ABSTRACT
We previously identified TDRG1 (testis developmental related gene 1), a novel gene with exclusive expression in testis, promoted the proliferation and progression of cultured human seminoma cells through PI3K/Akt/mTOR signaling. As increasing evidence reveal that aberrant activation of this signaling is involved in cisplatin resistance. Then, in this study, we further explored whether TDRG1 regulated the chemosensitivity of seminoma TCam-2 cells to cisplatin. Our researches showed TDRG1 could regulate the viability of TCam-2 cells following cisplatin treatment in vitro through control of both cell apoptosis and cell cycle. Mechanistically, we observed TDRG1 positively regulated the expression levels of the key elements in PI3K/Akt/mTOR pathway including p-PI3K, p-Akt and p-mTOR and also affected the translocation of nuclear p-Akt in TCam-2 cells during cisplatin treatment. Meanwhile, the levels of Bad, cytochrome c, caspase-9 ratio (activated/total), caspase-3 ratio (activated/total) and cleaved-PARP were negatively modulated by TDRG1, which meant the involvement of mitochondria-mediated apoptotic pathway. Furthermore, we found the effect of TDRG1 knockdown or TDRG1 overexpression could be reversed by IGF-1, a PI3K signaling activator, or LY294002, a inhibitor of this pathway, respectively. Similar effects of TDRG1 on cisplatin chemosensitivity and associated molecular mechanism were also confirmed in vivo by employing xenograft assays. In addition, the positive correlation between TDRG1 and p-PI3K, or p-Akt, was found in tumor tissues from seminoma patients. In conclusion, we uncover that TDRG1 regulates chemosensitivity of TCam-2 cells to cisplatin through PI3K/Akt/mTOR signaling and mitochondria-mediated apoptotic pathway both in vitro and in vivo.
Introduction
Pure seminoma is a well defined clinic and pathologic entity consisting approximately 50% of testicular germ cell tumors (TGCT), and its incidence is increasing worldwide.Citation1 Seminoma represents a paradigm for a curable neoplasm. Almost 90% of these patients initially diagnosed with clinical Stage I (testis involvement only) or with non-bulky Stage II disease (retroperitoneal lymphadenopathy measuring less than 5 cm in greatest dimension) were cured after undergoing radical inguinal orchiectomy followed by suitable radiotherapy.Citation2 Even for patients with further advanced stages, the complete response rate can reach 70% to 90% with standard first-line cisplatin (cis-dichlorodiammine platinum or CDDP)-based chemotherapy regimens.Citation3-5
However, a minority of patients in advanced period are either refractory to or will relapse after CDDP-based chemotherapy.Citation6-8 They commonly do not achieve a long-term palliation and eventually die of their disease. Though the absolute amount of deaths in these patients is small, the effects are devastating, with no less than 35 y (years) of life lost per patient.Citation8 On account of the centrality of CDDP in the current used chemotherapy regimens, the principal cause of therapeutic failures in these cases involves the phenomenon of resistance to this drug. Furthermore, in addition to the issue of CDDP resistance, approaches to reduce CDDP doses so as to limit the short and long-lasting side effects in young patients are also needed.Citation9 Nevertheless, up to date, neither the biological basis for the highly sensitivity to CDDP in the most seminoma nor the mechanisms underlying the rare phenomenon of CDDP resistance have been well elucidated, which representing a great challenge for the treating physician.
Testis developmental related gene 1 (TDRG1; GenBank ID, DQ168992) is a novel and characteristic gene identified by our group with exclusive expression in human spermatogenic cells.Citation10,11 Moreover, enhanced expression of TDRG1 protein is identified in testicular seminoma when compared to normal testicular tissues, and TDRG1 promotes the proliferation and invasion of seminoma cells through activating the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, indicating the carcinogenic role of the protein in seminoma.Citation12 The PI3K/Akt pathway is one of the crucial signaling cascades in human and involved in normal cellular processes such as cell survival, proliferation and apoptosis, and also in many physiologic processes.Citation13,14 And yet, aberrant activation of this signaling has been widely reported in human cancers, including seminoma.Citation15,16 What's more, numerous studies reported that abnormal activation of the PI3K/Akt signaling plays critical roles in the development of resistance to CDDP, and inhibition of this pathway increases the efficacy of CDDP in several kinds of human malignancies.Citation17 Additionally, a recent study also found overactivation of Akt in CDDP-resistant TGCT cells compared with sensitive cells and overexpression of p-Akt in CDDP-resistant choriocarcinoma orthotopic tumors versus their sensitive counterparts, highlighting the correlation of PI3K/Akt signaling with CDDP resistance in TGCT.Citation6 However, whether TDRG1 participates in the regulation of CDDP chemosensitivity via this signaling in seminoma is still largely unknown and further molecular mechanism needs to be unveiled.
Therefore, in the present study, we investigated the effects of TDRG1 protein on the growth of seminoma TCam-2 cells in vitro and on the tumor growth in vivo in nude mice, with or without CDDP. Meanwhile, we also evaluated the association of key components in PI3K/Akt signaling with TDRG1 in seminoma tissues. Our results demonstrated for the first time that TDRG1 regulates chemosensitivity of TCam-2 cells to CDDP via PI3K/Akt/mTOR signaling pathway and mitochondria-mediated apoptotic pathway, and p-PI3K and p-Akt are positively correlated with TDRG1 in seminoma.
Methods
Ethics statement
This study was approved by the Institutional Research Ethics Committee of The Third Xiangya hospital of Central South University. Informed consent conformed to the principles outlined in the Declaration of Helsinki were obtained from all patients for the use of their tissue samples and records. The in vivo experiments also conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the United National Institutes of Health.
Chemical compounds and antibodies
LY294002 (a PI3K inhibitor) and insulin-like growth factor-1 (IGF-1, a PI3K activator) were purchased from Selleck Chemicals Company (Houston, TX, USA). Both the above compounds were dissolved in dimethyl sulfoxide (DMSO). CDDP was obtained from Sigma-Aldrich (St. Louis, MO, USA) and diluted in sterile serum.
Antibodies against PI3K(the p85 subunit, 1:8000), p-PI3K (Tyr607 of the p85 subunit, 1:8000), p-Akt (Ser473, 1:4000), cytochrome c (1:200), caspase-3 (1:200), cleaved- PARP (1:300), Bad (1:1000), PLAP (1:400), OCT4 (1:100) were obtained from Abcam Biotechnology (Cambridge, UK); antibodies against caspase-9 (1:150) and GAPDH (1:8000) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Akt (1:600), p-mTOR (Ser2448, 1:4000), TDRG1 (1:1000) and Ki-67 (1:100) antibodies were purchased from Signalway Antibody Biotechnology (Baltimore, MD, USA), Cell Signaling Technology (Boston, MA, USA), Acris Biotechnology (San Diego, CA, USA) and GeneTex (Irvine, CA, USA), respectively.
Human tissue specimens, cell culture and drugs treatment
TDRG1, p-PI3K, p-Akt expression were analyzed on samples representative of 35 patients diagnosed with seminoma between April 2005 and April 2013 at our hospital. The formalin-fixed paraffin-embedded tissue sections were histopathologically confirmed and provided by Department of pathology. The detailed clinical information was extracted from the patients' electronic medical records.
Many research teams worldwide including us have failed to establish a seminoma cell line, which mainly due to the distinct property of seminoma cells to be prone to apoptosis in vitro. Hence, to date, only 3 lines have been reported and none of them can be purchased.Citation18 The human TCam-2 cell line was primarily derived from a testicular tumor sample of pure classical seminoma in 1992.Citation19 In this study, TCam-2 cells (kindly gifted from Dr. Riko Kitazawa, Department of Diagnostic Pathology, Ehime University Hospital, Matsuyama, Japan) were maintained in complete medium (Roswell Park Memorial Institute Medium-1640 with 10% fetal calf serum) in a humidified 5% CO2, 37°C incubator.
Different concentrations (0, 5, 10, 15, 25 and 30 µM) of CDDP were used to treat TCam-2 cells with different TDRG1 expression levels for dose-depended cell viability assay for 72 h (hours) and the 50% inhibitory concentrations (IC50) were calculated, respectively. As for time-depended viability curves and all followed experiments, the CDDP were used by IC50 of TCam-2 cells with normal TDRG1 expression, and IGF-1 (100 ng/ml) or LY294002 (20 uM) were added as indicated.
RNAi and overexpression
RNAi and overexpression vectors were described previously.Citation12 Briefly, 3 short hairpin interfering RNA (shRNA) targeting TDRG1 were designed and chemically synthesized, and inserted into the pGPU6/GFP/Neo vector. We selected one with a highest inhibition efficiency here (Forward primer: 5′-caccGCGCAGGATCAAGCTACAATGttcaagagaCATTGTAGCTTGATCCTGCGCttttttg-3′; Reverse primer: 5′-gatccaaaaaaGCGCAGGATCAAGCTACAATGtctcttgaaCATTGTAGCTTGATCCTGCGC-3′) and a shRNA-control vector containing non-silencing sequence (5′-GACTTCATAAGGCGCATGC-3′) was also used (Invitrogen Life Technologies, CA, USA). For overexpression, open reading frame of TDRG1 was cloned into the enhanced green fluorescent protein plasmid-C1 (pEGFP-C1) vector (Invitrogen Life Technologies).
The shRNA-TDRG1 vector, shRNA-control vector and pEGFP-C1-TDRG1 vector were transfected into Tcam-2 cells after reaching 70% confluence using Lipofectamine 2000 (Invitrogen Life Technologies) according to the manufacturer's instructions. To determine the transfection efficicency, cells expressing GFP protein were imaged using laser confocal scanning microscopy (Leica TCS-SP5, Germany). Then 400 ng/ml G418 (Sigma-Aldrich) was used for screening positively stable transfectants.
Quantitative real-time PCR
The detailed procedure was described previously.Citation12 The PCR primer sequences for TDRG1 and β-actin were as follows: TDRG1 forward primer 5′-GAAGAGGAGGGAGGCAGTCT-3′, reverse primer 5′-GGGAACCTAGA CCTGGGAAG-3′; β-actin forward primer 5′-CATTAAGGAGAAGCTGTGCT-3′, reverse primer 5′-GTTGAAGGTAGTTTCGTGGA-3′. A melting curve analysis of the amplified products was performed at the end of each PCR cycle. β-actin was used as the internal control, and gene expression was relatively quantified using 2−ΔΔCT method.Citation20
Cell viability assay
The cytotoxic effect of CDDP on TCam-2 cells with different TDRG1 expression levels was measured by MTT assay. Cells were seeded at a density of 104 cells/well in 96-well plates for 24 h. For dose-depended assay, different concentrations of CDDP were used to treat cells for another 72 h. Then cells were incubated with 20 µl MTT solution (final concentration, 5 mg/ml, Sigma-Aldrich) for 4 h at 37°C. While for time-depended curves, cells were treated with CDDP (10 µM), and with or without IGF-1 or LY294002 as indicated. At different time points (0, 12, 24, 48, 72, 96 h), MTT were added as described above. After that, DMSO was added to each well to dissolve the purple-blue formazan crystals by gentle agitation. For each well, the absorbance at 570 nm (A570) was estimated using a Microplate Reader (Bio-Tek ELX-800; Winooski, VT, USA), with DMSO used as blank.
Cell apoptosis analysis
Annexin V-FITC (fluorescein isothiocyanate)/PI staining was used to investigate cell apoptosis as described previously.Citation12 Briefly, after drugs treatment and incubation for 72 h, cells were collected, washed with ice-cold phosphate-buffered saline (PBS), and labeled with Annexin V and propidium iodide (PtdIns) in the dark using an Annexin V-FITC apoptosis detection Kit (Beyotime Biotechnology, Shanghai, China). Cell apoptosis was subsequently analyzed by a FACSCalibur flow cytometry (BD Biosciences, San Jose, CA, USA).
Cell cycle analysis
Cell cycle analysis was performed using the PI single staining method as described previously.Citation12 Briefly, after drugs treatment and cultivation for 72 h, the cells were collected, washed and centrifuged at 1000 rpm, and then fixed with 75% cold ethanol at 4°C overnight. After staining in PtdIns and RNase A at room temperature for 30 min (minutes) in the dark, the percentage of cells in each cell cycle phase was determined with Cell Quest software and ModiFit (Verity Software House, Topsham, USA) by a flow cytometer (BD Biosciences).
Western blotting
Western blotting (WB) was implemented as described previously.Citation12 Briefly, the cells and mice xenograft tumors were lysed in lysis buffer containing protease inhibitors. Protein concentration of lysates was determined by the bicinchoninic acid assay (Beyotime Biotechnology). Equal amounts of protein (20 µg) were separated by 10% SDS-PAGE electrophoresis and subsequently transferred onto PVDF membranes. The membranes were blocked with 5% non-fat dry milk in 0.2% Tween-20 in Tris-buffered saline (TBS-T) for 1 h at room temperature and then probed with primary antibodies. Immunoreactivity was detected after incubation with a horseradish peroxidase-conjugated secondary antibody by the enhanced chemiluminescence method (Thermo Scientific, Waltham, MA, USA). GAPDH was stained as a loading control.
Immunofluorescence assay
Immunofluorescence assay was performed as described previously.Citation21 Briefly, after drugs treatment and incubation for 72 h, the cells was fixed in 4% paraformaldehyde for 20 min and permeabilized with 0.3% Triton X-100 (Sigma-Aldrich) for 1 h at room temperature. Then cells were blocked with 1% BSA in PBS for 2 h, and incubated with primary antibody against TDRG1 or p-Akt at 4°C overnight. Finally, the cells were incubated with anti-Rabbit IgG (H+L) antibody labeled with Alexa 555 (Invitrogen Life Technologies, 1:200) for 1 h at room temperature in the dark, washed 3 times in PBS and mounted with DAPI. A confocal microscope (Leica TCS-SP5) was used to observe the cells.
Xenograft
Male athymic BALB/c nude mice (4–5 weeks old) were purchased from the Institute of Experimental Animal of Central South University (Changsha, China). Mice were maintained in a germ-free environment for one week. All animals had free access to standard laboratory mouse food and water. Then TDRG1 knockdown, TDRG1 overexpression or siRNA control TCam-2 cells (1 × 106 in 200µl medium) were injected subcutaneously into the groin area. After one week, mice were randomly chosen and assigned into 4 groups (6 mice per group) based on different TDRG1 expression levels in the injected cells, and CDDP (6mg/kg body weight, one times/week for 3 weeks, i.p.) was administrated as indicated: N.C. group, N.C. + CDDP group, TDRG1 knockdown + CDDP group and TDRG1 overexpression + CDDP group. Tumor parameters were measured every 4 days, and tumor volume was calculated by length × width2 × 0.5. After a 31 d follow-up period, mice were sacrificed and the tumors were removed. The tumors were fixed in 10% buffered formalin for immunhistochemistry analysis and in liquid nitrogen for WB.
Immunohistochemistry analysis
Immunohistochemistry (IHC) assays were carried out as described previously. Briefly, the sections were routinely deparaffinized in xylene, rehydrated in ethanol and finally rehydrated in double-distilled water. Antigen retrieval was performed by placing the sections in 0.01 M citrate buffer (pH 6.0) before microwave heating for 20 min. Samples were then blocked with 10% normal goat serum for 30 min and subsequently incubated with primary antibodies at 4°C overnight. The primary antibodies were visualized under the light microscope using the SP method according to the kit instructions (Maixin Biotechnology, Fuzhou, China).
Protein expression level was classified semiquantitatively combining the proportion and intensity of positively stained immunoreactive cells. For each section, 5 random fields were selected for scoring and a mean score was calculated in final analysis. The percentage of positive-staining cells was scored as follows: 0 (no positive cells), 1 (< 10% positive cells), 2 (10%–40% positive cells), 3 (40%–70% positive cells), and 4 (> 70% positive cells). The intensity of positive staining was also used in a 4 scoring system: 0 (negative staining), 1 (weak staining exhibited as light yellow), 2 (moderate staining exhibited as yellow brown), and 3 (strong staining exhibited as brown). Protein expression index = intensity score × positive score.
Data processing
Statistical analysis of the in vitro and in vivo results was analyzed using GraphPad Prism 5.01 software (GraphPad Software, CA, USA). The quantitative data were expressed as the mean ± standard deviation (mean ± SD) of 3 to 6 independent experiments. Differences among the means of multiple groups were compared based on one-way ANOVA, followed by a Newman–Keuls multiple comparison test. To test the correlation between TDR1 with p-PI3K or p-Akt in IHC assays, linear regression analysis was performed based on expression index of each protein. p values below 0.05 were considered statistically significant.
Result
Establishment of seminoma TCam-2 cells with TDRG1 knockdown or overexpression
To investigate the role of TDRG1 in regulating CDDP sensitivity in TCam-2 cells, separate knockdown and overexpression were done using either TDRG1 shRNA or pcDNA-TDRG1, and stable transfected cells were screened. Successful transfection of TCam-2 cells was determined by detecting GFP expression using fluorescence microscopy (Fig. S1). The results showed that short hairpin constructs against TDRG1 were efficient and specific in the ablation of the gene in TCam-2 cells at both mRNA level and protein level compared with vector control cells. In contrast, TDRG1 expression was significantly enhanced in TCam-2 cells treated with pcDNA-TDRG1 (Fig. S1). Thus, we successfully set up cell line models genetically manipulated for TDRG1 expression here.
TDRG1 regulates cell viability during CDDP treatment in TCam-2 cells
Firstly, we employed MTT assays to see the effect of TDRG1 on the chemosensitivity of TCam-2 cells to CDDP. As demonstrated in , after incubation with different concentrations of CDDP for 72 h, TDRG1 inhibition markedly decreased the cell viability in TCam-2 cells compared with negative control (N.C.). Furthermore, knockdown of TDRG1 also decreased the cell viability in TCam-2 cells following CDDP treatment in a time-dependent manner (). As expectedly, TDRG1 overexpression confered resistance to CDDP in TCam-2 cells in a both dose-dependent manner and time-dependent manner (). The 50% inhibitory concentration of CDDP for 72 h in the TDRG1 knockdown, TDRG1 overexpression and N.C. cells are shown in . The concentration from the N.C. cells in this study was similar with the IC50 of CDDP for 72 h in TCam-2 cells reported by Wermann H et al.Citation22
Figure 1. The effect of TDRG1 on cell viability of seminoma TCam-2 cells following CDDP treatment. (A) MTT assays were used to confirm the effects of different expression levels of TDRG1 on cell viability of TCam-2 cells following CDDP treatment. *p < 0.05, **p < 0.01, ***p < 0.0001 compared to negative control TCam-2 cells with CDDP treatment. (B) The effect of IGF-1, a PI3K signaling activator, on TCam-2 cells with TDRG1 knockdown and the effect of LY24002, a PI3Ksignaling inhibitor, on TCam-2 cells with TDRG1 overexpression were also evaluated during CDDP treatment. *p < 0.05, **p < 0.01, ***p < 0.0001 compared to negative control values.
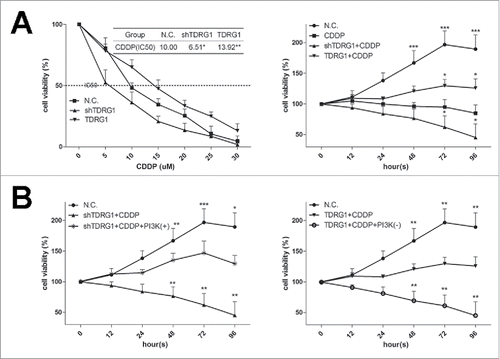
It was reported that PI3K signaling plays a critical role in the occurrence and development of seminoma.Citation15 Interestingly, we found that LY294002, a PI3K signaling inhibitor, re-sensitized TCam-2 cells with TDRG1 overexpression to CDDP, while IGF-1, a PI3K activator, reversed the positive effect of TDRG1 knockdown on the chemosensitivity of TCam-2 cells to CDDP ().
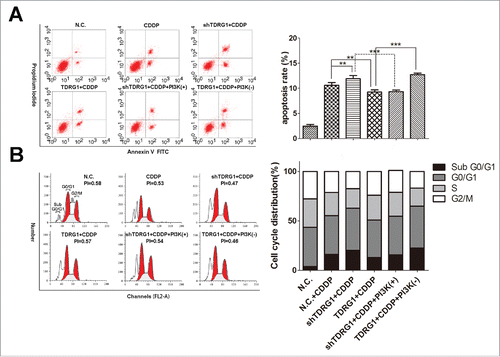
Considering either control of cell apoptosis or regulation of cell cycle may contribute to the modulatory effect of TDRG1 on the chemosenitivity, we then performed flow cytometry analysis. The data showed that CDDP alone for 72 h had a moderate effect on increasing early apoptosis rate and inducing cell cycle arrest in the G0/G1 phase, with a lower proliferation index (PI) in TCam-2 cells (). Similarly with the results in MTT assays, the combined therapy with TDRG1 inhibition enhanced the effect of CDDP on cell apoptosis and cell cycle, which could be hindered by IGF-1 (). Reversely, the joint application of TDRG1 overexpression and CDDP attenuated the therapeutic effect, which could be rescued by LY294002 (). Collectively, these findings suggest that TDRG1 regulates cell viability during CDDP treatment in TCam-2 cells via modulating both cell apoptosis and cell cycle, and PI3K signaling may be involved.
Figure 2. TDRG1 regulates early cell apoptosis and cell cycle distribution in seminoma TCam-2 cells following CDDP treatment for 72 hours. (A) Left: Cells were treated with or without CDDP, IGF-1 or LY24002 for 72 hours, followed by staining with Annexin V-fluorescein isothiocyanate and propidiumiodide and analyzed by flow cytometry. Right: Quantitative summary of early apoptosis rate in different groups. Compared as indicated, **p < 0.01, ***p < 0.0001. (B) Left: Cell cycle distribution in defferent groups was analyzed by flow cytometry. Right: Quantitative summary of cell cycle distribution in different groups.
TDRG1 regulates the PI3K/Akt/mTOR signal pathway during CDDP treatment in TCam-2 cells
Suppression of the PI3K/Akt/mTOR signaling has been demonstrated to overcome CDDP resistance in several kinds of human malignancy, indicating an important role of this signaling in regulating CDDP chemosensitivity.Citation23 Therefore, based on the above results, we then employed WB to test whether the PI3K/Akt/mTOR signaling was involved in the regulating effect of TDRG1 on CDDP sensitivity. It was widely accepted that disruption of the PI3K signaling is one of the molecular mechanisms of action during CDDP treatment.Citation24 Indeed, as shown in , we found the expression levels of phosphorylated PI3K, Akt and mTOR were decreased after CDDP treatment for 72 h in TCam-2 cells, while total PI3K, Akt and mTOR showed no significant changes. As expected, combined with the effect of CDDP, the expression levels of p-PI3K, p-Akt and p-mTOR were further inhibited in TCam-2 cells with TDRG1 inhibition, while the effect could be blocked by IGF-1. Additionally, the inhibitory effect of CDDP on phosphorylated PI3K, Akt and mTOR in TCam-2 cells with TDRG1 overexpression were attenuated and could be reversed by LY294002 ().
Figure 3. TDRG1 regulates PI3K/Akt/mTOR signaling and mitochondria-mediated apoptotic pathway in seminoma TCam-2 cells following CDDP treatment in vitro. (A) Protein expression of TDRG1, PI3K, p-PI3K, Akt, p-Akt and p-mTOR was measured by protein gel blotting at 72 hours after CDDP treatment in different groups. GAPDH served as the internal control. (B) Immunoflurescence staining was peformed to further detect the expression of TDRG1 and p-Akt in TCam-2 cells from different groups at 72 hours after CDDP treatment. TDRG1 affected the nuclear p-Akt expression. (C) The key elements in mitochondria-mediated apoptotic pathway including Bad, cytochrome c, caspase-9, caspase-3 and cleaved-PARP were also measured by western blotting at 72 hours after CDDP treatment in different groups. GAPDH served as an internal control.
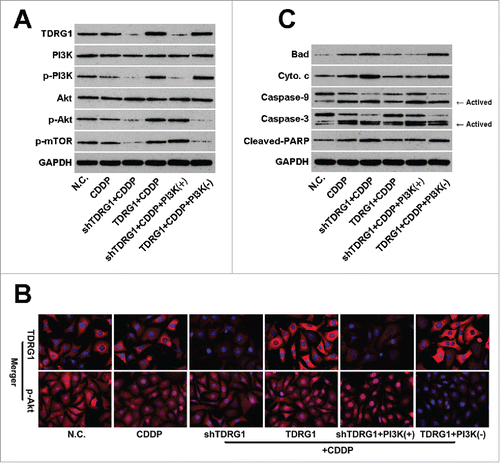
Akt is an important and central component in PI3K signaling.Citation13,14 Akt regulates downstream targets in the PI3K pathway, such as mTOR, and outside of the PI3K pathway, such as Bcl-2-asscociated proteins, and thus functions in many physiologic processes.Citation25 Moreover, Akt is also expressed in the nucleus, and increased levels of nuclear p-Akt have been seen in several human cancers.Citation26 Interestingly, as shown in , we found the expression of p-Akt in the nucleus of TCam-2 cells, which could be inhibited by CDDP treatment. Furthermore, TDRG1 knockdown inhibited the translocation of p-Akt from cytoplasm to the nucleus during CDDP treatment in TCam-2 cells, while TDRG1 overexpression enhanced the translocation. Identically, these effects also could be reversed by IGF-1 or LY294002, respectively ().
TDRG1 regulates mitochondria-mediated apoptotic pathway during CDDP treatment in TCam-2 cells
The mitochondria-associated apoptotic pathway participates in cellular response in tumors to many anticancer drugs including CDDP.Citation27 To see whether it is involved in TDRG1 induced alteration in sensitivity of TCam-2 cells to CDDP, we performed an analysis of pro-apoptotic proteins (Bad, cytochrome c). Furthermore, it is also believed that activation of caspase-9, caspase-3 and PARP follows and stimulates mitochondrial cell death signals, therefore we also investigated the effects of TDRG1 on the 3 proteins during CDDP treatment.Citation28 In , the WB results showed Bad, cytochrome c, caspase-9 ratio (activated/total), caspase-3 ratio (activated/total) and cleaved-PARP levels moderately increased after CDDP treatment in TCam-2 cells for 72 h. Moreover, compared to the vector control cells, TCam-2 cells with TDRG1 knockdown showed higher levels of Bad, cytochrome c, caspase-9 ratio, caspase-3 ratio and cleaved-PARP after treatment with CDDP, while TCam-2 cells with enhanced TDRG1 expression showed lower levels of these proteins or indexes. Consistently, these alterations could be respectively reversed by IGF-1 or LY294002 as well.
TDRG1 regulates seminoma growth in vivo during CDDP treatment
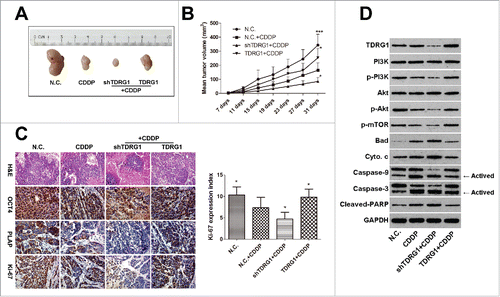
To further validate the effect of TDRG1 on the chemosensitivity of seminoma cells to CDDP in vivo, xenograft tumors were established by subcutaneous inoculation of TCam-2 cells in male BALB/c nude mice. Seen from , after 3 cycles of CDDP treatment, we found the mean volume of tumors derived from TCam-2 cells with TDRG1 knockdown was significantly smaller than these from the vector control cells. On the other hand, TDRG1 overexpression obviously enhanced the growth of TCam-2 tumors during CDDP treatment. Furthermore, TDRG1 knockdown caused more necrosis in some areas of the xenograft tumors during CDDP treatment (). Accordingly, a significant decrease of Ki-67 expression (a well-known cell proliferation marker)Citation29 was indicated in the TDRG1 knockdown + CDDP group compared to the CDDP group, while TDRG1 overexpression markedly increased the expression (). Similar results could also be found in the analysis of the expression of OCT4 and PLAP,Citation30 2 widely-accepted seminoma markers (). To elucidate the molecular mechanism in vivo, WB tests were then performed and also showed the involvement of PI3K/Akt/mTOR signaling and mitochondria-mediated apoptotic pathway, which was highly agreed with the results in vitro ().
Figure 4. TDRG1 regulates the chemosensitivity of seminoma to CDDP in vivo. (A) Representative tumors in nude mice from different groups. (B) The growth curves of transplanted tumors in nude mice (n = 6), *p < 0.05, ***p < 0.0001. (C) Representative photographs of hematoxylin and eosin (H&E) and immunohistochemistry (IHC) analysis of OCT4, PLAP and Ki-67, magnification, 200. The expression index of Ki-67 in different groups was analyzed, *p < 0.05. (D) Protein in mice xenograft tumors from different groups were collected. And the key elements in PI3K/Akt/mTOR signaling and mitochondria-mediated apoptotic pathway were measured by protein gel blotting. GAPDH served as a loading control.
Expression and correlation of TDRG1, p-PI3K and p-Akt in human seminoma tissues
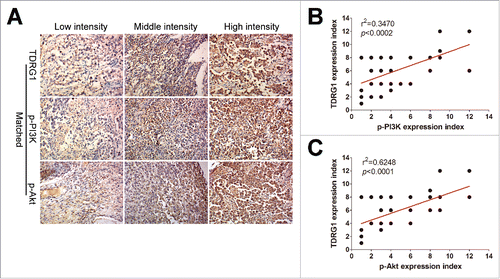
Finally, we detected the expression status of TDRG1, p-PI3K and p-Akt in human seminoma samples from 35 cases of seminoma patients by IHC staining. The positive staining of TDRG1 was indicated as brown precipitates in the cytoplasm, while the expression of p-PI3K and p-Akt could be detected both in the cytoplasm and nucleus. The intensity of TDRG1, p-PI3K and p-Akt staining was characterized as low, middle, or high, as illustrated in . We further analyzed the correlation between TDRG1 and p-PI3K or p-Akt expression in these samples and found that TDRG1 was significantly and positively correlated with p-PI3K (p = 0.0002) and p-Akt (p < 0.0001, ). It is also interesting to note that approximately 91% (32/35) of the patients expressed low (8/35) or moderate (24/35) levels of TDRG1.
Figure 5. Detect the expression of TDRG1, p-PI3K and p-Akt in tumor tissues from seminoma patients by immunohistochemistry (IHC) analysis. (A) Examples representative of low intensity, middle intensity and high intensity of positive TDRG1, p-PI3K and p-Akt immunostaining in matched seminoma patients samples. (B) The expression of TDRG1 in seminoma tissues was positive correlated with the expression of p-PI3K (r2 = 0.3470, p < 0.0002). (C) TDRG1 expression in seminoma tissues was also positive associated with p-Akt (r2 = 0.6248, p < 0.0001).
Discussion
Chemotherapy has been widely administrated in the treatment of a variety of cancers, including seminoma. So far, platinum is still the key agent in the chemotherapeutic regime for seminoma patients, and CDDP is one of the most commonly used.Citation31,32 The majority of seminoma patients present an extremely good response to CDDP-based chemotherapy and thus get the chance to be cured. In fact, yet a small group of patients are not sensitive to CDDP-based chemotherapy and suffer from pain and even inevitable death.Citation6-8,33 However, the mechanism accounting for the both phenomena is largely unclear. Recently, certain genes have been reported to be promising targets in regulating the sensitivity of tumor cells to CDDP, such as REV3L in cervical cancer cells, TFAP2a in bladder cancer cells.Citation27,34 In the present study, as part of our efforts to elucidate the above mechanism, TDRG1 was identified, for the first time, to be able to regulate the chemosenitivity of seminoma cells to CDDP, which was confirmed both in in vitro and in vivo experiments.
TDRG1 is a newly characterized human testis-specific gene and encodes a 100-amino-acid protein without any known protein domains.Citation10 Therefore, the function of TDRG1 protein still needs to be explored. Early studies indicated the protein functions in the process of spermatgenesis and in the tumorigenesis and development of TGCT.Citation11,12,21 Generally, several signaling pathways jointly participate in tumorigenesis. However, PI3K/Akt signal pathway, one of the most active signaling in malignancies, has been reported to play a key role in seminoma.Citation15 Wang et al. firstly reported that TDRG1 activates PI3K/Akt signaling and thus contributes to the progression of seminoma.Citation12 In this study, we employed MTT assays and further found TDRG1 positively regulated the cell viability of seminoma TCam-2 cells in vitro during CDDP treatment in a both dose-dependent and time-dependent manner. In consideration of the link of PI3K/Akt signaling with CDDP sensitivity, which has been confirmed in many human cancers, such as lung cancer,Citation35 bladder cancer,Citation25 ovary cancer and even in TGCT,Citation6,23 we then designed functional reverse tests using IGF-1, a PI3K signaling activator, and LY294002, a PI3K signaling inhibitor. As expected, the results showed the effect of TDRG1 knockdown or TDRG1 overexpression on the viability of TCam-2 cells during CDDP treatment could be well reversed by IGF-1 or LY294002, respectively. In vivo experiments also verified these findings, since TDRG1 could regulate the growth of xenograft seminoma cancer and affect the expression levels of Ki-67, OCT4 and PLAP in xenograft tumors. These markers commonly represent the proliferation ability and number of TCam-2 cells in tumors.Citation29,30
Furthermore, in vitro and in vivo results from western blotting further confirmed the involvement of PI3K/Akt signaling. PI3K is a heterodimer which consists of an 85 kDa regulatory subunit (p85) and a 110 kDa catalytic subunit (p110).Citation13 Consistent with the report from Wan et al., the expression of TDRG1 protein was positively correlated with the level of p-PI3K/p85 subunit in TCam-2 cells following CDDP treatment, while the total PI3K/p85 was not obviously changed.Citation12 The possible explanation might be that TDRG1 can act like a protein kinase and thus phosphorylate PI3K/p85. However, the action could also be indirect, thus more researches are needed to fully explain this phenomenon. Once p85 is phosphorylated, its regulatory activity decreases, leading to increased catalytic activity of p110. Activated PI3K further caused the phosphorylation of Akt.Citation36 Therefore, the level of p-Akt was consistently changed with TDRG1 as well. Moreover, it has been reported that the level of nuclear p-Akt increases during the progression of prostate cancer and increased level of nuclear Akt has been seen in tumors such as acute myeloid leukemia and lung, breast and pancreatic and thyroid cancers.Citation37,38 These evidence support an important role of nuclear Akt in tumorigenesis. Interestingly, from the in vitro immunofluorescence results, we observed p-Akt expression in nucleus of TCam-2 cells, and TDRG1 regulated its expression during CDDP treatment. Therefore, we postulated here aberrant nuclear p-Akt expression was involved in seminoma tumorigenesis and the response to CDDP.
mTOR is phosphorylated at Ser2448 via PI3K/Akt signaling and thus activated.Citation13 In turn, activated mTOR participates in mTORC2 complex, which phosphorylates Akt at Ser473 and also stimulates Akt phosphorylation at Thr308 by 3-phosphoinostitide dependent protein kinase-1, leading to full activation of Akt cascade.Citation39 Furthermore, mTOR also acts as a catalytic subunit in mTORC1 complex, the activation of which leads to the phosphorylation of p70 S6 protein kinase-1 (S6K1) and eIF4E-binding proteins (4E-BPs) and thus enhances the demand for protein synthesis to support cell cycle progression.Citation40 In accordance with these reports, here we found TDRG1 acted as a positive regulator of p-mTOR in TCam-2 cells during CDDP treatment and subsequently affected cell cycle progression in the G0/G1 phase. However, more detailed molecular mechanisms should be further explored. Furthermore, we also observed TDRG1 could regulate the early apoptosis rate in TCam-2 cells during CDDP treatment. As reported, induction of cell apoptosis also serves as an essential mechanism of the anti-tumor efficacy of CDDP.Citation32 Cell apoptosis is executed by members of the caspase family, which can be mainly activated by 2 main pathways, including the extrinsic death receptor pathway and the intrinsic mitochondria-related apoptotic pathway.Citation41 Bad, a pro-apoptotic protein, can be treated as a link between PI3K/Akt signaling and mitochondrial apoptotic pathway. In activation of PI3K/Akt signaling leads to dephosphorylation of Bad and resulting in its translocation from cytoplasm to the mitochondria, which is an important event triggering the release of cytochrome c from mitochondria.Citation42,43 Cytochrome c in cytoplasm then activates caspase-9 and caspase-3. Activation of either caspase can cleave PARP and trigger chromosomal DNA fragmentation, which is an apoptotic hallmark.Citation41 Herein, after confirming the involvement of PI3K/Akt signaling, we then examined the expression of Bad and cytochrome c in TCam-2 cells during CDDP treatment and found they were both positively correlated with TDRG1. Moreover, activation of the caspase cascade was also examined and it could also be regulated by TDRG1. These findings support that mitochondria-mediated apoptotic pathway can be modulated by TDRG1 in TCam-2 cells following CDDP treatment.
In this study, though we reported some primary findings, some limitations were inevitable. Firstly, we failed to obtain the rare tissues from seminoma patients with CDDP resistance, mainly due to its low incidence and the relatively unsound follow-up system in China. So we only detected the expression of TDRG1, p-PI3K and p-Akt in seminoma tissues. Interestingly, TDRG1 was positively correlated with p-PI3K and p-Akt in seminoma. What's more, the majority of patients presented low or moderate levels of TDRG1. We thus postulated this might, at least partially, contribute to the high sensitivity to CDDP in the most seminoma patients. In addition, because of no purchase channel and the difficulty in primary culture, we used only one seminoma cell line. Hence, if possible, more data from various kinds of seminoma cells including CDDP-resistant ones are needed.
However, we used both knockdown and overexpression of TDRG1 in TCam-2 cells, which might cover the shortage to some extent. All in all, here we provided both in vivo and in vitro evidence that TDRG1 regulates the chemosensitivity to CDDP in seminoma TCam-2 cell line through the PI3K/Akt/mTOR signaling and mitochondria-mediated apoptotic pathway. Moreover, the expression level of TDRG1 is positively linked with the activity of PI3K/Akt signaling in seminoma. Therefore, the therapy targeting TDRG1 may be a promising approach in seminoma patients receiving CDDP treatment.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_Informations.doc
Download MS Word (240.5 KB)Funding
This study was supported by the National Nature Science Foundation of China (No. 81372181) and China Scholarship Council (No. 201606370204). The authors thank Mr. Xiaohua Li for technical support.
References
- Le Cornet C, Lortet-Tieulent J, Forman D, Beranger R, Flechon A, Fervers B, Schüz J, Bray F. Testicular cancer incidence to rise by 25% by 2025 in Europe? Model-based predictions in 40 countries using population-based registry data. Eur J Cancer 2014; 50:831-9; PMID:24369860; http://dx.doi.org/10.1016/j.ejca.2013.11.035
- Giannatempo P, Greco T, Mariani L, Nicolai N, Tana S, Fare E, Raggi D, Piva L, Catanzaro M, Biasoni D, et al. Radiotherapy or chemotherapy for clinical stage IIA and IIB seminoma: a systematic review and meta-analysis of patient outcomes. Ann Oncol 2015; 26:657-68; PMID:25214543; http://dx.doi.org/10.1093/annonc/mdu447
- Horwich A, Shipley J, Huddart R. Testicular germ-cell cancer. Lancet 2006; 367:754-65; PMID:16517276; http://dx.doi.org/10.1016/S0140-6736(06)68305-0
- Hanna NH, Einhorn LH. Testicular cancer–discoveries and updates. N Eng J Med 2014; 371:2005-16; PMID:25409373; http://dx.doi.org/10.1056/NEJMra1407550
- Horwich A, Nicol D, Huddart R. Testicular germ cell tumours. Bmj 2013; 347:f5526; PMID:24065428; http://dx.doi.org/10.1136/bmj.f5526
- Juliachs M, Munoz C, Moutinho CA, Vidal A, Condom E, Esteller M, Graupera M, Casanovas O, Germà JR, Villanueva A, et al. The PDGFRbeta-AKT pathway contributes to CDDP-acquired resistance in testicular germ cell tumors. Clin Cancer Res 2014; 20:658-67; PMID:24277456; http://dx.doi.org/10.1158/1078-0432.CCR-13-1131
- Kobayashi T, Fujii T, Jo Y, Kinugawa K, Fujisawa M. Possible mechanism responsible for the acquisition of resistance to cis-diamminedichloroplatinum (II) by cultured human testicular seminoma cells. J Urol 2004; 171:1929-33; PMID:15076314; http://dx.doi.org/10.1097/01.ju.0000122901.70300.20
- Squillante CM, Vaughn DJ. Targeted therapies in germ cell tumors. Urol Oncol 2015; 33:363-9; PMID:25544153; http://dx.doi.org/10.1016/j.urolonc.2014.09.008
- Fung C, Vaughn DJ. Complications associated with chemotherapy in testicular cancer management. Nat Rev Urol 2011; 8:213-22; PMID:21403662; http://dx.doi.org/10.1038/nrurol.2011.26
- Jiang X, Li D, Yang J, Wen J, Chen H, Xiao X, et al. Characterization of a novel human testis-specific gene: testis developmental related gene 1 (TDRG1). Tohoku J Exp Med 2011; 225:311-8; PMID:22123530; http://dx.doi.org/10.1620/tjem.225.311
- Chen H, Sun J, He Y, Zou Q, Wu Q, Tang Y. Expression and localization of testis developmental related gene 1 (TDRG1) in human spermatozoa. Tohoku J Exp Med 2015; 235:103-9; PMID:25749352; http://dx.doi.org/10.1620/tjem.235.103
- Wang Y, Gan Y, Tan Z, Zhou J, Kitazawa R, Jiang X, et al. TDRG1 functions in testicular seminoma are dependent on the PI3K/Akt/mTOR signaling pathway. Onco Targets Ther 2016; 9:409-20; PMID:26855590; http://dx.doi.org/10.2147/OTT.S97294
- Cantley LC. The phosphoinositide 3-kinase pathway. Science 2002; 296:1655-7; PMID:12040186; http://dx.doi.org/10.1126/science.296.5573.1655
- Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 1995; 376:599-602; PMID:7637810; http://dx.doi.org/10.1038/376599a0
- Nakai Y, Nonomura N, Oka D, Shiba M, Arai Y, Nakayama M, Inoue H, Nishimura K, Aozasa K, Mizutani Y, et al. KIT (c-kit oncogene product) pathway is constitutively activated in human testicular germ cell tumors. Biochem Biophys Res Commun 2005; 337:289-96; PMID:16188233; http://dx.doi.org/10.1016/j.bbrc.2005.09.042
- Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene 2005; 24:7455-64; PMID:16288292; http://dx.doi.org/10.1038/sj.onc.1209085
- West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug resist Updat 2002; 5:234-48; PMID:12531180; http://dx.doi.org/10.1016/S1368-7646(02)00120-6
- Russell SM, Lechner MG, Mokashi A, Megiel C, Jang JK, Taylor CR, Looijenga LH, French CA, Epstein AL. Establishment and characterization of a new human extragonadal germ cell line, SEM-1, and its comparison with TCam-2 and JKT-1. Urology 2013; 81:464 e1-9; PMID:23374840; http://dx.doi.org/10.1016/j.urology.2012.09.029
- Mizuno Y, Gotoh A, Kamidono S, Kitazawa S. [Establishment and characterization of a new human testicular germ cell tumor cell line (TCam-2)]. Nihon Hinyokika Gakkai zasshi The japanese journal of urology 1993; 84:1211-8; PMID:8394948; http://dx.doi.org/10.5980/jpnjurol1989.84.1211
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method 2001; 25:402-8; PMID:11846609; http://dx.doi.org/10.1006/meth.2001.1262
- Gan Y, Yang J, Wang Y, Tan Z, Jiang X, Tang Y. study on shRNA-mediated reduction of testis developmental related gene 1 expression and its effects on the proliferation, invasion and apoptosis of NTERA-2 cells. Oncol Lett 2015; 10:61-6; PMID:26170977; http://dx.doi.org/10.3892/ol.2015.3219
- Wermann H, Stoop H, Gillis AJ, Honecker F, van Gurp RJ, Ammerpohl O, Richter J, Oosterhuis JW, Bokemeyer C, Looijenga LH. Global DNA methylation in fetal human germ cells and germ cell tumours: association with differentiation and cisplatin resistance. J Pathol 2010; 221:433-42; PMID:20593487; http://dx.doi.org/10.1002/path.2725
- Zhang HY, Zhang PN, Sun H. Aberration of the PI3K/AKT/mTOR signaling in epithelial ovarian cancer and its implication in cisplatin-based chemotherapy. Eur J Obstet Gynecol Reprod Biol 2009; 146:81-6; PMID:19540648; http://dx.doi.org/10.1016/j.ejogrb.2009.04.035
- Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia 2003; 17:590-603; PMID:12646949; http://dx.doi.org/10.1038/sj.leu.2402824
- Sun D, Sawada A, Nakashima M, Kobayashi T, Ogawa O, Matsui Y. MK2206 potentiates cisplatin-induced cytotoxicity and apoptosis through an interaction of inactivated Akt signaling pathway. Urol Oncol 2015; 33:111 e17-26; PMID:25499922; http://dx.doi.org/10.1016/j.urolonc.2014.10.018
- Neri LM, Borgatti P, Capitani S, Martelli AM. The nuclear phosphoinositide 3-kinase/AKT pathway: a new second messenger system. Biochim Biophys Acta 2002; 1584:73-80; PMID:12385889; http://dx.doi.org/10.1016/S1388-1981(02)00300-1
- Yang L, Shi T, Liu F, Ren C, Wang Z, Li Y, Tu X, Yang G, Cheng X. REV3L, a promising target in regulating the chemosensitivity of cervical cancer cells. PloS One 2015; 10:e0120334; PMID:25781640; http://dx.doi.org/10.1371/journal.pone.0120334
- Nicholson DW, Thornberry NA. Apoptosis. Life and death decisions. Science 2003; 299:214-5; PMID:12522239; http://dx.doi.org/10.1126/science.1081274
- Ciaputa R, Nowak M, Madej JA, Poradowski D, Janus I, Dziegiel P, Gorzynska E, Kandefer-Gola M. Inhibin-alpha, E-cadherin, calretinin and Ki-67 antigen in the immunohistochemical evaluation of canine and human testicular neoplasms. Folia Histochem Cytobiol 2014; 52:326-34; PMID:25511291; http://dx.doi.org/10.5603/FHC.a2014.0036
- Chieffi P, Chieffi S, Franco R, Sinisi AA. Recent advances in the biology of germ cell tumors: implications for the diagnosis and treatment. J Endocrinol Invest 2012; 35:1015-20; PMID:23143673; http://dx.doi.org/10.3275/8716
- Cost NG. Testicular germ cell tumors. Current concepts and management strategies. Minerva Urol Nefrol = The Italian journal of urology and nephrology 2013; 65:133-55; PMID:23703101
- Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 2014; 740:364-78; PMID:25058905; http://dx.doi.org/10.1016/j.ejphar.2014.07.025
- Voutsadakis IA. The chemosensitivity of testicular germ cell tumors. Cell Oncol 2014; 37:79-94; PMID:24692098; http://dx.doi.org/10.1007/s13402-014-0168-6
- Nordentoft I, Dyrskjot L, Bodker JS, Wild PJ, Hartmann A, Bertz S, Lehmann J, Orntoft TF, Birkenkamp-Demtroder K. Increased expression of transcription factor TFAP2alpha correlates with chemosensitivity in advanced bladder cancer. BMC Cancer 2011; 11:135; PMID:21489314; http://dx.doi.org/10.1186/1471-2407-11-135
- Wang Y, Chen L, Huang G, He D, He J, Xu W, Zou C, Zong F, Li Y, Chen B, et al. Klotho sensitizes human lung cancer cell line to cisplatin via PI3k/Akt pathway. PloS One 2013; 8:e57391; PMID:23437382; http://dx.doi.org/10.1371/journal.pone.0057391
- Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 1997; 387:673-6; PMID:9192891; http://dx.doi.org/10.1038/42648
- Ye ZW, Ghalali A, Hogberg J, Stenius U. Silencing p110beta prevents rapid depletion of nuclear pAkt. Biochem Biophys Res Commun 2011; 415:613-8; PMID:22074824; http://dx.doi.org/10.1016/j.bbrc.2011.10.120
- Van de Sande T, Roskams T, Lerut E, Joniau S, Van Poppel H, Verhoeven G, Swinnen JV. High-level expression of fatty acid synthase in human prostate cancer tissues is linked to activation and nuclear localization of Akt/PKB. J Pathol 2005; 206:214-9; PMID:15880754; http://dx.doi.org/10.1002/path.1760
- Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta 2011; 1813:1978-86; PMID:21440011; http://dx.doi.org/10.1016/j.bbamcr.2011.03.010
- Ekim B, Magnuson B, Acosta-Jaquez HA, Keller JA, Feener EP, Fingar DC. mTOR kinase domain phosphorylation promotes mTORC1 signaling, cell growth, and cell cycle progression. Mol Cell Biol 2011; 31:2787-801; PMID:21576368; http://dx.doi.org/10.1128/MCB.05437-11
- Estaquier J, Vallette F, Vayssiere JL, Mignotte B. The mitochondrial pathways of apoptosis. Adv Exp Med Biol 2012; 942:157-83; PMID:22399422; http://dx.doi.org/10.1007/978-94-007-2869-1_7
- Zeng KW, Wang XM, Ko H, Kwon HC, Cha JW, Yang HO. Hyperoside protects primary rat cortical neurons from neurotoxicity induced by amyloid beta-protein via the PI3K/Akt/Bad/Bcl(XL)-regulated mitochondrial apoptotic pathway. Eur J Pharmacol 2011; 672:45-55; PMID:21978835; http://dx.doi.org/10.1016/j.ejphar.2011.09.177
- Borner C. The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol 2003; 39:615-47; PMID:12493639; http://dx.doi.org/10.1016/S0161-5890(02)00252-3
