ABSTRACT
Mesenchymal stem cells (MSCs) hold promise as cellular vehicles for the delivery of therapeutic gene products because they can be isolated, expanded, and genetically modified in vitro and possess tumor-oriented homing capacity in vivo.Citation1 Hepatocyte nuclear factor 4α (HNF4α) is a dominant transcriptional regulator of hepatocyte differentiation and hepatocellular carcinogenesis (HCC).Citation2,3 We have previously demonstrated that overexpression of HNF4α activates various hepatic-specific genes and enhances MSC differentiation.Citation4 However, the extent that overexpression of HNF4α in MSCs influences HCC progression has yet to be examined. Here we sought to investigate what effect MSCs overexpressing HNF4α (MSC-HNF4α) have on human hepatoma cells in vitro and in vivo. Conditioned medium collected from in vitro MSC-HNF4α cultures significantly inhibited hepatoma cell growth and metastasis compared with controls. Additionally, nude mice administered MSC-HNF4α exhibited significantly smaller tumors compared with controls in vivo. Immunoblot analysis of HCC cells treated with MSC-HNF4α displayed downregulated β-catenin, cyclinD1, c-Myc, MMP2 and MMP9. Taken together, our results demonstrate that MSC-HNF4α inhibits HCC progression by reducing hepatoma cell growth and metastasis through downregulation of the Wnt/β-catenin signaling pathway.
Abbreviations
| MSCs | = | mesenchymal strem cells |
| HNF4α | = | hepatocyte nuclear factor 4α |
| HCC | = | hepatocellular carcinogenesis |
| MSC-HNF4α | = | MSCs overexpressing HNF4α |
| UC-MSCs | = | umbilical cord-derived mesenchymal stem cells |
| FZD | = | frizzled |
| PBS | = | phosphate-buffered saline |
| FBS | = | fetal bovine serum |
| OD | = | optical density |
| FITC | = | v-fluorescein isothiocyanate |
| PI | = | propidiumiodide |
| PVDF | = | polyvinylidene difluoride |
| NS | = | normal saline |
| SD | = | standard deviation |
| ANOVA | = | analysis of variance |
Introduction
With 660,000 new cases per year, liver cancer is the fifth most common form of cancer and one of the most devastating malignancies, as it is the third highest cause of cancer-related death.Citation5 Curative treatments are not possible and the prognosis is dismal for the majority of advanced HCC cases because of underlying cirrhosis or resistance of tumors to standard chemotherapy. Therefore, surgical intervention is the only available treatment for the majority of patients diagnosed at an intermediate or advanced tumor stage. Additionally, liver transplantation is problematic due to the low availability of donor organs and inherently long transplant waiting times, while the high rate of tumor recurrence also threatens successful treatment outcomes.Citation6 Thus, developing effective therapeutic strategies that specifically target malignant tissue is essential.
Mesenchymal stem cells (MSCs) were initially identified as a heterogeneous population of stromal cells in the bone marrow. They have now been isolated from a wide variety of additional tissues, such as adipose tissue, cartilage, umbilical cord, and even some solid tumors. MSCs are easier to obtain and propagate while fewer ethical concerns are associated with their use. Importantly, MSCs can differentiate into a variety of cell types that have unique immunological characteristics and persist in a xenogeneic environment.
Furthermore, MSCs have the ability to efficiently target sites of tissue injury including tumor environments. This phenomenon is expected because tumors are considered unresolved woundsCitation7 and their microenvironment is characterized by an increased local production of inflammatory mediators and chemo-attractants.Citation8 Each of these characteristics contributes to the potential application of MSCs as cell-based vehicles for tumor-targeted gene therapy. In humans, the umbilical cord is a richer source of MSCs than the bone marrow and allows for easy isolation with less risk of contamination. Furthermore, umbilical cord-derived mesenchymal stem cells (UC-MSCs) demonstrate low immunogenicity which allows them to better tolerate HLA mismatch.Citation9,10
Hepatocyte nuclear factor 4α (HNF4α) is a transcription factor which plays a key role in hepatocyte differentiation and the maintenance of hepatic function.Citation3 HNF4α is expressed in hepatocytes, colon, small intestine, epididymis, and kidney while also having links to a variety of human diseases, including diabetes, colitis, and cancers. Recent evidence supports an oncogenic role for HNF4a in intestinal cancer.Citation11 However, decreased expression of HNF4α in hepatocellular carcinomas has been demonstrated while its up-regulation dramatically blocked the development of hepatocellular carcinoma through various routes.Citation12-14 Previously, we have shown that the overexpression of HNF4α activates various hepatic-specific genes and enhances the differentiation of MSCs.Citation4
In this study, we show that overexpression of HNF4α in UC-MSCs confers antitumor activity to these UC-MSCs and therefore establishes the feasibility of using gene-enhanced MSCs in a cell-based neo-organoid therapeutic approach for cancer treatment.
Results
Isolation and characterization of human umbilical cord-derived mesenchymal stem cells
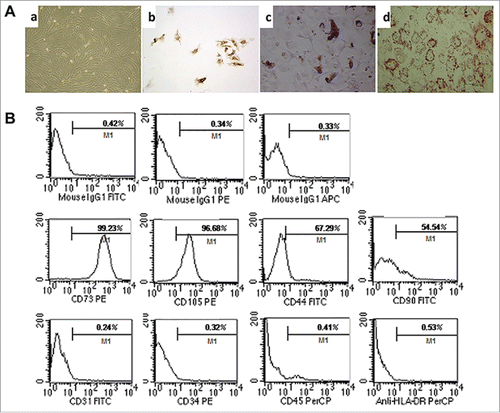
As we have shown previously, single fibroblast-like cells derived from umbilical cord and rapidly growing colonies exhibit a homogeneous morphology. When induced with conditioned medium for 2∼3 weeks, MSCs differentiate into chondrogenic, osteogenic, and adipogenic lineages as indicated by positive type II collagen, Alizarin red, and Oil Red O staining respectively (). Analysis of cultured MSCs was performed using flow cytometry to assess expression patterns of CD44, CD73, CD90, CD105, CD31, CD34, CD45, and HLA-DR ().
Figure 1. Characteristics and differentiation potential of MSCs derived from umbilicalcord tissues. (A) Morphology of UC-MSCs Magnification:×100. After chondrogenic differentiation conditions, MSCs differentiate into chondrogenic-like cells and immunohistochemically stained positive for type II Collagen; ×100. After osteogenic-specific induction, the MSCs were stained with Alizarin red; ×100. After inducing adipogenic differentiation, the cells showed many small lipid vacuoles, as assessed by Oil Red O staining;×100. (B) Flow cytometric analysis showing the MSC cells surface antigens: positive for mesenchymal lineage markers (CD44, CD73, CD90 and CD105), negative for haematopoietic and endothelial markers (CD31, CD34 and CD45), and negative for HLA-DR.
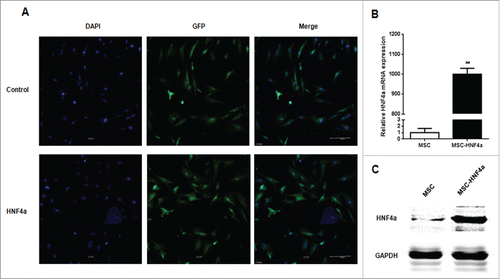
Stable transfection of HNF4α in MSCs
MSCs were transduced with either the pWIPIGFP (named MSC) or pWIPI-HNF4α-GFP (named MSC-HNF4α) lentiviral vector. After infection, approximately 95% of cultured cells were GFP-positive (). Real-time PCR and western blotting indicated that HNF4α expression was elevated in the MSC-HNF4α samples ().
Figure 2. HNF4α stably expressed in MSCs. (A) The transduction efficiency of MSCs infected with lentiviral vectors was assessed based on the GFP expression in MSCs by immunofluorescence staining, and more than 90% of MSCs stably expressed GFP; (B) Real-time PCR showed that the HNF4α mRNA expression was significantly up-regulation in MSC-HNF4α compare with MSC (p < 0.01); (C)Western blotting indicated that the HNF4α protein expression was elevated.
MSC-HNF4α inhibited HCC cell proliferation and invasion
To examine the effect of MSC-HNF4α on HCC, cells were incubated with culture medium and proliferation was measured using a CCK-8 assay. Compared with control groups (L02 and MSC), MSC-HNF4α conditioned medium significantly inhibited the proliferation of SK-Hep-1 and HepG2 cells (P<0.05). Conversely, when analyzing effects of the same conditioned mediums on the proliferation of control LO2 cells, we found no significant difference (). These results were confirmed using a colony formation assay. We obtained similar results for SK-Hep-1 and HepG2 ().
Figure 3. MSC-HNF4α inhibited HCC proliferation, migration and invasion. (A) Upper panel: CCK-8 assay showed that the OD value of SK-Hep-1 and HepG2 cells cultured with 50% MSC-HNF4α conditioned media was significantly decreased as compared to LO2 or MSC-conditioned media. Lower panel: Effect of conditioned-media on LO2 proliferation, no statistically significant difference was observed among 3 groups; (B) The colony formation assay showed that the proliferation of SK-Hep-1 and HepG2 cells treated with MSC-HNF4α conditioned media was significantly lower than that of the control group(L02) and MSC group; (C) The subcutaneous tumorigenicity assay showed that the weight and volume of SK-Hep-1 tumors treated with MSC-HNF4α were significantly decreased compared with those of the control group(NS) and MSC group; (D) The Matrigel invasion assay showed that MSC-HNF4α-conditioned medium significantly inhibits SK-Hep-1 and HepG2 cells invasion in vitro; (E) Immunofluorescence staining showed lower expression of MMP2 and MMP9 in HCC tissues (SK-Hep-1) following MSC-HNF4α treatment compared with the controls.×400; (F)Cell apoptosis assay showed that the different in each group was notstatistically significant. (*P < 0.05).
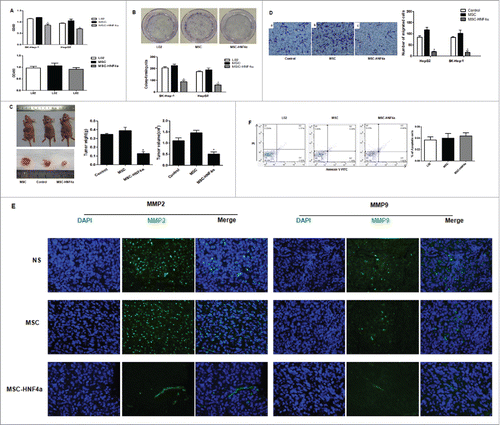
In order to investigate the efficacy of MSC-HNF4α in vivo, we subcutaneously injected SK-Hep-1 cells into nude mice to generate HCC with MSC-HNF4α and administered these cells intravenously 24 hours later as well as once every 7 days thereafter. Forty-two days later, tumor volume and mass were significantly lower in MSC-HNF4α-treated mice versus controls (). The results of the proliferation analysis demonstrated that MSC-HNF4α inhibited HCC growth. Using Matrigel invasion assays, we found MSC-HNF4α significantly reduced the migration and invasion potential of HepG2 and SK-Hep-1 cells compared to controls (). When examining the expression of matrix metalloproteinases in vivo, we found lower levels of MMP2 and MMP9 in MSC-HNF4α-treated subjects (). To determine if MSC-HNF4α could induce HCC cell apoptosis, we analyzed Annexin V staining using flow cytometry and found no significant difference between groups ().
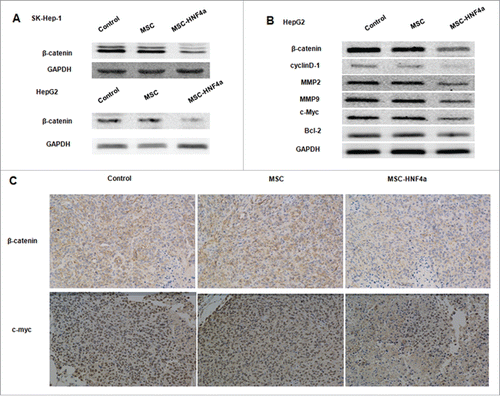
MSC-HNF4α down-regulates Wnt/β-catenin signaling pathway in HCC cells
When investigating whether MSC-HNF4α affects signal pathways commonly altered in malignancies like HCC, we found β-catenin signaling was markedly down-regulated in SK-Hep-1 and HepG2 cells treated with conditioned media from MSC-HNF4α cultures (). We next examined gene expression resulting from activation of the Wnt/β-catenin signaling pathway and found downregulation of β-catenin, cyclinD1, MMP2, MMP9 and c-myc in HepG2 cells treated with MSC-HNF4α conditioned media(). However, there was no significant difference in Bcl-2 expression observed in MSC-HNF4α vs. control treatment. Additionally, in vivo expression of β-catenin, c-myc, MMP2, and MMP9 was investigated in tumors using immunohistochemistry (). The results show that β-catenin, c-myc, MMP2, and MMP9 were noticeably decreased in the MSC-HNF4α-treated group. Taken together, these data are consistent with our hypothesis that soluble factors in conditioned media released from MSC-HNF4α cultures inhibit tumor cell proliferation and invasion via the Wnt/β-catenin signaling pathway.
Figure 4. MSC-HNF4α inhibited HCC proliferation and invasion by inhibiting the Wnt/β-catenin signaling pathway. HCC cells were cultured with culture medium for 48 h, (A) protein gel blotting assay for β-catenin in SK-Hep-1 and HepG2 was downregulated when cells were treated with MSC-HNF4α conditioned media. (B) Target genes of the Wnt/β-catenin pathways, β-catenin, cyclin D1, MMP2, MMP9 and c-Myc were also down-regulated in MSC-HNF4α group but Blc-2 did not demonstrate any significant changes between each group. (C) Expression of β-catenin and c-Myc in tumor were clearly decreased in MSC-HNF4a group by immunohistochemical assay.
Discussion
HCC can be cured by radical therapies if early diagnosis occurs when the tumor is still small in size. Unfortunately, diagnosis often comes late after the tumor has grown and spread. Thus, palliative approaches are usually applied instead, such as transarterial intrahepatic chemoembolization (TACE) or sorafenib, an anti-angiogenic agent and MAP kinase inhibitor. The latter is the only targeted therapy that has shown significant, although moderate, efficacy in some individuals with advanced HCC. This highlights the need to develop other targeted therapies and to achieve this goal we have to identify additional cell signaling pathways as potential targets.
Recently, researchers have made use of MSC as vehicles for tumor-targeted gene therapy due to their accessibility for genetic modification as well as their ability to be cultured and expanded in vitro.Citation15 MSCs are successfully engrafted into tissues under conditions of increased cell turnover triggered by tissue damage or neoplastic growth. They have the ability to efficiently target sites of tissue injury including tumor environments. The exact mechanisms governing this recruitment remain poorly understood. MSCs are thought to show a strong tropism for tumors because the body sees the tumor environment as the equivalent of a chronic wound, or for example “the wound that never heals.”Citation16,17 In this study, we intended to utilize human MSCs as a vehicle for gene delivery to cure HCC.
HNF4α is a key factor in determining the differentiation of hepatocyte-like cells derived from human MSCs. We previously reported that overexpression of HNF4α activates various hepatic-specific genes and enhances the differentiation status UC-MSC.Citation4 Moreover, findings from one recent study suggest that the upregulation of HNF4α in HCC effectively suppresses its progression.Citation18 With this concept in mind, we utilized gene transfection technology during this study to overexpress HNF4α in human MSCs in an attempt to inhibit the progression of HCC.
The Wnt signaling pathway plays an important role in cell metabolism, morphogenesis, differentiation, cell survival, and proliferation as well as in the migration/invasion capacity of cancer cells.Citation19-21 Activation of this pathway occurs when a Wnt ligand binds to a Frizzled (FZD) receptor at the cell membrane. Two different Wnt signaling cascades have been identified based on previous data, being the non-canonical and canonical pathways, with the latter involving the β-catenin protein. Downregulation of the Wnt pathway is an early event in hepatocarcinogenesis and has been associated with an aggressive HCC phenotype due to its role supporting in cell survival, proliferation, migration, and invasion. Thus, component proteins identified in this pathway are potential candidates for pharmacological intervention. Wnt/β-catenin signaling is often aberrantly activated in malignant tumors, especially HCC, and the c-Myc, cyclin-D, and MMP gene families are all targets of Wnt signaling.Citation22-24
There is evidence linking Wnt pathway activation with a malignant HCC cell phenotype, such as enhanced cell proliferation, migration, and invasion which suggests the possibility of targeting members of this signaling cascade as an attractive therapeutic approach for treatment of HCC.Citation25-27 One plausible therapeutic strategy would be to trap the endogenous Wnt ligands with the exogenous soluble form of FZD receptors. This approach was reported for FZD7 by Tanaka and colleagues in esophageal carcinoma cells and later confirmed in HCC cells.Citation28 This peptide decreased the viability of HCC cell lines with high specificity since normal hepatocytes were not sensitive to sFZD7. Additionally, sFZD7 cooperates with doxorubicin to reduce HCC cell proliferation in vitro and in a murine xenograft model as well. Interestingly, it has been shown to be highly efficient and independent of the β-catenin mutational status. More recently, IWP2, Wnt-C59, sFRP1, sFRP2, sFRP5, Wif1, and DKKs have been reported to inhibit tumors by interfering with the activation of Wnt signaling.Citation29-34
In summary, our data demonstrate the potential of using MSCs as targeted tumor therapy vehicles to enhance the delivery of therapeutically relevant levels of gene products that exert anti-neoplastic effects. We have shown here that the overexpression of HNF4α in human MSCs suppresses cancer cell proliferation and metastasis. Furthermore, when taken together our data suggest that MSC-HNF4α inhibits tumor cell proliferation and invasion via the Wnt/β-catenin signaling pathway. These findings provide a novel, efficacious, and clinically safe therapeutic approach to control HCC progression.
Materials and methods
Cell culture
With the informed consent of the tissue donor and following all ethical and institutional guidelines, fresh human umbilical cords were obtained from male or female neonates after birth, and 20 cords were collected in our experiment. The study was approved by the Institutional Review Board and Human Ethics Committee of Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China. Written consent for the use of the samples for research purposes was obtained from all patients. The samples were then maintained in phosphate-buffered saline (PBS) (Invitrogen) containing 100 U / mL penicillin / streptomycin (Gibco) at 4°C. Following disinfection in 75% ethanol for 1 min, the umbilical cord vessels were removed in PBS. The UC-MSCs were prepared as previously described. The mesenchymal tissue was diced into cubes of approximately 1 cm3. Following the removal of the supernatant fraction, the precipitate was washed with DMEM/F12 (Gibco) and centrifuged at 250×g for 5 min. The mesenchymal tissue was treated with collagenase II (Invitrogen) at 37°C for 1 h and further digested with 0.25% trypsin (Invitrogen) at 37°C for 30 min. Fetal bovine serum (FBS; Gibco) was added to the mesenchymal tissue to neutralize the excess trypsin. The dissociated mesenchymal cells were further dispersed by treatment with 10% FBS-DMEM/F12 and counted. The mesenchymal cells were then used directly for the cultures, and the media was changed twice per week. The fifth to eighth passages of UC-MSC were used in the following experiments.
The liver cancer cell lines HepG2 and SK-Hep-1 were obtained from the Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). All cells were cultured in high-glucose minimum essential medium (DMEM, Gibco) supplemented with 10% FBS maintained at 37°C and 5% CO2.
Flow cytometry analysis
Antibodies against the human antigens CD31, CD34, CD44, CD45, CD73, CD90, CD105, and HLA-DR were purchased from BD Sciences. A total of 1 × 106 cells were re-suspended in 200 μL of PBS and incubated with FITC- or PE- or APC- conjugated antibodies for 30 min at room temperature. The fluorescence intensity of the cells was evaluated by flow cytometry using a flow cytometer (BD Sciences), and the data were analyzed using the CELLQUEST Pro software (BD Sciences).
Chondrogenic osteogenic and adipogenic differentiation in vitro
To induce chondrogenic and osteogenic differentiation, 4th passage cells were treated with chondrogenic medium (STEMPRO CHONDRO DIFF KIT, Gibco) or osteogenic medium (STEMPRO OSTEO DIFF KIT, Gibco) for 21 days. To induce adipogenic differentiation, 4th passage cells were treated with adipogenic medium (STEMPRO ADIPO DIFF KIT, Gibco) for 14 days. The medium was changed twice per week. Chondrogenesis was assessed by immunohistochemical staining for type II collagen, osteogenesis was evaluated by Alizarin red staining, and adipogenesis was assessed by staining with Oil Red O.
Stable overexpression of HNF4α in UC-MSCs
HNF4α cDNA was generated via PCR amplification and confirmed by sequencing. The cDNA was inserted into lentivirus particles (LV-HNF4α) with green fluorescent protein to monitor that the transduction was stable. UC-MSCs were infected with lentiviral particles for 10 h; the supernatant contained 5 mg/mL polybrene to stably overexpress HNF4α (MSC-HNF4α), and cells transfected with a lentiviral vector containing only green fluorescent protein (MSC) were used as controls. The lentiviral transduction efficiency was monitored using a confocal laser scanning microscope and blotprotein gel blot analysis.
Treatment with conditioned medium
L02 cells, MSC, and MSC-HNF4α were cultured in FBS-DMEM/F12 until they reached 60∼80% confluence. The adherent cells were washed and further incubated in FBS-free DMEM/F12 for 48 h, and the medium was then collected and filtered through a 0.22 μm filter. The conditioned medium from the cells was harvested and stored at −80°C until use. (For all in vitro experiments, we used conditioned medium from L02 cells as control)
Colony formation assay and CCK-8 assay
The conditioned media were added to the culture medium of HCC cells to a final concentration of 50%. For the colony formation assay, 200 cells were cultured in 6-well plates. The experiment was performed in triplicate for each cell clone. The medium was changed twice per week. After 2–3 weeks, the cells were fixed in 4% paraformaldehyde and stained with 1% Crystal Violet, and colonies with a diameter exceeding 2 mm were counted.
The culture medium for the CCK-8 assay as the same as that used in the colony formation assay: a 96-well plate was inoculated with 100 μL of a cell suspension containing 8×103 to 1.5×104 cells. After incubation for 24 h to 72 h, 10 μl of the Cell Counting Kit solution (CCK-8) (Dojindo, Kumamoto, Japan) was added to the wells and incubated for a further 2.5 h with stirring before measuring the optical density (OD) of each well at 450 nm on a microplate reader.
Matrigel invasion assay
Prior to the start of the experiment, all cells were cultured in culture medium for 2 days before being collected. A Matrigel invasion assay was performed in triplicate to analyze cell invasion: 80 μl of serum-free DMEM/F12-diluted Matrigel (dilution 1:6, BD) was added to the Transwell filters of a Boyden chamber (Coning Costar, MA, USA) and incubated at 37°C for 2 h to form a gel matrix. The HCC cells were cultured in serum-free DMEM/F12 or conditioned medium 24 h, and 5×104 cells (200 μl) were then suspended in DMEM/F12 and seeded in the upper well of the transwell chamber. Eight hundred microliters of DMEM containing 10% FBS were then added to the lower chamber as the chemo-attractant. After incubation at 37°C for 24 h, the cells that had invaded across the Matrigel and passed through the transwell filter were stained with 1% Crystal Violet, and cells in 10 randomly selected fields (×200) in each well were counted.
Cell apoptosis assay
Cell apoptosis was detected by using an Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit according to the supplier's protocols. 48 hours post-transfection, cells were collected, centrifuged, and re-suspended in 500 μl of 1X binding buffer. Annexin V-FITC (5 μl) and 10 μl PtdIns were then added to each tube. The tubes were incubated in the dark at room temperature for 15 min. Cell apoptosis assay was performed immediately using flow cytometry. Each experiment was performed at least 3 times.
Reverse transcriptase-PCR (RT-PCR)
The total RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Reverse transcription was performed using a PrimeScript RT reagent kit with the gDNA Eraser kit (Takara). Real-time RT-PCR analyses were performed by using the SYBR Green Real-time PCR Master Mix (Takara, Japan) to determine the mRNA levels.
Western blots
All cells were lysed in RIPA buffer and a protease inhibitor cocktail at 4°C for 1 h. The cell lysates were centrifuged at 13,000 xg and 4°C for 20 minutes, and the protein concentration was determined using a BCA Protein Assay kit. Equal amounts of protein were heated to 100°C for 5 min, separated by 10% or 12% SDS-PAGE, and transferred to polyvinylidene difluoride membranes (PVDF, BioRad, Hercules, CA). The membranes were blocked with TBST containing 5% nonfat dried milk for 1 h and incubated with primary antibodies overnight at 4°C. The membranes were washed 3 times with TBST and then incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. After three additional washes with TBST, the signal intensity was quantified with the Quantity One software (Odyssey). The following mouse antibodies were used for western blots: GAPDH, HNF4α, c-Myc (Santa Cruz), Blc-2, β-catenin, cyclin-D1, MMP2, and MMP9 (Abcam).
Animals
Male nude mice aged 4–6 weeks were purchased from the Shanghai Experimental Center of Chinese Science Academy and housed under standard animal laboratory conditions in the experimental animal center of the RenJi Hospital Shanghai Jiao Tong University Medical School. SK-Hep-1 cells (5×106) were subcutaneously implanted into nude mice. Twenty-four hours after implantation, 6 mice in each group were intravenously injected with 1×106 MSC or MSC-HNF4α once per week, while the other 6 mice were injected with 0.9% Normal saline (NS) as controls. After approximately 35 days, the animals were sacrificed and the subcutaneous tumors were removed and weighed and subsequently subjected to histology, immunoblotting, and immunofluorescence analyses.
Immunofluorescence analysis
The tissue pieces were fixed in 4 % paraformaldehyde, embedded in paraffin, and cut into transverse sections. A standard histological hematoxylin-eosin staining procedure was performed. The expression levels of MMP2 and MMP9 were examined in HCC cells by immunofluorescence analysis.
Statistical analysis
The data in this study are expressed as the means ± standard deviation (SD). The differences between groups were analyzed by using Student's t-test or ANOVA and compared by using analysis of variance (ANOVA) as well as Tukey's post hoc test. All statistical analyses were conducted by using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). All tests were 2-sided, and the statistical significance level was defined as p < 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank the Central Laboratory and experimental animal center of the RenJi Hospital Shanghai Jiao Tong University Medical School for the technical support.
Funding
This project was supported by the National Natural Science Foundation of China (No. 81570561), the National Natural 415 Science Foundation of China (No. 81472243), the Science Technology Commission Medical Foundation of Shanghai (No. 134119a9501) and Technology Commission Medical Foundation of Nanjing No. 201308014.
References
- Porada CD, Almeida-Porada G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv Drug Deliv Rev 2010; 62:1156-66; PMID:20828588; http://dx.doi.org/10.1016/j.addr.2010.08.010
- DeLaForest A, Nagaoka M, Si-Tayeb K, Noto FK, Konopka G, Battle MA, Duncan SA. HNF4A is essential for specification of hepatic progenitors from human pluripotent stem cells. Development 2011; 138:4143-53; PMID:21852396; http://dx.doi.org/10.1242/dev.062547
- Tanaka T, Jiang S, Hotta H, Takano K, Iwanari H, Sumi K, Daigo K, Ohashi R, Sugai M, Ikegame C, et al. Dysregulated expression of P1 and P2 promoter-driven hepatocyte nuclear factor-4alpha in the pathogenesis of human cancer. J Pathol 2006; 208:662-72; PMID:16400631; http://dx.doi.org/10.1002/path.1928
- Hang H, Yu Y, Wu N, Huang Q, Xia Q, Bian J. Induction of highly functional hepatocytes from human umbilical cord mesenchymal stem cells by HNF4alpha transduction. PloS one 2014; 9:e104133; PMID:25137413; http://dx.doi.org/10.1371/journal.pone.0104133
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. The Lancet 2003; 362:1907-17; PMID:14667750; http://dx.doi.org/10.1016/S0140-6736(03)14964-1
- Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Annal Surg 2006; 243:229-35; PMID:16432356; http://dx.doi.org/10.1097/01.sla.0000197706.21803.a1
- Bayo J, Marrodan M, Aquino JB, Silva M, Garcia MG, Mazzolini G. The therapeutic potential of bone marrow-derived mesenchymal stromal cells on hepatocellular carcinoma. Liver Int 2014; 34:330-42; PMID:24112437; http://dx.doi.org/10.1111/liv.12338
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454:436-44; PMID:18650914; http://dx.doi.org/10.1038/nature07205
- Can A, Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem cells 2007; 25:2886-95; PMID:17690177; http://dx.doi.org/10.1634/stemcells.2007-0417
- Koh SH, Kim KS, Choi MR, Jung KH, Park KS, Chai YG, Roh W, Hwang SJ, Ko HJ, Huh YM, et al. Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res 2008; 1229:233-48; PMID:18634757; http://dx.doi.org/10.1016/j.brainres.2008.06.087
- Saandi T, Baraille F, Derbal-Wolfrom L, Cattin AL, Benahmed F, Martin E, Cardot P, Duclos B, Ribeiro A, Freund JN, et al. Regulation of the tumor suppressor homeogene Cdx2 by HNF4alpha in intestinal cancer. Oncogene 2013; 32:3782-8; PMID:22986531; http://dx.doi.org/10.1038/onc.2012.401
- Ning BF, Ding J, Liu J, Yin C, Xu WP, Cong WM, Zhang Q, Chen F, Han T, Deng X, et al. Hepatocyte nuclear factor 4alpha-nuclear factor-kappaB feedback circuit modulates liver cancer progression. Hepatology 2014; 60:1607-19; PMID:24752868; http://dx.doi.org/10.1002/hep.27177
- Ning BF, Ding J, Yin C, Zhong W, Wu K, Zeng X, Yang W, Chen YX, Zhang JP, Zhang X, et al. Hepatocyte nuclear factor 4 alpha suppresses the development of hepatocellular carcinoma. Cancer Res 2010; 70:7640-51; PMID:20876809; http://dx.doi.org/10.1158/0008-5472.CAN-10-0824
- Hatziapostolou M, Polytarchou C, Aggelidou E, Drakaki A, Poultsides GA, Jaeger SA, Ogata H, Karin M, Struhl K, Hadzopoulou-Cladaras M, et al. An HNF4alpha-miRNA inflammatory feedback circuit regulates hepatocellular oncogenesis. Cell 2011; 147:1233-47; PMID:22153071; http://dx.doi.org/10.1016/j.cell.2011.10.043
- Hodgkinson CP, Gomez JA, Mirotsou M, Dzau VJ. Genetic engineering of mesenchymal stem cells and its application in human disease therapy. Hum Gene Ther 2010; 21:1513-26; PMID:20825283; http://dx.doi.org/10.1089/hum.2010.165
- Kidd S, Spaeth E, Klopp A, Andreeff M, Hall B, Marini FC. The (in) auspicious role of mesenchymal stromal cells in cancer: be it friend or foe. Cytotherapy 2008; 10:657-67; PMID:18985472; http://dx.doi.org/10.1080/14653240802486517
- Kucerova L, Altanerova V, Matuskova M, Tyciakova S, Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res 2007; 67:6304-13; PMID:17616689; http://dx.doi.org/10.1158/0008-5472.CAN-06-4024
- Yin C, Lin Y, Zhang X, Chen YX, Zeng X, Yue HY, Hou JL, Deng X, Zhang JP, Han ZG, et al. Differentiation therapy of hepatocellular carcinoma in mice with recombinant adenovirus carrying hepatocyte nuclear factor-4alpha gene. Hepatology 2008; 48:1528-39; PMID:18925631; http://dx.doi.org/10.1002/hep.22510
- Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer research 2005; 65:7554-60; PMID:16140917; http://dx.doi.org/10.1158/0008-5472.CAN-05-1317
- Gonzalez-Sancho JM, Aguilera O, Garcia JM, Pendas-Franco N, Pena C, Cal S, Garcia de Herreros A, Bonilla F, Munoz A. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene 2005; 24:1098-103; PMID:15592505; http://dx.doi.org/10.1038/sj.onc.1208303
- Kozinski K, Dobrzyn A. [Wnt signaling pathway–its role in regulation of cell metabolism]. Postepy Hig Med Dosw 2013; 67:1098-108; PMID:24379251; http://dx.doi.org/10.5604/17322693.1077719
- Jiang GX, Liu W, Cui YF, Zhong XY, Tai S, Wang ZD, Shi YG, Li CL, Zhao SY. Reconstitution of secreted frizzled-related protein 1 suppresses tumor growth and lung metastasis in an orthotopic model of hepatocellular carcinoma. Digest Dis Sci 2010; 55:2838-43; PMID:20033841; http://dx.doi.org/10.1007/s10620-009-1099-3
- Huang CL, Liu D, Ishikawa S, Nakashima T, Nakashima N, Yokomise H, Kadota K, Ueno M. Wnt1 overexpression promotes tumour progression in non-small cell lung cancer. Eur J Cancer 2008; 44:2680-8; PMID:18790633; http://dx.doi.org/10.1016/j.ejca.2008.08.004
- Cadoret A, Ovejero C, Saadi-Kheddouci S, Souil E, Fabre M, Romagnolo B, Kahn A, Perret C. Hepatomegaly in transgenic mice expressing an oncogenic form of beta-catenin. Cancer Res 2001; 61:3245-9; PMID:11309273
- Bai XL, Zhang Q, Ye LY, Liang F, Sun X, Chen Y, Hu QD, Fu QH, Su W, Chen Z, et al. Myocyte enhancer factor 2C regulation of hepatocellular carcinoma via vascular endothelial growth factor and Wnt/beta-catenin signaling. Oncogene 2015; 34:4089-97; PMID:25328135; http://dx.doi.org/10.1038/onc.2014.337
- Pez F, Lopez A, Kim M, Wands JR, Caron de Fromentel C, Merle P. Wnt signaling and hepatocarcinogenesis: molecular targets for the development of innovative anticancer drugs. J Hepatol 2013; 59:1107-17; PMID:23835194; http://dx.doi.org/10.1016/j.jhep.2013.07.001
- Chen L, Li M, Li Q, Wang CJ, Xie SQ. DKK1 promotes hepatocellular carcinoma cell migration and invasion through beta-catenin/MMP7 signaling pathway. Mol Cancer 2013; 12:157; PMID:24325363; http://dx.doi.org/10.1186/1476-4598-12-157
- Merle P, de la Monte S, Kim M, Herrmann M, Tanaka S, Von Dem Bussche A, Kew MC, Trepo C, Wands JR. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology 2004; 127:1110-22; PMID:15480989; http://dx.doi.org/10.1053/j.gastro.2004.07.009
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 2009; 5:100-7; PMID:19125156; http://dx.doi.org/10.1038/nchembio.137
- Proffitt KD, Madan B, Ke Z, Pendharkar V, Ding L, Lee MA, Hannoush RN, Virshup DM. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res 2013; 73:502-7; PMID:23188502; http://dx.doi.org/10.1158/0008-5472.CAN-12-2258
- Hu J, Dong A, Fernandez-Ruiz V, Shan J, Kawa M, Martinez-Anso E, Prieto J, Qian C. Blockade of Wnt signaling inhibits angiogenesis and tumor growth in hepatocellular carcinoma. Cancer Res 2009; 69:6951-9; PMID:19690140; http://dx.doi.org/10.1158/0008-5472.CAN-09-0541
- Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Gen 2004; 36:417-22; PMID:15034581; http://dx.doi.org/10.1038/ng1330
- Lu W, Lin C, King TD, Chen H, Reynolds RC, Li Y. Silibinin inhibits Wnt/beta-catenin signaling by suppressing Wnt co-receptor LRP6 expression in human prostate and breast cancer cells. Cell Signal 2012; 24:2291-6; PMID:22820499; http://dx.doi.org/10.1016/j.cellsig.2012.07.009
- Lu W, Lin C, Roberts MJ, Waud WR, Piazza GA, Li Y. Niclosamide suppresses cancer cell growth by inducing Wnt co-receptor LRP6 degradation and inhibiting the Wnt/beta-catenin pathway. PloS One 2011; 6:e29290; PMID:22195040; http://dx.doi.org/10.1371/journal.pone.0029290
