ABSTRACT
Von Hippel–Lindau (VHL) disease is a rare autosomal dominant inherited cancer syndrome that is characterized by hemangioblastomas in the central nervous system and retina, renal cell carcinoma and cysts, pancreatic tumors and cysts, and pheochromocytoma. The underlying gene in this disease is the VHL tumor suppressor gene. We characterized a family with 2 affected siblings. The brother and sister displayed VHL type 2B and type 2A features, respectively. Renal lesions in the brother exhibited 3 different phenotypes, including simple renal cysts, multilocular cystic renal cell carcinoma and clear cell renal cell carcinoma. The phenotypes of the 3 concurrent renal lesions were first reported in this study. Mutation detection of the VHL gene revealed 2 recurrent mutations, namely c.256C>T (p.P86S) and c.340 + 5G > C. The former was predicted to be deleterious and to destabilize the hydrophobic core and lead to VHL dysfunction; however, the latter was predicted to be a benign variant. Our findings provided new data for the genotype-phenotype of VHL diseases and elucidated the pathogenic mechanism with in silico analysis.
Introduction
Von Hippel–Lindau (VHL, MIM #193300) disease is an autosomal dominant inherited cancer syndrome. Errors in the VHL gene (Gene ID: 7428) are believed to be responsible for this disease.Citation1,2 This disease has a variety of clinical phenotypes and is routinely classified into type 1 (without pheochromocytoma) and type 2 (with pheochromocytoma). Type 1 displays typical VHL manifestations with hemangioblastomas and clear cell renal cell carcinomas (ccRCC), while type 2 is subdivided into 2A (with low risk of ccRCC), 2B (with increased risk of ccRCC), and 2C (pheochromocytoma only).Citation3,4 Those patients can also develop epididymal or broad ligament cystadenomas and other cystic changes in the kidney, pancreas and liver.Citation1,2 The same family or the same mutation could exhibit different subtypes of VHL disease.Citation1 However, different subtypes of renal cell carcinoma in one person with multilocular cystic renal cell carcinoma (mcRCC) concurrent with ccRCC are very rare.Citation5 In this study, we present 2 VHL patients with type 2B and type 2A in a Chinese family, where the former is concurrent with simple renal cyst, mcRCC and ccRCC. To our knowledge, this is the first report of a VHL case. Mutation detection and bioinformatics predictions in the VHL gene identified the errors in this family.
Patients and clinical description
The brother
A 42-year-old man was admitted to our Urology unit with a one-year history of repeating left lumbago. Ten years ago, this patient received gamma knife treatment for cerebral hemangioblastomas. There was no history of hypertension disease (blood pressure at admission: 134/79mmHg). Eyesight and fundus examinations were normal.
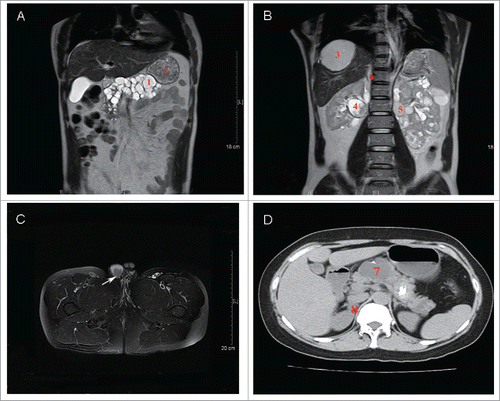
Prior to the operation, levels of blood creatinine and urea nitrogen were normal (82 μmol/L and 6.05 mmol/L, respectively). However, the routine urine test showed an elevated level of RBCs with 9,298 cells per liter. Common tumor markers such as AFP, CEA, and PSA were normal. Magnetic resonance (MR) of the abdomen showed multiple solid lesions in the bilateral kidneys (53 mm was the largest diameter) with enhanced density after contrast, multilocular renal cysts, a right adrenal nodule, multiple cysts and a solid lesion in the pancreas (42.7 mm was the largest diameter), and multiple cysts in the liver with 2 highly vascular lesions located in the S7/8 and S2 sections (). Special tests for adrenal lesions included plasma cortisol, renin angiotensin aldosterone, and urinary VMA, which were normal. The patient underwent 3 consecutive surgeries. The first surgery was partial nephrectomy that removed most of the tumors in the right kidney. Three weeks later, the second surgery removed most of the tumors on the surface of the left kidney, and radiofrequency ablation guided by ultrasound aided in treating the inner tumors. Two weeks after the second surgery, the patient received an emergency nephrectomy on the left side kidney due to a severe hemorrhage.
Figure 1. Imaging findings of 2 patients. (A) and (B) Abdomen magnetic resonance (MR) of the brother (II:1) showed a solid lesion (1) and multiple cysts (2) in the pancreas; liver vascular lesion located in the S7/8 section (3); solid lesions (4) and multilocular renal cysts (5) in the kidney; and a right adrenal nodule (6). (C) Scrotum magnetic resonance (MR) of the brother (II:1) showed a malignant tumor in the right side epididymis (arrow). (D) Abdomen computed tomography (CT) of the sister (II:6) showed multiple cysts in the pancreas (7) and a right adrenal nodule (8).
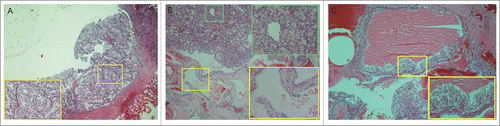
Pathologic reviews and an immunohistochemical test (IHC) were performed to confirm the diagnosis. Tumor sections from the right side kidney stained with hematoxylin and eosin (H&E) showed sheet-like, solid clear cells in the tumor lesions and cysts lined by clear cells with uniform nuclei in some areas (). The pathological manifestations confirmed ccRCC concurrent with simple renal cysts. The left side kidney showed multilocular cysts lined by clear cells with plenty of plasm and high nuclear to cytoplasmic ratios by microscopy. Clusters of clear cells were present in the septal walls (), and mcRCC was confirmed pathologically. The IHC staining of the bilateral kidney tumor was positive for CD10, CK and Vim (data not shown).
Figure 2. Pathological images of the brother. (A) Clear cell renal cell carcinoma (ccRCC) from the right kidney. The tumor consists of sheet-like, solid clear cells (H&E 40×).The image in the yellow-boxed area displays part (B). Multilocular renal cysts with clear cell renal carcinoma from the right kidney. The yellow-boxed area displays a renal cyst that is lined by a single-layer of clear cells with uniform nuclei (H&E 100×). Additionally, the green-boxed area displays part of the ccRCC with sheet-like, solid clear cells. (C) Cystic renal cell carcinoma from the left kidney. Cysts are lined by clear cells with uniform nuclei. Clusters of clear cells are present in the septal walls (H&E 40×). The image in the yellow-boxed area displays the aggregates of similar-appearing clear cells scattered without permeating the septa and forming solid nodules (H&E 100×).
During this patient's hospitalization, a mass was palpable in his right side scrotum, which had no tenderness, redness or itching. The scrotum MR suspected a malignant tumor on the right side epididymis (). However, the patient refused to accept a biopsy of this mass.
The sister
The younger sister of the proband was 34 y old. Six years ago, she also received gamma knife treatment for cerebral hemangioblastomas and had a normal blood pressure.
Computed tomography (CT) of the abdomen showed a right adrenal nodule (17 mm in diameter) (), multiple cysts in the pancreas (52 mm was the largest diameter) (), and multiple cysts in the liver at the S5/8 section. However, there were no renal lesions. A cranium CT scan showed 2 novel lesions located at the supra sella cistern and cisterna magna that were 23 mm and 8.6 mm in diameter, respectively. No recurrence was found in the primary operative site. Additionally, an ophthalmic fundus examination showed no retinal hemorrhage or mass in either eye.
The younger brother of the proband, a 36-year-old, was also diagnosed with cerebral hemangioblastomas and underwent gamma knife treatment 15 y ago. A relapse of cerebral hemangioblastomas was found 3 y ago. Because he was not our outpatient, we could not complete a detailed clinical examination; however, a family member said that he did not have any other symptoms so far. The father of the patients committed suicide at the age of 60, because he was blind in both eyes (unknown reason). Their mother was normal, without a remarkable history of disease.
Results
Clinical diagnoses
The clinical features conformed to the diagnosis criteria of VHL disease in both of the patients.Citation2 The brother displayed the full spectrum of VHL disease, including hemangioblastomas in the central nervous system, RCC, pheochromocytoma, and other typical VHL manifestations, and was diagnosed with VHL type 2B. At this time, the sister only had hemangioblastomas in the central nervous system, pheochromocytoma and liver cysts, but no RCC. She was diagnosed with VHL type 2A. Mutation analysis was performed on this family.
Mutation detection and bioinformatics analysis
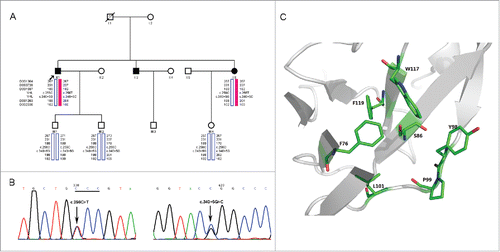
Direct sequencing of both DNA strands in the brother (II:1) and sister (II:6) () revealed 2 recurrent mutations of c.256C > T (p.P86S) and c.340 + 5G > C in exon 1 ().Citation2 Haplotype construction displayed that the c.256C > T (p.P86S) mutation in cis with c.340 + 5G > C were located on the disease haplotype (). However, their children did not inherit both of the mutations and the disease haplotype (). The missense mutation, c.256C > T (p.P86S), was predicted as deleterious using PolyPhen-2 and Mutation Taster. However, the c.340 + 5G > C mutation was predicted as benign according to the Human Splicing Finder.
Figure 3. Genetic Analyses of the Family. (A) Pedigree of the VHL family and haplotype analysis. Markers used in haplotype construction are listed in the upper left corner. Red solid bars depict the disease haplotype, and blue hollow bars are the normal haplotype. (B) Partial nucleotide sequences of the VHL gene in the brother (II:1). Left panel: forward sequences in exon 1. Arrow points to the c.256C > T (p.P86S) mutation, mutated codon is underlined. Right panel: forward sequence in exon 1. Arrow depicts the c.340 + 5G > C mutation. (C)Structural modeling of the VHL P86S mutant. VHL and hydrophobic residues are shown as cartoon and stick, respectively. Structure display was performed by PyMOL (http://www.pymol.org/).
Structure modeling of the mutant protein
The VHL protein has an N-terminal disordered domain (residues 1–53) and a C-terminal ordered domain consisting of an α-helical domain (residues 155–192) and a mainly β-sheet domain (residues 63–154 and 193–204).Citation6,7 Its β-sheet domain contains a small hydrophobic core composed of F76, P86, Y98, P99, L101, W117 and F119, and mutations in this region were reported to dramatically impair VHL function, such as F119S.Citation6,8 P86 is located in the center of the hydrophobic core, and its mutation to the hydrophilic residue serine may severely destabilize the hydrophobic core and lead to VHL dysfunction ().
Discussion
Von Hippel-Lindau disease is a rare and progressive multi-system neoplastic disorder that is an autosomal dominant genetic condition caused by inactivation of the VHL tumor suppressor gene.Citation1,2,9 VHL disease demonstrates marked phenotypic variability and age-dependent penetrance, including retinal and central nervous system hemangioblastomas, RCC, pheochromocytoma, pancreatic islet tumors and endolymphatic sac tumors, in addition to renal, liver and pancreatic cysts.Citation1,2,9 RCC is considered a leading cause of mortality in VHL syndrome, and the average age at clinical diagnosis is approximately 60 y old.Citation2,10 The family in this study had 3 family members exhibiting VHL manifestations with initial stages of central nervous system hemangioblastomas; however, none of them had any ocular symptoms. Moreover, the brother and his sister were considered as subclinical pheochromocytoma or normotensive pheochromocytoma because of serendipitous discoveries by radiologic examinationCitation11 and negative endocrine subjects, including urine VMACitation12. They were diagnosed with type 2B and type 2A, respectively, although the renal lesions in the brother were rare and concurrent with renal cysts, ccRCC and mcRCC.
Mutilocular cystic RCC is a rare renal cell carcinoma characterized by low-grade renal neoplasm with an excellent prognosis. It is a subtype of clear cell renal cell carcinoma according to its characteristics from immunohistochemistry and genetics.Citation13-15 Mutilocular renal cysts are common in VHL disease.Citation2 However, they are unlike the benign cysts in the general population and might give rise to RCC when the epithelial lining is dysplastic or shows carcinoma-in-situ.Citation2,16 Therefore, it is presumed that renal cysts are precursors to RCC; however, the transformation of a simple renal cyst to ccRCC is unusual.Citation17 Our patient (II:1) harbored 3 different renal lesions with simple cysts, mcRCC and ccRCC. It is possible that renal cysts gradually evolve to cystic tumors (mcRCC) and then solid tumors (ccRCC). Therefore, we speculate that this patient's 3 different renal lesions could be associated with the short observation time. Yet, it is difficult to track with imaging and to perform pathological co-localization.
VHL, a tumor suppressor gene, was considered to be a causative gene.Citation1,2 Patients carrying one mutant VHL allele in every cell are inclined to have tumor development with a loss of heterozygosity in somatic cells.Citation2 This gene codes 2 VHL protein (pVHL) products, namely p30 (NP_000542) and p19 (NP_937799).Citation2 The former has 213 amino acids and consists of 3 domains (N-terminal, β-sheet and C-terminal domains).Citation6,7 In our study, we found 2 point mutations in the VHL gene, specifically c.256C > T (p.P86S) and c.340 + 5G > C, both of which were considered pathogenic mutations in the past.Citation2,18 However, the c.340 + 5G > C mutation, which was associated with VHL type 1B disease,Citation2,18 has always been disputed because some researchers suggest that it is a benign polymorphism.Citation19,20 Our in silico analysis also revealed that it did not affect the splicing site alteration. The other missense mutation, c.256C > T (p.P86S), was previously reported in VHL type 1 or type 2B patients.Citation2 In our patients with VHL type 2A and type 2B, the bioinformatics analysis revealed that this mutation might severely destabilize the hydrophobic core of pVHL and cause VHL manifestations; therefore, it may be a deleterious mutation. Furthermore, the haplotype analysis displayed the c.340 + 5G > C mutation in cis with the deleterious mutation (c.256C > T) as the disease haplotype was inherited, which also indicated that the c.340 + 5G > C mutation was a benign supporting variant in autosomal dominant inheritance.Citation21 Conversely, the brother in this study exhibited different phenotypes from past reports,Citation2 especially regarding the mcRCC and epididymal tumors. Moreover, it was not excluded that the sister with type 2A will develop to type 2B according to delayed dominance. The younger brother and their deceased father had hemangioblastomas in the central nervous system and ocular diseases, respectively. Despite having no information of detailed clinical data, it is reasonable to assume that they had VHL diseases.
In summary, the VHL disease patient had 3 different phenotypes of renal lesions, including simple renal cysts, clear cell renal carcinoma, and multilocular cystic renal cell carcinoma, which may represent the whole progression from benign lesion to precancerous lesion and finally to renal carcinoma. Mutations and in silico analysis of the VHL gene identified the genotype-phenotype consistencies. It is important for family members to be directed to clinical genetic counseling.
Material and methods
The study was approved by the Ethics Committee of Human Study at Sun Yat-sen University and was conducted in accordance with the Declaration of Helsinki. All patients were counseled and signed a consent form approved by the ethics committee.
Mutation detection and bioinformatics analysis
Genomic DNA was extracted from the peripheral blood of 2 affected individuals and 3 unaffected individuals using the QIAamp® DNA Blood Mini kit (Germany) (). Mutation detection in the VHL gene was completed by sequencing the polymerase chain reaction (PCR) products of all 3 exons and flanking intronic regions with primers designed by Oligo 6.0 (http://www.oligo.net/down-loads.html) (primer sequences and PCR conditions are available upon request). Each of the variants was identified by searching the following databases: the Human Gene Mutation (http://www.hgmd.org/), dbSNP (http://www.ncbi.nlm.nih.gov/snp) and 1000 Genomes Project databases (http://www.1000genomes.org/). Mutation nomenclature recommended by den Dunnen and Antonarakis (http://www.hgvs.org/mutnomen/) was adopted with +1 corresponding to the A of the ATG translation initiation codon (GenBank cDNA sequence NM_000551). Haplotypes were constructed with microsatellite markers (D3S1304, D3S3728, D3S1597, D3S1263 andD3S2338; Applied Biosystems, Foster City, CA) spanning the VHL locus and the mutations identified in the family members by Cyrillic 2.1 (http://www.cyrillicsoftware.com/). The effects of the sequence variants were also predicted using PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/), Mutation Taster (http://www.mutationtaster.org/) and the Human Splicing Finder version 2.4 (http://www.umd.be/HSF/).
Structure modeling of the mutant protein
The P86S mutant structure was constructed based on the crystal structure of wild-type VHL (PDB ID: 4WQO) by selecting a proper rotamer of serine using the PyMOL Mutagenesis module (http://www.pymol.org/).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We acknowledge the patients and Prof. Yiming Wang from the Department of Medical Genetics and Genome Research Center of Sun Yat-sen University. This work was supported by the Fundamental Research Funds for the Central Universities (13ykpy29), the Natural Science Foundation of Guangdong Province (2014A030310158), the Medical Scientific Research Foundation of Guangdong Province (A2015117), Grant [2013]163 from Key Laboratory of Malignant Tumor Molecular Mechanism and Translational Medicine of Guangzhou Bureau of Science and Information Technology, Grant KLB09001 from the Key Laboratory of Malignant Tumor Gene Regulation and Target Therapy of Guangdong Higher Education Institutes and the National Natural Science Foundation of China (81402168).
References
- Kaelin WG. Von Hippel-Lindau disease. Annu Rev Pathol 2007; 2:145-73; PMID:18039096; http://dx.doi.org/10.1146/annurev.pathol.2.010506.092049
- Nordstrom-O'Brien M, van der Luijt RB, van Rooijen E, van den Ouweland AM, Majoor-Krakauer DF, Lolkema MP, van Brussel A, Voest EE, Giles RH. Genetic analysis of von Hippel-Lindau disease. Hum Mutat 2010; 31:521-37; PMID:20151405; http://dx.doi.org/10.1002/humu.21219
- Crossey PA, Richards FM, Foster K, Green JS, Prowse A, Latif F, Lerman MI, Zbar B, Affara NA, Ferguson-Smith MA, et al. Identification of intragenic mutations in the von Hippel-Lindau disease tumour suppressor gene and correlation with disease phenotype. Hum Mol Genet 1994b; 3:1303-8; PMID:7987306; http://dx.doi.org/10.1093/hmg/3.8.1303
- Garcia A, Matias-Guiu X, Cabezas R, Chico A, Prat J, Baiget M, De Leiva A. Molecular diagnosis of von Hippel-Lindau disease in a kindred with predominance of familial phaeochromocytoma. ClinEndocrinol (Oxf) 1997; 46:359-63; PMID:9156047; http://dx.doi.org/10.1046/j.1365-2265.1997.00149.x
- Chen S, Jin B, Xu L, Fu G, Meng H, Liu B, Li J, Xia D. Cystic renal cell carcinoma: a report of 67 cases including 4 cases with concurrent renal cell carcinoma. BMC Urol 2014; 14:87; PMID:25381150; http://dx.doi.org/10.1186/1471-2490-14-87
- Stebbins CE, Kaelin WG Jr, Pavletich NP. Structure of the VHL-ElonginC-ElonginB complex: implications for VHL tumor suppressor function. Science 1999; 284:455-61; PMID:10205047; http://dx.doi.org/10.1126/science.284.5413.455
- Nguyen HC, Yang H, Fribourgh JL, Wolfe LS, Xiong Y. Insights into Cullin-RING E3 ubiquitin ligase recruitment: structure of the VHL-EloBC-Cul2 complex. Structure 2015; 23:441-9; PMID:25661653; http://dx.doi.org/10.1016/j.str.2014.12.014
- Gossage L, Pires DE, Olivera-Nappa Á, Asenjo J, Bycroft M, Blundell TL, Eisen T. An integrated computational approach can classify VHL missense mutations according to risk of clear cell renal carcinoma. Hum Mol Genet 2014; 23:5976-88; PMID:24969085; http://dx.doi.org/10.1093/hmg/ddu321
- Maher ER, Neumann HP, Richard S. von Hippel-Lindau disease: a clinical and scientific review. Eur J Hum Genet 2011; 19:617-23; PMID:21386872; http://dx.doi.org/10.1038/ejhg.2010.175
- Roupret M, Hopirtean V, Mejean A, Thiounn N, Dufour B, Chretien Y, Chauveau D, Richard S. Nephron sparing surgery for renal cell carcinoma and von Hippel-Lindau's disease: a single center experience. J Urol 2003; 170:1752-5; PMID:14532769; http://dx.doi.org/10.1097/01.ju.0000092780.85876.de
- Agarwal A, Gupta S, Mishra AK, Singh N, Mishra SK. Normotensive pheochromocytoma: institutional experience. World J Surg 2005; 29:1185-8; PMID:16091986; http://dx.doi.org/10.1007/s00268-005-7839-4
- Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, Keiser HR, Goldstein DS, Eisenhofer G. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA 2002; 287:1427-34; PMID:11903030; http://dx.doi.org/10.1001/jama.287.11.1427
- Eble JN. Multilocular cystic renal cell carcinoma. In: Eble JN, Sauter G, Epstein JI, et al, eds. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon, France: IARC Press. 2004; World Health Organization Classification of Tumours.
- Halat S, Eble JN, Grignon DJ, Lopez-Beltran A, Montironi R, Tan PH, Wang M, Zhang S, MacLennan GT, Cheng L. Multilocular cystic renal cell carcinoma is a subtype of clear cell renal cell carcinoma. Mod Pathol 2010; 23:931-6; PMID:20348877; http://dx.doi.org/10.1038/modpathol.2010.78
- Srigley JR, Delahunt B, Eble JN, Egevad L, Epstein JI, Grignon D, Hes O, Moch H, Montironi R, Tickoo SK, et al. The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. Am J SurgPathol 2013; 37:1469-89; PMID:24025519; http://dx.doi.org/10.1097/PAS.0b013e318299f2d1
- Kaelin WG Jr. The von Hippel-Lindau tumor suppressor gene and kidney cancer. Clin Cancer Res 2004; 10:6290S-5S; PMID:15448019; http://dx.doi.org/10.1158/1078-0432.CCR-sup-040025
- Choyke PL, Glenn GM, Walther MM, Zbar B, Weiss GH, Alexander RB, Hayes WS, Long JP, Thakore KN, Linehan WM. The natural history of renal lesions in von Hippel-Lindau disease: a serial CT study in 28 patients. AJR Am J Roentgenol 1992; 159:1229-34; PMID:1442389; http://dx.doi.org/10.2214/ajr.159.6.1442389
- Hwang S, Ku CR, Lee JI, Hur KY, Lee MS, Lee CH, Koo KY, Lee JS, Rhee Y. Germline mutation of Glu70Lys is highly frequent in Korean patients with von Hippel-Lindau (VHL) disease. J Hum Genet 2014; 59:488-93; PMID:25078357; http://dx.doi.org/10.1038/jhg.2014.61
- Dandanell M, Friis-Hansen L, Sunde L, Nielsen FC, Hansen TV. Identification of 3 novel VHL germ-line mutations in Danish VHL patients. BMC Med Genet 2012; 13:54; PMID:22799452; http://dx.doi.org/10.1186/1471-2350-13-54
- Erlic Z, Hoffmann MM, Sullivan M, Franke G, Peczkowska M, Harsch I, Schott M, Gabbert HE, Valimäki M, Preuss SF, et al. Pathogenicity of DNA variants and double mutations in multiple endocrine neoplasia type 2 and von Hippel-Lindau syndrome. J Clin Endocrinol Metab 2010; 95:308-13; PMID:19906784; http://dx.doi.org/10.1210/jc.2009-1728
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17:405-24; PMID:25741868; http://dx.doi.org/10.1038/gim.2015.30
