of combination therapy of a VEGFR inhibitor, sorafenib, with an mTOR inhibitor, sirolimus.
This result may have potential to deliver a new treatment option and inhibiting the mTOR pathway combined with inhibiting the VEGF pathways may be a useful strategy for malignant PEComas.
Abbreviations
| CT | = | computed tomographic |
| HMB-45 | = | human melanoma black-45 |
| mTOR | = | mammalian target of rapamycin |
| PEComa | = | perivascular epithelioid cell tumor |
| PET-CT | = | positron emission tomography-CT |
| VEGFR | = | vascular endothelial growth factor receptor |
Introduction
Perivascular epithelioid cell tumor (PEComa) was first proposed by Zamboni et al. in 1996,Citation1,2 and classified as a group of mesenchymal tumors originating from perivascular epithelioid cells by the World Health Organization (WHO) in 2002.Citation2-4 PEComas have since been reported to derive from various locations, including the uterus, lung, liver, retroperitoneum, prostate and kidney.Citation5-8 As the number of malignant PEComa cases reported increases, the common metastatic sites of uterine PEComa are found in the lung, liver, but rare in kidney.Citation9 PEComa is commonly asymptomatic, and therefore its diagnosis may be difficult on the images in clinic. It is diagnosed based on its pathological and immunohistochemical characteristics.Citation10 The surgical excision may be an only way of the curable treatment for malignant PEComa. Although chemotherapy and/or radiotherapy have been reported in a small number of cases, the treatment of advanced malignant PEComas is still challenging, and no uniform regimen has been proposed for patients with metastatic or recurrent disease. Recently the mammalian target of rapamycin (mTOR) inhibitor, sirolimus (rapamycin), has been used for treatment of the patients with metastatic PEComa with mixed results.Citation11-13 Sorafenib, a vascular endothelial growth factor receptor (VEGFR) inhibitor, showed some objective responses in patients with advanced soft tissue sarcomas.Citation14,15 However, because of its relative rarity, experience with mTOR inhibitor or VEGFR inhibitor in treating malignant PEComas is limited. To our knowledge, there are no reports on the use of an mTOR inhibitor combined with a VEGFR inhibitor as palliative therapy in malignant PEComa. Herein, we report a rapidly progressive advanced malignant PEComa of uterus occurring in a middle-aged female patient with rare kidney and lung metastases, who had a remarkable response on the treatment of mTOR inhibitor, sirolimus, combining with VEGFR inhibitor, sorafenib.
Case report
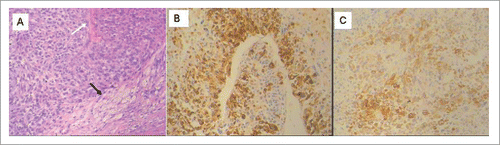
A 47-year-old woman with a right lung nodule was admitted to our hospital in March 2014 (). Two years previously, she had undergone uterectomy for “uterine leiomyosarcoma,” and nephrectomy for the left renal metastasis 2 months ago at another hospital. One week late, the patient underwent a pulmonary metastasectomy at our hospital. Macroscopically, the mass was measured at 4 × 3.5 × 2 cm. The section of the mass was gray-white in color and had areas of hemorrhage. Specimens consisted of a sheet-like pattern of epithelioid cells. The tumor cells were moderate and had a high degree of nuclear pleomorphism and macronucleoli (). The mitotic count was 1 per 50 under high power field. Lymphovascular invasion was detected. Immunohistochemically, pulmonary lesion showed a positive for human melanoma black (HMB)-45 () and Melan A (), which were same as retrospective specimens of resection for kidney lesion and initial uteric tumor (data not shown). The tumor cells were negative for S-100, Desmin, Vimentin. Based on these features, she was diagnosed as malignant uteric PEComa with renal and pulmonary metastases.
Figure 1. Response of sorafenib combined with sirolimus for metastatic PEComa in the lungs (A) CT demonstrated a mass in the right lung before surgery. (B, C) PET-CT showed pulmonary metastases one month after surgery. (D-F) CT showed complete response of pulmonary metastases after combination therapy.
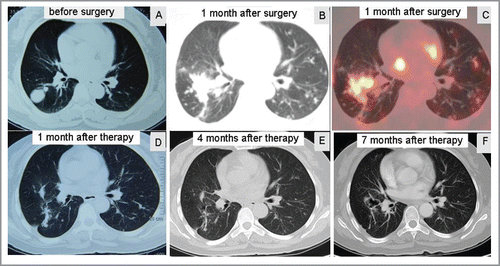
Figure 2. Pathological and immunohistochemistry features of pulmonary metastasis of uterine PEComa. (A) Tumor cells with prominent nucleoli and irregular mitoses were arranged radially around the blood vessels (white arrow), HE, × 100. Focal fat cells (black arrow) were found within tumor, HE × 100. (B, C) The tumor cells were positive for HMB45 (B), Melan-A (C) by immunohistochemistry staining, IHC × 100.
One month after the pulmonary surgery, she complained of chest distress and pain in abdomen, and positron emission tomography-CT (PET-CT) scan showed strong F-18-2-fluoro-2-deoxyglucose (FDG) uptake in multiple sites of lungs (), and live, left adrenal gland, retroperitoneal lymph node, and complicated by tumor thrombus in left and right upper pulmonary artery, and the inferior vena cava (data not shown). After a discussion with the patient, she was administrated on sirolimus at 200 mg for 7 d then 300 mg per day combining with sorafenib at 400 mg twice per day, respectively. One month after the combination therapy, the abdominal pain was complete response. CT scan showed remarkable shrinkage of metastatic lesions, and some had complete response on the assessment (). Four months and 7 month after the continuing combination therapy, the patient showed a complete response on the following-up assessment (), respectively. After that, the patient went back home and lost follow-up.
Discussion
Perivascular Epithelioid Cell Tumor (PEComa) is a very rare type of mesenchymal malignant tumor. The common metastatic sites of malignant PEComa might be involved in liver, lung, bone, and lymph nodes, but fewer kidney metastases were described. To our knowledge, this is the second case of metastatic PEComa in kidney reported.Citation8
Since there are no imaging characteristics for the identification of malignant PEComa before surgery, the diagnosis of this patient was confirmed immunohistochemically by the positive stains to the markers including the positive HMB-45 and Melan A.16 The clinical pathological characteristics in our case was that the rapid multiple progressive metastases developed just in one month after pulmonary metastasectomy (). For such swift progressive case, because of the rarity of disease, there currently is no standard therapy to be recommended. The cytotoxic chemotherapy agents showed minimal response or no response. Sirolimus might be one of the most common options of treatment for advanced PEComas.Citation17 Sorafenib had a response in patients with sarcomas.Citation14,18 These encouraged us to choose combination therapy of sirolimus with sorafenib in the treatment of this patient with advanced PEComa. The results showed a remarkable response of metastatic tumor on CT scan after the first one-month of treatment (), and there was no CT evidence of disease progression for another 6 months (), suggesting that combination target therapy of sorafenib with sirolimus had a successful treatment for this rapidly progressive advanced PEComa.
Although their precise mechanisms of combination therapy of sirolimus with sorafenib are unclear, their different mechanisms of action in pathways of cell proliferation and angiogenesis present a rationale for their combination. Sirolimus is the prototypical inhibitor of mammalian target of rapamycin (mTOR), a key regulator of protein translation. PEComa has been specially related to the mTOR pathway. The development of PEComa may be induced by dysfunction in tumor suppressors TSC1/2 and the subsequent upregulation of mTORC1.Citation19 Sirolimus has proved the role of antitumor activityCitation13,19-21 and inhibiting mTOR kinase activity by interacting with FK506 binding protein (FKBP12), which is one of the proteins that form mTORC1. This induces cell cycle arresting in G1 phase and the subsequent inhibition in proliferation and cell growth. On the other hand, sorafenib is a multikinase inhibitor targeting VEGF-R 2 and 3, PDGFR, Raf, and KIT, and has antitumor activity in a variety of malignancies, including angiosarcoma,Citation15 advanced renal cell carcinoma,Citation18 advanced hepatic carcinoma.Citation22 and Several preclinical studies have demonstrated efficacy in different soft-tissue and bone sarcoma cell lines.Citation23 Maki et al. published a phase II trial of sorafenib in 145 patients with different types of advanced sarcomas.Citation24 Of them, 37 patients with angiosarcoma had 14% of response rate (RR) primary end point planned for the study. However, because of its very rarity, there to date is no any clinical trial report of combination therapy of sorafenib with sirolimus for PEComa. A preclinical study showed that sirolimus combined with sorafenib prolonged survival and induced tumor shrinkage comparing with treatment of sirolimus or sorafenib alone in vivo.Citation25 In order to verify the safety of combination therapy of sirolimus with sorafenib, Gangadhar et al. examined pharmacokinetically a difference in Cmax for sirolimus and sorafenib in patients with various carcinomas. Their results showed no significant differences in Cmax for any of the agents alone compared to the Cmax during combination therapy of sirolimus with sorafenib and no unexpected toxicity noted in patients with sirolimus combined with sorafenib compared to those expected for either drug alone, implying that sirolimus combined with sorafenib could be safely used to treat the patients with carcinomas.Citation26 Therefore, we used a multikinase inhibitor, sorafenib, combined with an mTOR inhibitor, sirolimus, to treat the patient with multiple metastases of uteric PEComa and illustrated a remarkable response of metastatic diseases. This suggests that combined targeting the VEGF pathway with inhibiting the mTOR pathway may be a useful strategy for the treatment of malignant PEComa.
Conclusions
For a very low incidence and a rarely curable disease, the finding of new therapy is essential. Although the targeted agents have been increasingly developed in the last years, very few are administrated and have response in malignant PEComa. One of the strategies is to explore a way of combination target therapy. We here firstly report that combination therapy of sorafenib with sirolimus had a rapidly remarkable response in a patient with advanced PEComa. This suggested that the strategy of inhibiting the mTOR pathway combined with inhibiting the VEGF pathways may be an additional option in patients with malignant PEComas. The future study should explore the biologic rationale of sorafenib inducing this additive clinical efficacy, especially should focus on the examination of changes of VEGF pathway and mTOR pathway induced by sorafenib and sirolimus, respectively.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The work was supported by the Foundation of Health and Family Planning Commission of Shandong Province (Grant Nos. 2013WSC02041 and YG201519) and Shandong Natural Science Foundation (Grant Nos. ZR2012HL33).
References
- Zamboni G, Pea M, Martignoni G, Zancanaro C, Faccioli G, Gilioli E, Pederzoli P, Bonetti F. Clear cell "sugar" tumor of the pancreas. A novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J Surg Pathol 1996; 20:722-30; PMID:8651352; http://dx.doi.org/10.1097/00000478-199606000-00010
- Armah HB, Parwani AV. Perivascular epithelioid cell tumor. Arch Pathol Lab Med 2009; 133:648-54; PMID:19391667; http://dx.doi.org/10.1043/1543-2165-133.4.648
- Folpe AL, McKenney JK, Li Z, Smith SJ, Weiss SW. Clear cell myomelanocytic tumor of the thigh: report of a unique case. Am J Surg Pathol 2002; 26:809-12; PMID:12023589; http://dx.doi.org/10.1097/00000478-200206000-00018
- Folpe AL, Weiss SW. Lipoleiomyosarcoma (well-differentiated liposarcoma with leiomyosarcomatous differentiation): a clinicopathologic study of nine cases including one with dedifferentiation. Am J Surg Pathol 2002; 26:742-9; PMID:12023578; http://dx.doi.org/10.1097/00000478-200206000-00007
- Bleeker JS, Quevedo JF, Folpe AL. Malignant perivascular epithelioid cell tumor of the uterus. Rare Tumors 2012; 4:e14; PMID:22532912; http://dx.doi.org/10.4081/rt.2012.e14
- Bergamo F, Maruzzo M, Basso U, Montesco MC, Zagonel V, Gringeri E, Cillo U. Neoadjuvant sirolimus for a large hepatic perivascular epithelioid cell tumor (PEComa). World J Surg Oncol 2014; 12:46; PMID:24575738; http://dx.doi.org/10.1186/1477-7819-12-46
- Wu JH, Zhou JL, Cui Y, Jing QP, Shang L, Zhang JZ. Malignant perivascular epithelioid cell tumor of the retroperitoneum. Int J Clin Exp Pathol 2013; 6:2251-6; PMID:24133607
- Shi H, Cao Q, Li H, Zhen T, Lai Y, Han A. Malignant perivascular epithelioid cell tumor of the kidney with rare pulmonary and ileum metastases. Int J Clin Exp Pathol 2014; 7:6357-63; PMID:25337291
- Bleeker JS, Quevedo JF, Folpe AL. "Malignant" perivascular epithelioid cell neoplasm: risk stratification and treatment strategies. Sarcoma 2012; 2012:541626; PMID:22619565; http://dx.doi.org/10.1155/2012/541626
- Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol 2005; 29:1558-75; PMID:16327428; http://dx.doi.org/10.1097/01.pas.0000173232.22117.37
- Benson C, Vitfell-Rasmussen J, Maruzzo M, Fisher C, Tunariu N, Mitchell S, Al-Muderis O, Thway K, Larkin J, Judson I. A retrospective study of patients with malignant PEComa receiving treatment with sirolimus or temsirolimus: the Royal Marsden Hospital experience. Anticancer Res 2014; 34:3663-8; PMID:24982384
- Scheppach W, Reissmann N, Sprinz T, Schippers E, Schoettker B, Mueller JG. PEComa of the colon resistant to sirolimus but responsive to doxorubicin/ifosfamide. World J Gastroenterol 2013; 19:1657-60; PMID:23539498; http://dx.doi.org/10.3748/wjg.v19.i10.1657
- Dickson MA, Schwartz GK, Antonescu CR, Kwiatkowski DJ, Malinowska IA. Extrarenal perivascular epithelioid cell tumors (PEComas) respond to mTOR inhibition: clinical and molecular correlates. Int J Cancer 2013; 132:1711-7; PMID:22927055; http://dx.doi.org/10.1002/ijc.27800
- Lee DW, Chang H, Kim YJ, Kim KM, Lee HJ, Lee JS. Sorafenib-induced tumor response in a patient with metastatic epithelioid angiomyolipoma. J Clin Oncol 2014; 32:e42-5; PMID:24449236; http://dx.doi.org/10.1200/JCO.2012.48.1960
- Ray-Coquard I, Italiano A, Bompas E, Le Cesne A, Robin YM, Chevreau C, Bay JO, Bousquet G, Piperno-Neumann S, Isambert N, et al. Sorafenib for patients with advanced angiosarcoma: a phase II Trial from the French Sarcoma Group (GSF/GETO). Oncologist 2012; 17:260-6; PMID:22285963; http://dx.doi.org/10.1634/theoncologist.2011-0237
- Filho Jdo E, Meneses de Amorim D, Sweet GM, Rodrigues de Freitas LA, Athanazio PR, Athanazio DA. Renal epithelioid angiomyolipoma with epithelial cysts: demonstration of Melan A and HMB45 positivity in the cystic epithelial lining. Ann Diagn Pathol 2012; 16:397-401; PMID:21676635; http://dx.doi.org/10.1016/j.anndiagpath.2011.03.004
- Palleschi G, Pastore AL, Evangelista S, Silvestri L, Rossi L, Di Cristofano C, Porta N, Petrozza V, Tomao S, Carbone A. Bone metastases from bladder perivascular epithelioid cell tumor - an unusual localization of a rare tumor: a case report. J Med Case Rep 2014; 8:227; PMID:24965209; http://dx.doi.org/10.1186/1752-1947-8-227
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007; 356:125-34; PMID:17215530; http://dx.doi.org/10.1056/NEJMoa060655
- Wagner AJ, Malinowska-Kolodziej I, Morgan JA, Qin W, Fletcher CD, Vena N, Ligon AH, Antonescu CR, Ramaiya NH, Demetri GD, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol 2010; 28:835-40; PMID:20048174; http://dx.doi.org/10.1200/JCO.2009.25.2981
- Gennatas C, Michalaki V, Kairi PV, Kondi-Paphiti A, Voros D. Successful treatment with the mTOR inhibitor everolimus in a patient with perivascular epithelioid cell tumor. World J Surg Oncol 2012; 10:181; PMID:22943457; http://dx.doi.org/10.1186/1477-7819-10-181
- Italiano A, Delcambre C, Hostein I, Cazeau AL, Marty M, Avril A, Coindre JM, Bui B. Treatment with the mTOR inhibitor temsirolimus in patients with malignant PEComa. Ann Oncol 2010; 21:1135-7; PMID:20215136; http://dx.doi.org/10.1093/annonc/mdq044
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10:25-34; PMID:19095497; http://dx.doi.org/10.1016/S1470-2045(08)70285-7
- Peng CL, Guo W, Ji T, Ren T, Yang Y, Li DS, Qu HY, Li X, Tang S, Yan TQ, et al. Sorafenib induces growth inhibition and apoptosis in human synovial sarcoma cells via inhibiting the RAF/MEK/ERK signaling pathway. Cancer biology & therapy 2009; 8:1729-36; PMID:19633425; http://dx.doi.org/10.4161/cbt.8.18.9208
- Maki RG, D'Adamo DR, Keohan ML, Saulle M, Schuetze SM, Undevia SD, Livingston MB, Cooney MM, Hensley ML, Mita MM, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol 2009; 27:3133-40; PMID:19451436; http://dx.doi.org/10.1200/JCO.2008.20.4495
- Lee N, Woodrum CL, Nobil AM, Rauktys AE, Messina MP, Dabora SL. Rapamycin weekly maintenance dosing and the potential efficacy of combination sorafenib plus rapamycin but not atorvastatin or doxycycline in tuberous sclerosis preclinical models. BMC Pharmacol 2009; 9:8; PMID:19368729; http://dx.doi.org/10.1186/1471-2210-9-8
- Gangadhar TC, Cohen EE, Wu K, Janisch L, Geary D, Kocherginsky M, House LK, Ramirez J, Undevia SD, Maitland ML, et al. Two drug interaction studies of sirolimus in combination with sorafenib or sunitinib in patients with advanced malignancies. Clin Cancer Res 2011; 17:1956-63; PMID:21447721; http://dx.doi.org/10.1158/1078-0432.CCR-10-2061
