ABSTRACT
Despite advances in screening and prevention strategies, colorectal cancer (CRC) remains the second-leading cause of cancer-related death in the United States. Given this continued public health burden of CRC, there is a clear need for improved disease prevention. CRC initiates and progresses over decades, canonically proceeding via a series of stepwise molecular events that turn a normal epithelium into a dysfunctional epithelium, then subsequently into an adenoma, and finally an invasive adenocarcinoma. An emerging paradigm suggests that guanylyl cyclase C (GUCY2C) functions as a tumor suppressor in the intestine, and that the loss of hormone ligands for this receptor causes epithelial dysfunction and represents an important step in the disease process. In that context, GUCY2C ligand replacement therapy has been proposed as a strategy to prevent colorectal cancer, a translational opportunity that is underscored by the recent regulatory approval of the oral GUCY2C ligand linaclotide (Linzess™, Forest Laboratories and Ironwood Pharmaceuticals, Inc.).
Abbreviations
| CRC | = | colorectal cancer |
| GUCY2C | = | guanylyl cyclase C |
| USPSTF | = | United States Preventive Services Task Force |
| ST | = | heat-stable enterotoxin |
| ETEC | = | enterotoxigenic E. coli |
| cGMP | = | cyclic GMP |
| CFTR | = | cystic fibrosis transmembrane conductance receptor |
| CIN | = | chromosomal instability pathway |
| FAP | = | familial adenomatous polyposis coli syndrome |
| MMR | = | mismatch repair |
| HNPCC | = | hereditary nonpolyposis colorectal cancer syndrome |
| CIMP | = | CpG island methylator phenotype |
| AOM | = | azoxymethane |
| IBS-C | = | constipation-predominant irritable bowel syndrome |
| CIC | = | chronic idiopathic constipation |
| IBD | = | inflammatory bowel disease |
| MPE | = | molecular pathologic epidemiology |
Introduction
Colorectal cancer (CRC) remains a huge public health burden, representing the second leading cause of cancer-related death.Citation1 In addition to morbidity and mortality, CRC takes a large financial toll on health care expenditures, a cost which is projected to balloon to over 14 billion dollars annually in the next decade.Citation2 An old medical adage suggests that “A penny of prevention is worth a pound of cure.” Despite this age-old ideal of preventative medicine, actual development and implementation of preventative strategies has proven difficult. Some diseases have seen astounding success in preventing disease (such as cardiovascular disease where modifiable risk factors can prevent disease), while still others, notably cancer, have lagged behind. Colorectal cancer is relatively unique among cancers as there has been a successful prevention campaign put in place through endoscopic screening, but patient compliance (due to perceived inconvenience of colonoscopy) and effectiveness remain an issue.Citation3,4 Currently, CRC prognosis is driven by disease stage at presentation. Whereas advanced metastatic disease is incurable, localized disease offers the potential for definitive and curative surgery. Despite screening programs, nearly one fourth of patients present with metastatic disease. Thus, although these interventions have lowered mortality, the continued overall health burden of CRC demands still further efforts to prevent disease and save additional lives.Citation5
Unfortunately, despite a litany of promising preclinical chemopreventative agents, translating preventative findings into clinical benefit has remained an elusive goal.Citation5 In fact, only aspirin has emerged as a recommended chemopreventive agent against CRC by the United States Preventative Services Task Force (USPSTF), but its use is somewhat controversial in low-risk patients due to GI toxicity.Citation6 As such, there is currently no FDA-approved, generally-accepted strategy for chemoprevention of CRC. In that context, the present review highlights the FDA-approved oral GUCY2C ligand linaclotide, an emerging candidate for chemoprevention. Leveraging a large body of preclinical data on safety and efficacy, linaclotide is now being translated into human chemopreventive studies.
Guanylyl cyclase C
Linaclotide functions through engagement of its receptor, guanylyl cyclase C (GUCY2C) in the intestine. GUCY2C is a particulate guanylyl cyclase receptor expressed primarily in intestinal epithelial cellsCitation7-9 and in the brain.Citation8,10-12 The GUCY2C receptor possesses several cognate ligands which are all structurally similar peptides. These ligands include the endogenous hormones guanylin and uroguanylin as well as exogenous ligands such as the heat-stable enterotoxins (STs) produced by enterotoxigenic E. coli (ETEC).Citation13,14 Importantly, linaclotide is structurally derived from naturally-occurring GUCY2C ligands,Citation15 and like these other peptides has a similar ability to increase cyclic GMP (cGMP) levels through GUCY2C.Citation16 In intestine, GUCY2C is localized to the apical brush border membranes, where it has a well-characterized role in fluid homeostasis.Citation8,13,17 Upon engagement of the extracellular ligand binding domain of GUCY2C by its ligands, the cytoplasmic catalytic domain catalyzes GTP into cGMP. Cyclic GMP acts as a second messenger to direct cell behavior, including through cGMP-dependent protein kinase (PKG) II, which phosphorylates the cystic fibrosis transmembrane conductance regulator (CFTR) inducing secretion of salt, and therefore water, leading to diarrhea.Citation18 More recent evidence which is reviewed below has suggested that GUCY2C also functions as a tumor suppressor in the intestine.
Colorectal cancer initiation and progression
Colorectal cancer is thought to arise by a series of mutations that in a stepwise fashion transforms normal epithelium into a malignant tumor. In most cases of sporadic colorectal cancer, chromosomal instability (CIN) leading to biallelic APC loss in intestinal stem cells initiate transformation.Citation19 Indeed, 80–90% of colorectal tumors harbor biallelic APC mutations,Citation20 and these are sufficient to cause disease.Citation21 Consistent with this model, germline mutations in APC in humans result in the familial colorectal syndrome known as familial adenomatous polyposis coli (FAP).Citation22 Canonically, APC functions as a key component of the destruction complex that targets β catenin for degradation.Citation23 In turn, this attenuates Wnt signaling and limits its oncogenic functions through downstream proto-oncogenes such as c-MYC and Cyclin D1.Citation23 Conversely, loss of APC function and dysregulation of Wnt signaling leads to adenoma formation.Citation23 Subsequently, mutations to established tumor suppressors and oncogenes, such as p53 and KRAS, drive progression from adenoma to invasive adenocarcinoma.Citation23
Mismatch repair (MMR) deficiency is another pathway that can lead to familial CRC (Lynch Syndrome; Hereditary Non-Polyposis Colorectal Cancer HNPCC) or, more rarely (∼15% cases), sporadic CRC in a pathway characterized by microsatellite instability.Citation24 These tumors originate through mutations in mismatch repair genes including MLH1, MSH2, MSH6 or PMS2.Citation24 Deficient MMR leads to an accumulation of somatic mutations that cause disease, including many of the molecular targets discussed above. Yet another pathway, the CpG island methylator phenotype (CIMP), silences tumor suppressor genes via promoter methylation, and functionally resembles MMR tumorigenesis.Citation25
Although colorectal cancer is traditionally considered a disease of irreversible mutational events, the paracrine hormone hypothesis of cancer highlights an alternative model of tumorigenesis in which the loss of the paracrine hormone guanylin potentiates tumorigenesis by silencing the GUCY2C tumor suppressor. Indeed, guanylin expression is universally lost early in colorectal tumorigenesis in an event which is conserved across species.Citation26,27 This observation suggests that the loss of GUCY2C ligands may represent an important mechanistic event in colorectal tumorigenesis. Interestingly, worldwide there is an inverse epidemiological association between the incidence of colorectal cancer and the risk of being colonized with ETEC expressing ST, consistent with a model in which chronic ETEC supplementation of GUCY2C ligands protect against the development of CRC.Citation28,29
The functional significance of this guanylin loss is underscored by studies done in knockout mice in which GUCY2C expression is eliminated. These mice exhibit epithelial dysfunction in the intestine characterized by defects in proliferation, migration, metabolic reprogramming, differentiation and genomic integrity along the crypt-villus axis.Citation30,31 These homeostatic processes that are corrupted when GUCY2C is silenced are the canonical pathways which are universally defective in all cancers in the “Hallmarks of Cancer” model.Citation32 Consistent with this tumorigenic phenotype, GUCY2C knockout mice also display a marked increase in in vivo tumorigenesis in both an azoxymethane (AOM) carcinogen model of intestinal tumorigenesis as well as the genetic APCmin tumor model. Taken together, these findings suggest that the loss of the paracrine hormone guanylin is an initiating event in intestinal tumorigenesis, presenting a unique opportunity for oral hormone replacement to prevent CRC. This paradigm transforms our understanding of colorectal tumorigenesis from a disease of irreversible stepwise mutational events into one of reversible signaling events.
GUCY2C signaling prevents colorectal cancer
The paracrine hormone hypothesis suggests that colorectal cancer can be prevented by oral administration of exogenous GUCY2C ligands which replace the lost guanylin. Consistent with that hypothesis, GUCY2C signaling produces growth arrest of normal human intestinal cells and human colon carcinoma cells in vitro and ex vivo.Citation33-35 Mechanistically, GUCY2C activation was associated with reduced expression of markers of cell cycle progression including β catenin, cyclin D1, pRb, and increased expression of cell cycle inhibitors including p27. Functionally, GUCY2C signaling restricted transition through the G1/S interface, limiting cell cycle progression without inducing apoptosis.Citation33-35 These findings suggest that during colorectal carcinogenesis, GUCY2C persists as a dormant tumor-suppressing receptor following loss of its ligand(s), and that it can be re-activated through administration of exogenous ligand which rescues cell cycle regulation and restricts aberrant growth.
Translating these findings, several animal studies have provided pivotal proof-of-concept for administration of GUCY2C ligand to prevent colorectal cancer. One such study utilized oral administration of uroguanylin and demonstrated increased apoptosis and attenuated tumorigenesis in a mouse model of CRC.Citation28 Further studies utilized a genetic approach, generating transgenic mice to overexpress guanylin under the control of an inducible, intestinal-specific Cre recombinase.Citation36,37 Importantly, transgenic guanylin expression eliminated tumorigenesis in mice subjected to a carcinogen-obesity model of colorectal tumorigenesis.Citation32 Further, obesity-associated epithelial dysfunction in the intestine was ameliorated in these animals, characterized by reversal of the observed defects in proliferation, migration, metabolic reprogramming, differentiation and genomic integrity along the crypt-villus axis.Citation32 Additionally, guanylin transgenic mice exhibited persistent GUCY2C signaling over the lifetime of the animal, without obvious attenuation or desensitization.Citation37 Importantly, administration of GUCY2C ligand was well-tolerated; no adverse events were observed inside or outside of the GI tract, thus further underscoring the safety of GUCY2C ligand administration.Citation28,36,37 These studies provide compelling preclinical evidence to support the GUCY2C ligand linaclotide as a safe, effective and novel approach to prevent colorectal cancer.
Clinical translation of GUCY2C ligands for colorectal cancer chemoprevention
Although there are 3 similar peptide agonists of GUCY2C in clinical development [linaclotide (Linzess™, Forest Laboratories and Ironwood Pharmaceuticals, Inc.) and SP-304 (plecanatide) and SP-333 (Synergy Pharmaceuticals, Inc.)],Citation16,38,39 only linaclotide is currently approved by the FDA for treatment of constipation-predominant irritable bowel syndrome (IBS-C) and chronic idiopathic constipation (CIC or CC).Citation40 Administration of linaclotide in mice increased cGMP production and induced fluid secretion, consistent with GUCY2C activation.Citation16 Further, linaclotide exhibited negligible bioavailability, confining its pharmacologic effects to the intestine and minimizing systemic side effects.Citation16
Several large-scale clinical trials supported the use of linaclotide in treating IBS-C, demonstrating the ability of the compound to improve colonic transit, improve abdominal pain and ameliorate pathologic bowel habits when compared to placebo.Citation41-47 Moreover, linaclotide's side effect profile was favorable, with the most common adverse event being diarrhea (in approximately 20% of patients), perhaps not surprising given GUCY2C's role in traveler's diarrhea. Other less common events included abdominal pain and distention, flatulence, gastroenteritis and headache.Citation48 However, none of these events were considered serious enough to discontinue development of the drug, and the FDA and international agencies approved its use in 2012.Citation41,48,49
SP-304, another GUCY2C ligand in clinical development, is an analog of the endogenous GUCY2C ligand uroguanylin.Citation50 Like linaclotide, SP-304 was designed to exert its effect in the intestine with minimal systemic exposure. In early phase IIa clinical trials, patients with chronic constipation exhibited a marked improvement in bowel habits compared with placebo.Citation51 Yet a third ligand in development, SP-333, is structurally similar to SP-304 but possesses several amino acid substitutions which render it resistant to proteolysis. This resistance is predicted to increase the dose of active drug passing through the small intestine and into the colon, thus giving it potentially greater utility in treating colonic disorders.Citation52,53 SP-333 is therefore also currently in clinical trials for the treatment of inflammatory bowel disease (IBD), a disease with a typically large colonic component.Citation53,54
Given the established role for the loss of GUCY2C signaling in colorectal tumorigenesis, linaclotide and other GUCY2C ligands may provide a unique opportunity for cancer chemoprevention ().Citation34,35,37,55-58 In that context, linaclotide is FDA-approved and possesses a favorable toxicity profile to support its use in healthy patients for disease prevention.
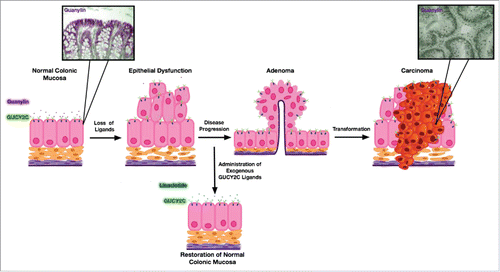
Figure 1. Preventing colorectal cancer by exploiting the paracrine hormone hypothesis of colorectal cancer. Colorectal cancer is a stepwise disease whereby a normal epithelium is transformed over a period of years into first an adenoma until finally becoming an adenocarcinoma. Importantly, guanylin expression is lost early and universally in this transformative continuum and contributes to disease progression as depicted in the diagram. Insets to this cartoon show adapted immunohistochemistry images (guanylin depicted in purple, nuclei/hematoxylin depicted in black) of normal human colonic epithelium as contrasted with human adenocarcinoma which demonstrate complete loss of guanylin staining in the adenocarcinoma (inset images adapted from Wilson etal. Biomarkers & Prevention, 2014). Importantly, GUCY2C ligand loss in colorectal cancer provides a therapeutic opportunity for chemoprevention through exogenous administration of GUCY2C ligands.
Given all of these factors, we have partnered with the Mayo Clinic and the National Cancer Institute (NCI)'s Division of Chemoprevention to undertake a phase 1 study to evaluate linaclotide as a chemopreventative agent for colorectal cancer (Linaclotide Acetate in Preventing Colorectal Cancer in Healthy Volunteers NCT01950403). The goal of this study is to evaluate the dose of linaclotide required to induce GUCY2C and increase cGMP levels in the colorectum of healthy volunteers. Secondary endpoints include measures of adverse events and toxicity as well as the activation of downstream tumor suppressive GUCY2C signaling. Tissue analysis will be conducted in biopsies collected via rectosigmoidoscopy, once at baseline and then again following 7 days of linaclotide treatment. Similar to the clinical path of NSAIDs/aspirin to chemoprevention, encouraging results from these studies must be confirmed in larger phase 2 and 3 trials that quantify actual tumor prevention over longer periods of follow-up.Citation6
Interestingly, evidence also suggests that GUCY2C activation may have a role in preventing multiple forms of cancer outside the intestine. Mechanistically, this prevention is achieved through GUCY2C's role in regulating intestinal barrier function. Indeed, multiple studies have shown that silencing GUCY2C induces barrier deficiency, while its activation restores barrier integrity.Citation37,57 Thus, conditions which silence GUCY2C also are likely to result in a leaky barrier which allows environmental toxins and carcinogens to leak into the systemic circulation, promoting inflammation and DNA damage in other organs outside the intestine.Citation37 Further studies are necessary, but these findings represent an intriguing avenue for multi-focal chemoprevention with a well-tolerated, intestine-specific approach.
Identification of populations for GUCY2C-targeted prevention
One major challenge to developing chemoprevention strategies lies in identifying suitable at-risk populations for treatment. In addition to known genetic CRC syndromes (FAP and HNPCC), there are established environmental risk factors, including obesity (body weight, diet and physical activity level), advanced age, intestinal inflammation, cigarette smoking and alcohol abuse.Citation59 Beyond these established epidemiological factors, further work must be done to identify populations who would likely accrue the most benefit from linaclotide treatment. In that context, recent studies have raised the possibility that guanylin loss is an important molecular event in colorectal cancer associated with inflammatory bowel disease and obesity, both well-known clinical risk factors for CRC.Citation36,60,61
The paradigm of cancer prevention is expanding into molecular pathologic epidemiology (MPE). Whereas traditional epidemiologic studies attempt to link risk factors to disease (i.e. a clinical association), MPE adds additional depth by considering the molecular underpinnings of the disease itself.Citation62,63 This approach applies these epidemiologic findings to achieve more precise patient management. Importantly, loss of guanylin silencing GUCY2C in colorectal cancer is universal, suggesting that this event is involved in the pathogenesis of multiple molecular subtypes of CRC.Citation26 Despite this prevalence, additional studies are required to identify which specific molecular subtypes of CRC are most affected by GUCY2C silencing, thereby identifying which at-risk patients to target.
Conclusions and future directions
There has been a gradual shift in the balance of cancer research in recent years from a more treatment-centric approach to prevention. This change in strategy may perhaps reflect an improvement in our understanding of causative mechanisms of disease, or alternatively be attributed to the lack of improvement in clinical outcomes for most cancers in recent decades, despite billions of dollars in research spending.Citation64 This failure in approach is perhaps best exemplified by small molecule inhibitors for cancer treatment, which generally speaking have yielded only months of survival benefit despite costing billions of dollars to develop.Citation48,49,65 Although research into curative interventions is still essential, this shift in focus toward chemoprevention has the potential to provide substantial clinical benefit to at-risk patient populations (summarized in ).
Currently, there is no FDA-approved, generally accepted strategy for such chemoprevention of CRC in the general population. However, recent advances in several preventative strategies, including linaclotide, have made the chemoprevention of CRC within striking distance of becoming a reality. Remarkably, activation of linaclotide's receptor, GUCY2C, eliminates tumorigenesis in mouse models of CRC, and these findings are currently being evaluated in human studies. Future studies should evaluate the efficacy of GUCY2C-directed chemoprevention in patients with known risk factors. In addition to chemoprevention of at-risk populations, linaclotide's favorable toxicity profile may even allow its use in the general population, a proposition which has previously been controversial in aspirin chemoprevention due to toxicity concerns.
Disclosure of potential conflicts of interest
SAW is the Chair (uncompensated) of the Scientific Advisory Board of Targeted Diagnostics & Therapeutics, Inc. which provided research funding that, in part, supported this work and has a license to commercialize inventions related to this work.
Funding
Supported by grants from NIH [CA170533] and Targeted Diagnostic and Therapeutics, Inc. ESB received an F30 Ruth Kirschstein MD-PhD Fellowship Award [CA180500]. SAW is the Samuel MV Hamilton Professor of Thomas Jefferson University.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66:7-30; PMID:26742998; http://dx.doi.org/10.1017/S0009840X15002851
- Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst 2011; 103:117-28; PMID:21228314; http://dx.doi.org/10.1093/jnci/djq495
- Willyard C. Screening: early alert. Nature 2015; 521:S4-5; PMID:25970456; http://dx.doi.org/10.1038/521S4a
- Van Gossum A, Munoz-Navas M, Fernandez-Urien I, Carretero C, Gay G, Delvaux M, Lapalus MG, Ponchon T, Neuhaus H, Philipper M, et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med 2009; 361:264-70; PMID:19605831; http://dx.doi.org/10.1056/NEJMoa0806347
- Gravitz L. Prevention: tending the gut. Nature 2015; 521:S6-8; PMID:25970457; http://dx.doi.org/10.1038/521S6a
- Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer 2016; 16(3):173-86; PMID:26868177; http://dx.doi.org/10.1038/nrc.2016.4
- Carrithers SL, Parkinson SJ, Goldstein S, Park P, Robertson DC, Waldman SA. Escherichia coli heat-stable toxin receptors in human colonic tumors. Gastroenterology 1994; 107:1653-61; PMID:7958675
- Schulz S, Green CK, Yuen PS, Garbers DL. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell 1990; 63:941-8; PMID:1701694; http://dx.doi.org/10.1016/0092-8674(90)90497-3
- Laney DW, Jr., Mann EA, Dellon SC, Perkins DR, Giannella RA, Cohen MB. Novel sites for expression of an Escherichia coli heat-stable enterotoxin receptor in the developing rat. Am J Physiol 1992; 263:G816-21; PMID:1359797
- Begg DP, Steinbrecher KA, Mul JD, Chambers AP, Kohli R, Haller A, Cohen MB, Woods SC, Seeley RJ. Effect of guanylate cyclase-C activity on energy and glucose homeostasis. Diabetes 2014; 63(11):3798-804; DB_140160; PMID:24898144; http://dx.doi.org/10.2337/db14-0160
- Gong R, Ding C, Hu J, Lu Y, Liu F, Mann E, Xu F, Cohen MB, Luo M. Role for the membrane receptor guanylyl cyclase-C in attention deficiency and hyperactive behavior. Science 2011; 333:1642-6; PMID:21835979; http://dx.doi.org/10.1126/science.1207675
- Valentino MA, Lin JE, Snook AE, Li P, Kim GW, Marszalowicz G, Magee MS, Hyslop T, Schulz S, Waldman SA. A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. J Clin Invest 2011; 121:3578-88; PMID:21865642; http://dx.doi.org/10.1172/JCI57925
- Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev 2000; 52:375-414; PMID:10977868
- Canani RB, Castaldo G, Bacchetta R, Martin MG, Goulet O. Congenital diarrhoeal disorders: advances in this evolving web of inherited enteropathies. Nat Rev Gastroenterol Hepatol 2015; 12:293-302; PMID:25782092; http://dx.doi.org/10.1038/nrgastro.2015.44
- Donia MS, Fischbach MA. HUMAN MICROBIOTA. Small molecules from the human microbiota. Science 2015; 349:1254766; PMID:26206939; http://dx.doi.org/10.1126/science.1254766
- Bryant AP, Busby RW, Bartolini WP, Cordero EA, Hannig G, Kessler MM, Pierce CM, Solinga RM, Tobin JV, Mahajan-Miklos S, et al. Linaclotide is a potent and selective guanylate cyclase C agonist that elicits pharmacological effects locally in the gastrointestinal tract. Life Sci 2010; 86:760-5; PMID:20307554; http://dx.doi.org/10.1016/j.lfs.2010.03.015
- Schulz S, Lopez MJ, Kuhn M, Garbers DL. Disruption of the guanylyl cyclase-C gene leads to a paradoxical phenotype of viable but heat-stable enterotoxin-resistant mice. J Clin Invest 1997; 100:1590-5; PMID:9294128; http://dx.doi.org/10.1172/JCI119683
- Li P, Lin JE, Marszlowicz GP, Valentino MA, Chang C, Schulz S, Pitari GM, Waldman SA. GCC signaling in colorectal cancer: Is colorectal cancer a paracrine deficiency syndrome? Drug News Perspect 2009; 22:313-8; PMID:19771320; http://dx.doi.org/10.1358/dnp.2009.22.6.1395254
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009; 457:608-11; PMID:19092804; http://dx.doi.org/10.1038/nature07602
- Brannon AR, Vakiani E, Sylvester BE, Scott SN, McDermott G, Shah RH, Kania K, Viale A, Oschwald DM, Vacic V, et al. Comparative sequencing analysis reveals high genomic concordance between matched primary and metastatic colorectal cancer lesions. Gen Biol 2014; 15:454; PMID:25164765; http://dx.doi.org/10.1186/s13059-014-0454-7
- Cheung AF, Carter AM, Kostova KK, Woodruff JF, Crowley D, Bronson RT, Haigis KM, Jacks T. Complete deletion of Apc results in severe polyposis in mice. Oncogene 2010; 29:1857-64; PMID:20010873; http://dx.doi.org/10.1038/onc.2009.457
- Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 2013; 13:11-26; PMID:23258168; http://dx.doi.org/10.1038/nrc3419
- Dow LE, O'Rourke KP, Simon J, Tschaharganeh DF, van Es JH, Clevers H, Lowe SW. Apc restoration promotes cellular differentiation and reestablishes crypt homeostasis in colorectal cancer. Cell 2015; 161:1539-52; PMID:26091037; http://dx.doi.org/10.1016/j.cell.2015.05.033
- Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol 2010; 7:153-62; PMID:20142816; http://dx.doi.org/10.1038/nrclinonc.2009.237
- Colussi D, Brandi G, Bazzoli F, Ricciardiello L. Molecular pathways involved in colorectal cancer: implications for disease behavior and prevention. Int J Mol Sci 2013; 14:16365-85; PMID:23965959; http://dx.doi.org/10.3390/ijms140816365
- Wilson C, Lin JE, Li P, Snook AE, Gong J, Sato T, Liu C, Girondo MA, Rui H, Hyslop T, et al. The paracrine hormone for the GUCY2C tumor suppressor, guanylin, is universally lost in colorectal cancer. Cancer Epidemiol Biomarkers Prevent 2014; 23:2328-37; PMID:25304930; http://dx.doi.org/10.1158/1055-9965.EPI-14-0440
- Steinbrecher KA, Tuohy TM, Heppner Goss K, Scott MC, Witte DP, Groden J, Cohen MB. Expression of guanylin is downregulated in mouse and human intestinal adenomas. Biochem Biophys Res Commun 2000; 273:225-30; PMID:10873591; http://dx.doi.org/10.1006/bbrc.2000.2917
- Shailubhai K, Yu HH, Karunanandaa K, Wang JY, Eber SL, Wang Y, Joo NS, Kim HD, Miedema BW, Abbas SZ, et al. Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer Res 2000; 60:5151-7; PMID:11016642
- Pitari GM, Zingman LV, Hodgson DM, Alekseev AE, Kazerounian S, Bienengraeber M, Hajnóczky G, Terzic A, Waldman SA. Bacterial enterotoxins are associated with resistance to colon cancer. Proc Natl Acad Sci USA 2003; 100:2695-9; PMID:12594332; http://dx.doi.org/10.1073/pnas.0434905100
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 2010; 143:134-44; PMID:20887898; http://dx.doi.org/10.1016/j.cell.2010.09.016
- Ghaleb AM, McConnell BB, Kaestner KH, Yang VW. Altered intestinal epithelial homeostasis in mice with intestine-specific deletion of the Kruppel-like factor 4 gene. Dev Biol 2011; 349:310-20; PMID:21070761; http://dx.doi.org/10.1016/j.ydbio.2010.11.001
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/10.1016/j.cell.2011.02.013
- Pitari GM, Di Guglielmo MD, Park J, Schulz S, Waldman SA. Guanylyl cyclase C agonists regulate progression through the cell cycle of human colon carcinoma cells. Proc Natl Acad Sci USA 2001; 98:7846-51; PMID:11438734; http://dx.doi.org/10.1073/pnas.141124698
- Li P, Lin JE, Chervoneva I, Schulz S, Waldman SA, Pitari GM. Homeostatic control of the crypt-villus axis by the bacterial enterotoxin receptor guanylyl cyclase C restricts the proliferating compartment in intestine. Am J Pathol 2007; 171:1847-58; PMID:17974601; http://dx.doi.org/10.2353/ajpath.2007.070198
- Lin JE, Li P, Snook AE, Schulz S, Dasgupta A, Hyslop TM, Gibbons AV, Marszlowicz G, Pitari GM, Waldman SA. The hormone receptor GUCY2C suppresses intestinal tumor formation by inhibiting AKT signaling. Gastroenterology 2010; 138:241-54; PMID:19737566; http://dx.doi.org/10.1053/j.gastro.2009.08.064
- Lin JE, Colon-Gonzalez F, Blomain E, Kim GW, Aing A, Stoecker B, Rock J, Snook AE, Zhan T, Hyslop TM, et al. Obesity-induced colorectal cancer is driven by caloric silencing of the Guanylin-GUCY2C paracrine signaling axis. Cancer Res 2016; 76:339-46; PMID:26773096; http://dx.doi.org/10.1158/0008-5472.CAN-15-1467-T
- Lin JE, Snook AE, Li P, Stoecker BA, Kim GW, Magee MS, Garcia AV, Valentino MA, Hyslop T, Schulz S, et al. GUCY2C opposes systemic genotoxic tumorigenesis by regulating AKT-dependent intestinal barrier integrity. Plos one 2012; 7:e31686; PMID:22384056; http://dx.doi.org/10.1371/journal.pone.0031686
- Harris LA, Crowell MD. Linaclotide, a new direction in the treatment of irritable bowel syndrome and chronic constipation. Curr Opin Mole Ther 2007; 9:403-10; PMID:17694454
- Vazquez Roque M, Camilleri M. Linaclotide, a synthetic guanylate cyclase C agonist, for the treatment of functional gastrointestinal disorders associated with constipation. Expert Rev Gastroenterol Hepatol 2011; 5:301-10; PMID:21651347; http://dx.doi.org/10.1586/egh.11.30
- Videlock EJ, Cheng V, Cremonini F. Effects of linaclotide in patients with irritable bowel syndrome with constipation or chronic constipation: a meta-analysis. Clin Gastroenterol Hepatol 2013; 11:1084-92.e3; quiz e68; PMID:23644388; http://dx.doi.org/10.1016/j.cgh.2013.04.032
- Johnston JM, Shiff SJ, Quigley EM. A review of the clinical efficacy of linaclotide in irritable bowel syndrome with constipation. Curr Med Res Opin 2013; 29:149-60; PMID:23198977; http://dx.doi.org/10.1185/03007995.2012.754743
- Quigley EM, Tack J, Chey WD, Rao SS, Fortea J, Falques M, Diaz C, Shiff SJ, Currie MG, Johnston JM. Randomised clinical trials: linaclotide phase 3 studies in IBS-C - a prespecified further analysis based on European Medicines Agency-specified endpoints. Aliment Pharmacol Ther 2013; 37:49-61; PMID:23116208; http://dx.doi.org/10.1111/apt.12123
- Chey WD, Lembo AJ, Lavins BJ, Shiff SJ, Kurtz CB, Currie MG, MacDougall JE, Jia XD, Shao JZ, Fitch DA, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol 2012; 107:1702-12; PMID:22986437; http://dx.doi.org/10.1038/ajg.2012.254
- Rao S, Lembo AJ, Shiff SJ, Lavins BJ, Currie MG, Jia XD, Shi K, MacDougall JE, Shao JZ, Eng P, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol 2012; 107:1714-24; quiz p 25; PMID:22986440; http://dx.doi.org/10.1038/ajg.2012.255
- Johnston JM, Kurtz CB, Macdougall JE, Lavins BJ, Currie MG, Fitch DA, O'Dea C, Baird M, Lembo AJ. Linaclotide improves abdominal pain and bowel habits in a phase IIb study of patients with irritable bowel syndrome with constipation. Gastroenterology 2010; 139:1877-86.e2; PMID:20801122; http://dx.doi.org/10.1053/j.gastro.2010.08.041
- Andresen V, Camilleri M, Busciglio IA, Grudell A, Burton D, McKinzie S, Foxx-Orenstein A, Kurtz CB, Sharma V, Johnston JM, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology 2007; 133:761-8; PMID:17854590; http://dx.doi.org/10.1053/j.gastro.2007.06.067
- Castro J, Harrington AM, Hughes PA, Martin CM, Ge P, Shea CM, Jin H, Jacobson S, Hannig G, Mann E, et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3′,5prime;-monophosphate. Gastroenterology 2013; 145:1334-46 e1-11; PMID:23958540; http://dx.doi.org/10.1053/j.gastro.2013.08.017
- Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012; 366:707-14; PMID:22356324; http://dx.doi.org/10.1056/NEJMoa1112302
- Carter CA, Kelly RJ, Giaccone G. Small-molecule inhibitors of the human epidermal receptor family. Expert Opin Invest Drugs 2009; 18:1829-42; PMID:19938898; http://dx.doi.org/10.1517/13543780903373343
- Solinga RKM, Busby R, Currie M. A comparison of the physical and pharmacological properties of plecanatide (SP-304) and the human hormone uroguanylin. ACJ 2011; 11:332
- Shailubhai KLB, Talluto C, Comiskey S, Foss J, Feng R, Joslyn A, Jacob G. Plecanatide, a guanylate cyclase C agonist, improves bowel habits and symptoms associated with chronic constipation in a phase IIa clinical study. ACJ 2011; 11:1174
- Graham Zhang KPA, Foss JA, Comiskey SJ, Shailubhai K. SP-333, a proteolysis-resistant agonist of guanylate cyclase-C, inhibits activation of NF-κB and suppresses production of inflammatory cytokines to ameliorate DSS-induced colitis in mice. 77th annual meeting of American College of Gastroenterology.
- SynergyPharmaceuticals. Pipeline: SP-333. http://www.synergypharmacom/pipeline/sp-333 2013.
- SynergyPharmaceuticals. Single ascending dose study to evaluate the safety, tolerability and pharmacokinetics (PK) of SP-333 tablets in healthy adult subjects. ClinicalTrialsgov; Identifier: NCT01705938.
- Steinbrecher KA, Wowk SA, Rudolph JA, Witte DP, Cohen MB. Targeted inactivation of the mouse guanylin gene results in altered dynamics of colonic epithelial proliferation. Am J Pathol 2002; 161:2169-78; PMID:12466132; http://dx.doi.org/10.1016/S0002-9440(10)64494-X
- Li P, Schulz S, Bombonati A, Palazzo JP, Hyslop TM, Xu Y, Baran AA, Siracusa LD, Pitari GM, Waldman SA. Guanylyl cyclase C suppresses intestinal tumorigenesis by restricting proliferation and maintaining genomic integrity. Gastroenterology 2007; 133:599-607; PMID:17681179; http://dx.doi.org/10.1053/j.gastro.2007.05.052
- Han X, Mann E, Gilbert S, Guan Y, Steinbrecher KA, Montrose MH, Cohen MB. Loss of guanylyl cyclase C (GCC) signaling leads to dysfunctional intestinal barrier. PloS One 2011; 6:e16139; PMID:21305056; http://dx.doi.org/10.1371/journal.pone.0016139
- Lubbe WJ, Zuzga DS, Zhou Z, Fu W, Pelta-Heller J, Muschel RJ, Waldman SA, Pitari GM. Guanylyl cyclase C prevents colon cancer metastasis by regulating tumor epithelial cell matrix metalloproteinase-9. Cancer Res 2009; 69:3529-36; PMID:19336567; http://dx.doi.org/10.1158/0008-5472.CAN-09-0067
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg 2009; 22:191-7; PMID:21037809; http://dx.doi.org/10.1055/s-0029-1242458
- Harmel-Laws E, Mann EA, Cohen MB, Steinbrecher KA. Guanylate cyclase C deficiency causes severe inflammation in a murine model of spontaneous colitis. PloS One 2013; 8:e79180; PMID:24244444; http://dx.doi.org/10.1371
- Brenna O, Bruland T, Furnes MW, Granlund A, Drozdov I, Emgard J, Brønstad G, Kidd M, Sandvik AK, Gustafsson BI. The guanylate cyclase-C signaling pathway is down-regulated in inflammatory bowel disease. Scand J Gastroenterol 2015; 50:1241-52; PMID:25979109; http://dx.doi.org/10.3109/00365521.2015.1038849
- Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut 2011; 60:397-411; PMID:21036793; http://dx.doi.org/10.1136/gut.2010.217182
- Ogino S, Lochhead P, Chan AT, Nishihara R, Cho E, Wolpin BM, Meyerhardt JA, Meissner A, Schernhammer ES, Fuchs CS, et al. Molecular pathological epidemiology of epigenetics: emerging integrative science to analyze environment, host, and disease. Mod Pathol 2013; 26:465-84; PMID:23307060; http://dx.doi.org/10.1038/modpathol.2012.214
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10-29; PMID:22237781; http://dx.doi.org/10.1017/S0009840X11002678
- Kaitin KI. Deconstructing the drug development process: the new face of innovation. Clin Pharmacol Ther 2010; 87:356-61; PMID:20130565; http://dx.doi.org/10.1038/clpt.2009.293
