ABSTRACT
Lapatinib, a novel tyrosine kinase inhibitor of HER2/EGFR, is used to treat HER2-positive breast cancer. However, acquired drug resistance has limited the clinical therapeutic efficacy of lapatinib. Our previous study found that inhibition of autophagy can reduce the proliferation, DNA synthesis, and colony-forming capacity of lapatinib-resistant cells. Berberine has attracted extensive attention due to its wide range of biochemical and pharmacological effects in breast cancer treatment. It has been reported that berberine can induce oxidative stress and the mitochondrial-related apoptotic pathway in human breast cancer cells. In our current study, we found that a new combination therapy of berberine with lapatinib overcame lapatinib resistance. Furthermore, we found that berberine induced apoptosis of lapatinib-resistant cells through upregulating the level of ROS. Specially, lapatinib activated both the c-Myc/pro-Nrf2 pathway and GSK-3β signaling to stabilize Nrf2 and maintain a low level of ROS in resistant cells. However, berberine can upset the ROS balance by downregulating c-Myc to reverse the lapatinib resistance. Our finding provides a novel strategy of using berberine to overcome lapatinib resistance.
Abbreviations
| Ros | = | reactive oxygen species |
| HER | = | epidermal growth factor receptor |
| MTT | = | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| EdU | = | 5-ethynyl-2′-deoxyuridine |
Introduction
Breast cancer is a global public health burden, with more than 234,000 new cases and 40,000 deaths worldwide in 2015. Based on gene detection, breast cancer is classified into 4 main subtypes (luminal A, luminal B, HER2+ and basal-like). Human epidermal growth factor receptor (HER) is overexpressed in an estimated 2025%– of total cases and is associated with enhanced tumor aggressiveness and reduced patient survival compared to HER2-negative patients.Citation1,2 HER2 overexpression is correlated with tumor size, lymph node positivity, high tumor grade and aneuploidy.Citation3 During the past 2 decades, significant effort has been put into generating therapeutic drugs that specifically target HER2. There are 2 main types of HER2-targeted therapy; these are monoclonal antibodies (mAbs) and tyrosine kinase inhibitors (TKIs).Citation4 Trastuzumab, pertuzumab (mAbs), trastuzumab emtansine (T-DM1, an antibody-drug conjugate) and lapatinib (TKI) have been approved by the U.S Food and Drug Administration and European Medicines Agency and are now used to treat patients with HER2-positive breast cancer.
Lapatinib, the dual-tyrosine kinase inhibitor of HER2 and EGFR,Citation5 acts by blocking the intracellular ATP binding site of the tyrosine kinase domain, leading to reduced receptor phosphorylation and activation.Citation6,7 It has proven successful in inhibiting the phosphorylation of HER receptors and its downstream targets such as Akt and MAPKCitation8,9 and in slowing tumor progression in vitro and in vivo.Citation10 Moreover, lapatinib has weak cardiotoxicity and can easily enter cells and penetrate the blood-brain barrier during the treatment of breast cancer brain metastases. However, like other molecular targeting drugs, primary and acquired resistance dramatically limits the efficiency of lapatinib. A few studies have proven that a majority of patients develop acquired resistance within 6 months when using lapatinib alone.Citation11 Primary or acquired resistance severely reduces the clinical therapeutic effect of lapatinib; therefore, exploring the mechanism by which resistance to lapatinib develops and investigating new strategies for lapatinib use may be helpful in increasing its therapeutic efficacy. Berberine has been isolated from many types of medicinal plants, such as Hydrastis Canadensis, Berberis aristata, Coptis chinensis, Coptis rhizome, Coptis japonica, Phellondendron amurense, and Phellondendron chinense schneid,Citation12 and now berberine has been proven to possess a wide variety of pharmacological and biological activities, including antimicrobial, anti-helminthic, and anti-inflammatory effects.Citation13 In recent years, research has focused on the anti-cancer activity of berberine due to evidence of anti-neoplastic properties, and researchers have demonstrated that berberine can suppress tumor cell proliferation and induce tumor cell apoptosis in a variety of human cancer both in vitro and in vivo.Citation14 It has been reported that berberine can decrease TPA-induced angiogenesis and migration factors, including VEGF and FN, in breast cancer cells.Citation15 In a current study, berberine decreased side population (SP) cells in breast cancer cells that were associated with a decrease in ABCG2 expression.Citation16 Additionally, berberine is used in combination with and shows strong synergy with some chemotherapy drugs. In neuroblastoma cells, As2o3 was used to enhance berberine-mediated apoptosis.Citation17 Some researchers found that combining berberine with estrogen receptor (ER) antagonists can improve anticancer efficacy in MCF-7 cells (ER+).Citation18 However, little experimental or clinical research has focused on the combination of lapatinib and berberine, and thus we sought to explore the effects of combining lapatinib and berberine in the treatment of breast cancer.
The anti-tumor mechanism of berberine in breast cancer has received increasing attention. ROS are produced by all aerobic cells to regulate cell development, growth, survival, and death. ROS normally exist in balance with biochemical antioxidants in all aerobic cells.Citation19,20 When this critical balance is disrupted by excess ROS production and antioxidant depletion, oxidative stress may develop and influence cell viability by modifying intracellular or extracellular macromolecules or producing hyper- or hypo-functionality of the signaling pathways, which finally leads to pathological effects or alteration of physiological action.Citation21 It has been reported that many chemotherapeutic drugs may be selectively toxic to cancer cells by inducing oxidative stress.Citation22 A few studies of berberine-induced ROS in breast cancer indicated that berberine increases ROS production and induces cell apoptosis through activation of the pro-apoptotic JNK signaling and downregulation of the expression of anti-apoptotic protein Bcl-2 concomitant with upregulation of the expression of pro-apoptotic protein Bax.Citation23 The transcription factor NF-E2-related factor 2 (Nrf2) was originally identified as a critical regulator of intracellular antioxidants and phase II detoxification enzymes by the transcriptional upregulation of many ARE-containing genes.Citation24,25 Since its discovery, Nrf2 has been viewed as a “good” transcription factor that protects us from many diseases. The Nrf2-knockout mouse is prone to acute damage induced by acetaminophen, ovalbumin, cigarette smoke, pentachlorophenol and 4-vinylcyclohexene diepoxide.Citation26-29 In addition, the Nrf2-knockout mouse shows increased tumor formation when exposed to carcinogens such as benzo[a]pyrene, diesel exhaust and N-nitrosobutyl (4-hydroxybuty) amine .Citation30-32 However, the dark side of Nrf2 was recently revealed when Nrf2 was found to be constitutively upregulated in several types of human cancer tissues and cancer cell lines and found to protect tumors and cell lines from chemotherapeutic drugs.Citation33,34 The relationship between berberine and Nrf2 is controversial. Some researchers believe that berberine can enhance the level of Nrf2 to protect cells from inflammation and oxidant stress,Citation35 whereas others see berberine as an anti-tumor agent based on its increased ROS production.
Based on this concept, we hypothesize that a combination of lapatinib and berberine could reverse lapatinib resistance in lapatinib-resistant breast cancer by increasing ROS production. Therefore, we established lapatinib-resistant cell lines BT-474LapR and AU-565LapR and found that berberine sensitizes lapatinib-resistant breast cancer cells to the anti-tumor effect of lapatinib. Additionally, we described the level of ROS production in the BT-474LapR cells and AU-565LapR cells when treated with and without berberine and lapatinib. Finally, we attempted to explain the sensitization of berberine through the Nrf2 pathway.
Results
Identification of lapatinib-resistant cell lines
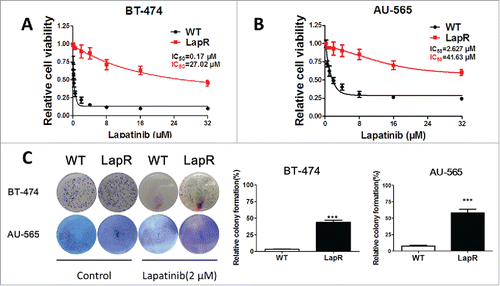
As in our previous report (Tumor Biology, 2015), we established lapatinib-resistant breast cancer models in vitro by treating BT-474WT and AU-565WT cells with lapatinib (2 μM) for 12 months. After continually passaging the cells, we identified those that showed resistance to lapatinib. The MTT assay was used to detect the half maximal inhibitory concentration (IC50) of lapatinib in the cells (BT-474WT, BT-474LapR, AU-565WT, and AU-565LapR). Cells were plated in 96-wells and cultured in DMEM and RPMI 1640 with 10% FBS. When cells grew to 60% confluence, the deferent dose of lapatinib was added to the cells. We performed the MTT assay after 2 d of lapatinib treatment. The results showed that the inhibitory rate of cell proliferation depended on the concentrations of lapatinib in both wild-type cells and in lapatinib-resistant cells (). However, the IC50 of lapatinib differed between BT-474 WT and BT-474LapR cells, where the IC50 in BT-474LapR cells increased about approximately 35-fold (P < 0.01). A similar result was observed in AU-565 cells. Furthermore, a colony formation assay was used to demonstrate lapatinib-resistance, and wild-type cells were used as the control. After being plated 200/well in 6-well plates, both cells were cultured in DMEM and RPMI 1640 with 10% FBS for 1 week. Lapatinib was added to the cells every 3 days, and the colony formation assay was performed after 2 weeks of treatment. As with the MTT assay, the results of the colony formation assay showed that lapatinib-resistant cells showed stronger drug resistance to lapatinib than the wild-type cells (). All the above results indicate that lapatinib-resistant cells (BT-474LapR and AU-565LapR) have been established and identified.
Figure 1. Establishing lapatinib-resistant cell lines. To establish stable lapatinib-resistant cell lines, we treated HER2-positive breast cancer cells BT-474 and AU-565 with lapatinib (2 μM) for 12 months. Then, the lapatinib-resistant cells were cultured in a medium containing 10% fetal bovine serum. (A) An MTT assay was used to analyze the sensitivity of BT-474WT and BT-474LapR cells treated with various concentrations of lapatinib (0–32 μM) for 3 d. (B) An MTT assay was used to analyze the sensitivity of AU-565wt and AU-565LapR cells to various concentrations of lapatinib (0-32 μM) for 3 d. (C) A colony formation assay was used to demonstrate the sensitivity of both BT-474 and AU-565 wild-type and resistant cells to lapatinib. The cells were treated with lapatinib (2 μM) every 3 days, and detection was performed 2 weeks after lapatinib treatment. ***p < 0.001.
Using a combination of lapatinib and berberine to reverse lapatinib resistance
To confirm the effect of a combination of lapatinib and berberine treatment in breast cancer cells that have acquired resistance to lapatinib, BT-474LapR cells and AU-565LapR cells were treated with both lapatinib and berberine, and an MTT assay was used to compare the effects with those of the control group, which was treated with lapatinib only. Cell viability was investigated after treatment with a single concentration of lapatinib combined with varying concentrations of berberine. With an increasing concentration of berberine, cell viability of the BT-474LapR cells and AU-565LapR cells was significantly lower than the control group (). Synthesising these results with those of the MTT assay, we can conclude that berberine can sensitize resistant cells to the anti-tumor effect of lapatinib. Furthermore, cell apoptosis of the BT-474LapR cells and AU-565LapR cells treated with a combination of lapatinib and berberine was observed by flow cytometry. As shown in , apoptosis in the group treated with combination therapy was obviously higher than that among other groups. All these results show that a combination of lapatinib and berberine inhibits lapatinib-resistant breast cancer. The colony formation assay was used to strengthen this conclusion. Given the flow cytometry results, the colony assay confirmed that the combination of lapatinib and berberine can reverse lapatinib resistance (). Moreover, the EDU assay also showed that a combination of lapatinib and berberine resulted in greater death among lapatinib-resistant cells ().
Figure 2. Berberine synthesized with lapatinib reversed lapatinib resistance in both BT-474LapR cells and AU-565LapR cells. The cells were treated with lapatinib alone, berberine alone and a combination of lapatinib and berberine. (A) An MTT assay was used to demonstrate sensitization of berberine to lapatinib in BT474LapR cells and AU-565LapR cells. The cells were treated various concentrations of berberine (0-16 μM) synthesized with lapatinib (2 μM). (B) Flow cytometry was used to demonstrate sensitization of berberine to lapatinib. The cells were treated with lapatinib (2 μM), berberine (2 μM) and both (L+B), and the control group was untreated. The right figure shows the relative apoptosis rate of cells. *p < 0.05, **p < 0.01. (C) Colony formation was used to prove that berberine could sensitize lapatinib-resistant cells to lapatinib. The cells were treated with lapatinib (2 μM), berberine (2 μM) and a combination of both (L+B), and the control group was untreated. The right figure shows the relative colony formation rate of cells. *p < 0.05, **p < 0.01.
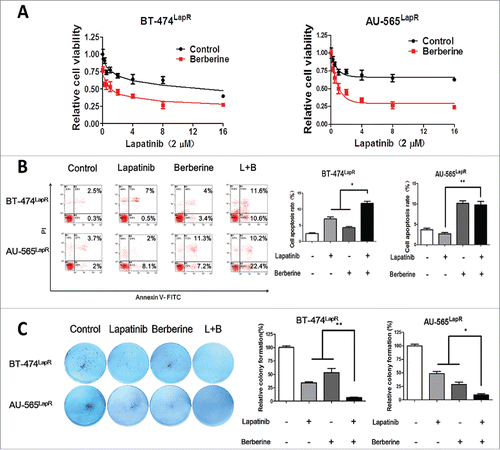
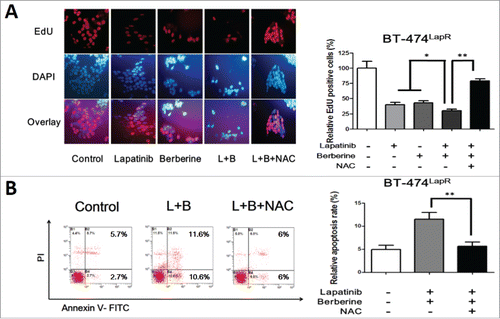
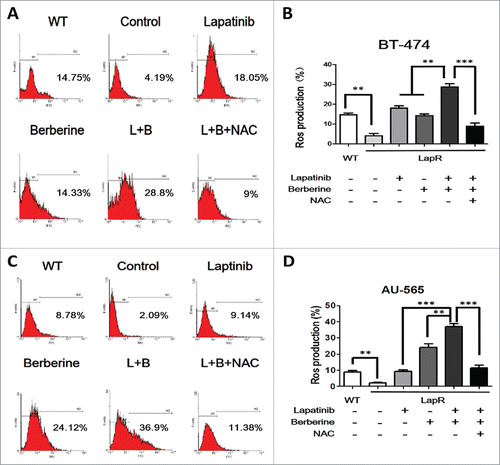
Figure 4. ROS involved in berberine-induced apoptosis. Lapatinib-resistant cells were treated with lapatinib alone (2 μM), berberine alone (2 μM), a combination of lapatinib and berberine (L+B), and a combination of lapatinib, berberine and NAC (2 mM) (L+B+NAC). Apoptosis of the cells was detected. (A) EdU staining was performed and showed a significantly difference between the presence and absence of NAC in berberine-induced apoptosis in BT-474LapR cells. Meanwhile, this provides additional evidence for sensitization of berberine. (B) The column shows that NAC can decrease the apoptosis rate in BT-474LapR cells treated with lapatinib and berberine. *p < 0.05, **p < 0.01. A line spanning 2 groups indicates that the effect of combination treatment differs significantly from that of individual treatment. (C) Flow cytometry was used to demonstrate the effect of berberine-induced apoptosis in BT-474LapR cells. (D) The column shows the flow cytometry results, **p < 0.01.
Berberine re-sensitizes resistant cells to lapatinib by increasing the upregulation of the ROS level
To investigate the role of ROS in lapatinib resistance, the Reactive Oxygen Species Assay Kit was used to detect the level of ROS in cells (BT-474 WT, BT-474LapR, AU-565WT, and AU-565LapR). As predicted, the ROS-positive rate in lapatinib-resistant cells was significantly lower than that in wild-type cells (). Therefore, we considered that lapatinib-resistant cells have much stronger drug tolerance to lapatinib than wild-type cells. Consequently, whether the anti-resistance effect of a combination of lapatinib and berberine can be attributed to ROS was examined. The level of ROS was examined in lapatinib-resistant cells treated with lapatinib, berberine and a combination of lapatinib and berberine. As shown in , compared with lapatinib or berberine treatment alone, a combination of lapatinib and berberine increased the level of ROS in lapatinib cells. These resulted indicate that ROS could play an important role in the mechanism of lapatinib resistance and reverse the effect of a combination of lapatinib and berberine. To provide additional evidence for these results, N-acetyl-L-cysteine, a type of antioxidant that can effectively reduce the ROS level, was added to lapatinib-resistant cells treated with a combination of lapatinib and berberine. The ROS level assay showed that NAC could significantly decrease ROS production in lapatinib-resistant cells treated with a combination of lapatinib and berberine. Additionally, after NAC treatment, the apoptosis rate of cells was reduced (), which is similar to the result provided by the EdU assay (). Consistent with these results, we can conclude that berberine can reverse lapatinib resistance and induce apoptosis in lapatinib-resistant cells by increasing ROS production.
Figure 3. The level of ROS production was observed in both lapatinib-resistant cells (BT-474LapR and AU-565LapR) and wild-type cells (BT-474WT and AU-565WT). We used the Reactive Oxygen Species Assay Kit, and the relative levels of fluorescence were quantified by flow cytometry (530 nm). The cells were treated with lapatinib (2 μM) alone, berberine (2 μM) alone, a combination of lapatinib and berberine (L+B), and a combination of lapatinib, berberine and NAC (2 mM) (L+B+NAC). The untreated wild-type cells were used as the control group (A) The relative level of ROS production was detected by flow cytometry in BT-474 cells. (B) The BT-474LapR cells showed lower levels of ROS production. Treatment with berberine and lapatinib alone and in combination increased ROS production, but the combination of berberine and lapatinib showed stronger effects. Conversely, NAC could clear the ROS production induced by berberine and lapatinib. A line spanning 2 groups indicates that the effect of combination treatment was significantly different than individual treatment. **p < 0.01, ***p < 0.001. (C) The relative level of ROS production was detected by flow cytometry in AU-565 cells. d The result of relative ROS production in AU-565 cells was similar to that of BT-474 cells. *p < 0.05, **p < 0.01, ***p < 0.001.
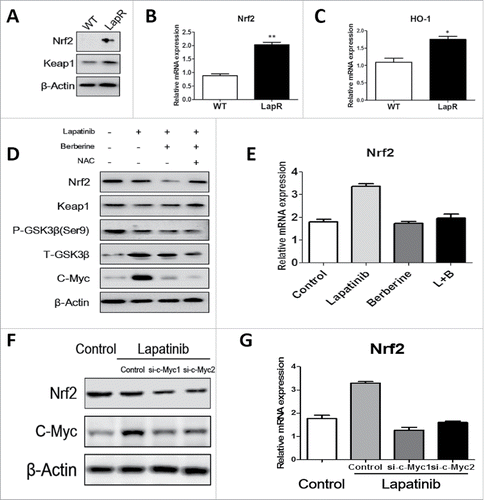
Berberine reverses lapatinib resistance by inhibiting the Nrf2 signaling pathway
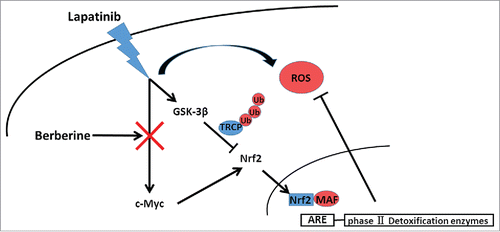
Although a few previous studies reported that berberine-induced apoptosis by ROS depended on activation of the pro-apoptotic JNK signaling Citation23, it is unclear how berberine increases ROS production. Therefore, we focused on the relationship between berberine and Nrf2. As shown in , western blot indicated that Nrf2 protein in lapatinib-resistant cells is higher than in wild-type cells; in addition, Q-PCR demonstrated that mRNA expression of Nrf2 in lapatinib-resistant cells is approximately 0.88 ± 0 .07-fold, and the mRNA expression of Nrf2 in wild-type cells is approximately 2.03 ± 0 .09-fold (). Furthermore, the mRNA expression of HO-1, a type of phase II detoxification enzyme that is regulated by Nrf2, also indicated significant differences between lapatinib-resistant cells and wild-type cells (). These results show that lapatinib cells have a greater ability to clear ROS production and thus counteract lapatinib. When lapatinib was added, the protein expression of Nrf2 decreased. Moreover, when a combination of lapatinib and berberine was used in lapatinib-resistant cells, the protein expression of Nrf2 was greatly reduced (). Therefore, we concluded that berberine induces downregulation of Nrf2 to decrease lapatinib resistance in lapatinib-resistant cells. To explain this phenomenon, we tested Keap1 protein, which is the classic E3-like ligase that promotes Nrf2 ubiquitination. However, the level of Keap1 protein was not what we anticipated; the GSK3-β pathway, which is a new pathway for Nrf2 ubiquitination, was observed. Lapatinib can greatly enhance GSK3-β signaling and inhibit its phosphorylation, though there was no significant difference between the presence and absence of berberine. Additionally, c-Myc, a transcriptional activation factor of Nrf2, was seen (). To further prove the important effect of c-Myc, we used siRNA to knockdown c-Myc when cells were treated lapatinib. And qPCR and Western blot confirmed that Nrf2 was downregulated when c-Myc siRNA added ().The results showed that c-Myc played an important role in lapatinib resistance. Maybe lapatinib promotes c-Myc to transcriptionally activate Nrf2, but berberine blocked this process, and the mRNA level of Nrf2 assessed by Q-PCR verified these results (). In summary, lapatinib accelerated ubiquitination of Nrf2 by a Keap1-independent pathway, and cells upregulated c-Myc to transcriptionally activate Nrf2 for feedback regulation. However, berberine blocked the feedback progress and further reduced Nrf2.
Figure 5. Berberine reversed lapatinib resistance via the c-Myc/Nrf2 pathway. Western blot and Q-PCR were used to analyze Nrf2 pathway activity. (A) Both wild-type and lapatinib-resistant BT-474 cells were collected, and antibodies against Nrf2, Keap1 and β-actin were detected. (B) Q-PCR was used to detect the level of Nrf2 mRNA in both BT-474WT cells and BT-474LapR cells. **p < 0.01 (C) Q-PCR was used to detect the level of HO-1 mRNA in both BT-474WT cells and BT-474LapR cells. *p < 0.05 (D) We treated the BT-474LapR cells with different drugs and collected cells for protein gel blotting with antibodies against Nrf2,Keap1, p- GSK-3β(ser9), GSK-3β, c-Myc and β-actin. (E) Q-PCR was used to observe Nrf2 mRNA in the absence or presence of berberine synthesized with lapatinib in BT-474LapR cells. (F) Q-PCR was used to detect level of Nrf2 when siRNA-c-Myc was used. (G) Western blotting was used to detect level of Nrf2 when siRNA-c-Myc was used.
Discussion
Lapatinib has been used clinically to treat HER2-positive breast cancer for many years, and its remarkable therapeutic advantage has been confirmed by many clinical trials.Citation36 However, as a molecular targeting drug, primary and acquired resistance dramatically limits the efficiency of lapatinib. Until now, this problem has confounded researchers and clinicians. Therefore, understanding the mechanism of lapatinib resistance and developing a strategy to increase its therapeutic efficacy is important for clinical medicine. In our report, a lapatinib-resistant human breast cancer model was established in vitro by constantly exposing BT-474WT and AU-565WT cells to lapatinib for 12 months. Although several mechanisms of lapatinib resistance have been reported, we demonstrate a low level of ROS production in lapatinib-resistant cells. Additionally, we applied a new strategy of combining lapatinib and berberine to reverse the lapatinib resistance. We believe that these results provide evidence for rational strategies that can overcome clinical lapatinib resistance.
In recent years, researchers have demonstrated several molecular mechanisms of lapatinib resistance, including the activation of AXL, Src, CXCR$, RON, and PI3K.Citation37-39 Our previous study reported that protective autophagy induced resistance after long-term treatment with lapatinib.Citation40 In this study, we confirmed that the level of ROS in lapatinib-resistant cells is significantly lower than in wild-type cells, and because of its stronger ability to inhibit ROS production, BT-474LapR and AU-565LapR cells maintained their internal environment to survive lapatinib therapy. To date, there is a consensus that berberine can suppress tumor cell proliferation and induce tumor cell apoptosis in breast cancer,Citation23,41 but there is no report showing that berberine can counteract lapatinib resistance. In our study, a dramatic difference in survival was observed between cells treated with lapatinib only and those treated with a combination of lapatinib and berberine. The question is whether the apoptosis induced by a combination of lapatinib and berberine is dependent on their synergy or on their individual actions. Here, we analyzed data from the MTT assay, colony formation assay and flow cytometry and found that the amount of apoptosis induced by a combination of lapatinib and berberine is higher than that induced by lapatinib or berberine alone. Thus, we conclude that berberine sensitized lapatinib-resistant cells to lapatinib. Interestingly, a similar tendency of ROS production was detected in lapatinib-resistant cells treated with a combination of lapatinib and berberine. Lapatinib increased the ROS level when used with berberine. To clarify the effect of ROS in apoptosis induced by a combination of lapatinib and berberine, NAC was used to oppose ROS in lapatinib-resistant cells treated with a combination of lapatinib and berberine. Consistent with our prediction, apoptosis of BT-474LapR cells was reduced when the ROS production was removed. Coincidently, Juan Xie reported that berberine-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species generation and the mitochondrial-related apoptotic pathway.Citation23 Meanwhile, S Mohanty reported that in comparison with sensitive tumors, resistant cancer cells maintained low levels of ROS. At a low ROS condition during genotoxic insult, the ATM/sumoylated-IKKγ interaction induced NFκB activation that resisted JNK-mediated apoptosis, whereas increasing cellular ROS restored ATM/JNK apoptotic signaling.Citation42 We also demonstrated a lower level of ROS in lapatinib resistant cells. And lapatinib-berberine combination induced high level of ROS may be one reason of lapatinib resistant cells apoptosis. Although it should not be assumed that ROS is the only cause of cellular apoptosis, an inseparable relationship between ROS and apoptosis induced by a combination of lapatinib and berberine was observed.
ROS is known as to trigger and modulate apoptosis,Citation43 and they have been shown to play an important role in the therapeutic principle of many chemotherapeutics such cisplatin and bleomycin.Citation44 In cancer, many signaling pathways regulate berberine-induced apoptosis, including JNK, mitochondria and DNA damage pathways.Citation45 However, the mechanism of how berberine regulates ROS is unclear. In our current study, we observed that BT-474LapR cells activate Nrf2, a key molecule that activates the phase II detoxification enzymes and clears ROS production. HO-1, a type of phase II detoxification enzyme, was also increased in BT-474LapR cells. Therefore, the BT-474LapR cells could preserve a low ROS level even when treated with lapatinib, though this balance was upset by berberine. Due to the effect of berberine, the ROS level increased greatly with decreasing Nrf2, and lapatinib resistance was reversed.
In our study, we also attempted to elucidate the mechanism by which berberine blocks the Nrf2 pathway. It is known that Nrf2 protein is suppressed by an association with Keap1 under homeostatic conditions, but it is activated when cells are exposed to oxidative or electrophilic stress.Citation46 Indeed, the level of Keap1 was not what we predicted, and thus we assumed that inhibition of Nrf2 is independent of Keap1. In fact, another pathway independent of Keap1 that promotes Nrf2 ubiquitination has been reported. Activation of GSK-3β signaling promoted phosphorylation of Nrf2, and Nrf2 is degraded by the E3 ligase TRCP.Citation47 Indeed, we observed that lapatinib activated GSK-3β signaling and slightly decreased Nrf2. Conversely, GSK-3β signaling did not differ significantly between cells treated with lapatinib alone and those treated with a combination of lapatinib and berberine. Thus, we demonstrated that the upstream oncogene c-Myc can activate Nrf2.Citation48 To our surprise, c-Myc was paradoxically activated by lapatinib and inhibited by berberine. In other words, c-Myc may be a feedback mechanism with which to antagonize ROS in lapatinib-resistant cells treated with lapatinib to maintain the anti-ROS and pro-ROS balance. A recent article reported that lapatinib-induced c-Myc expression is critical for reducing sensitivity of breast cancer cells to lapatinib. Additionally, inducible c-Myc knockdown acted in synergy with lapatinib to suppress the growth of cancer cells and increase their sensitivity to lapatinib.Citation49 Our finding also confirmed that the downregulated c-Myc induced by lapatinib-berberine combination increased lapatinib sensitivity of resistant cells. Especially, Zhang et al demonstrated that both cyclin D1 and c-Myc were down regulated when cells were treated with 0, 25, 50 and 100 μM beberine for 4 hrs.Citation50 In our study, we also confirmed c-Myc inhibition and ROS induction by berberine. Therefore, c-Myc inhibition might be the key reason of berberine to reverse lapatinib resistance.
In a summary, BT-474LapR cells had higher Nrf2 levels and thus were able to halt ROS production for lapatinib resistance, and both the pro-Nrf2 pathway of c-Myc and the anti-Nrf2 pathway of GSK-3β are activated to stabilize Nrf2 and maintain a low level of ROS. The combination of lapatinib and berberine resulted in inhibition of c-Myc, and thus berberine increased ROS production and reversed lapatinib resistance in the lapatinib-resistant cancer cells (). In fact, the mechanism of GSK-3β regulation by lapatinib and berberine remains unknown, and thus our future work will explore specific mechanism by which lapatinib and berberine regulate c-Myc and GSK-3β. Moreover, we plan to test and verify the effects of lapatinib and berberine in an in vivo model and in the clinic.
Materials and methods
Cell culture
The human breast cancer cell lines BT-474WT and AU-565WT were purchased from ATCC. BT-474LabR cells and AU-565LapR cells were developed by continually exposing parental cells (WT) to 2 μM of lapatinib for 12 months. BT-474 cells (WT and LapR) and AU-565 (WT and LapR) cells were cultured in Dulbecco's modified Eagle's medium and RPMI 1640 supplemented with 10% fetal bovine serum and incubated in a humidified atmosphere of 5% CO2 at 37°C.
MTT assay
Cells (BT-474WT, BT-474LabR, AU-565WT, and AU-565LapR) were harvested from exponentially growing cultures, counted, and plated in 96-well plates at 1×104 cells per well with the presence or absence of lapatinib or berberine. Thereafter, 10 μl of 5 mg/ml MTT in PBS was added to each well, and the cells were incubated for 4 h. The culture medium was then removed, and 150 μl of DMSO was added. Optical density was measured at 490 nm.
RNA preparation and qRT-PCR
Total cellular RNA was extracted from cultured cells with RNAiso for TRIzol reagent (TaKaRA, Dalian, China). The cDNA was the reverse transcription from 1 μg of total RNA using a reverse transcription kit (TaKaRA, Dalian, China). Real-time Q-PCR analyses were conducted using SYBR Prime Script™ mRNA RT-PCR Kit (TaKaRA, Dalian, China). All protocols were performed according to the manufacturers' instructions. The mRNA results were normalized to the expression of β-actin, and the primer sequences were as follows: Nrf2 forward primer, TTCCCGGTCACATCGAGAG; Nrf2 reverse primer, TCCTGTTGCATACCGTCTAAATC; HO-1 forward primer, AAGACTGCGTTCCTGCTCAAC; HO-1 reverse primer, AAAGCCCTACAGCAACTGTCG.
siRNA and transfection
siRNA-c-Myc kit was purchased from Shanghai Genepharma Co., Ltd. The sequences were as follows:
siRNA-c-Myc-1094: 5'-GCUUGUACCUGCAGGAUCUTTAGAUCCUGCAGGUACAAGCTT-3′
siRNA-c-Myc-1475: 5′-UCUCCACACAUCAGCACAATTUUGUGCUGAUGUGUGGAGATT-3′
BT474-R cells were plated in 60 mm dishes, and transfected using TurboFect Transfection Reagent (Thermo Fisher Scientific, Wilmington, DE) according to the manufacturer's instructions.
Western blotting
Cells were lysed with RIPA buffer containing 1% cocktail protease inhibitors for 10 min at 4°C, and lysates were then centrifuged at 12000×g for 15 min at 4°C. The supernatants were collected to determine protein concentrations using a BCA Protein Assay Kit. Cell lysates were separated using SDS-PAGE and transferred to nitrocellulose membranes. After blocking with 5% non-fat dry milk in Tris-buffered saline with Tween (TBST) for 1 h at room temperature, the membranes were incubated with primary antibodies against Nrf2, GSK-3β, p- GSK-3β(ser9), c-Myc, β-actin (Cell Signaling Technology, Danvers, MA, USA), and Keap1 (Santa Cruz Biotechnology, USA) .
EdU assay
EdU was obtained from Invitrogen. To optimize UV-induced UDS by EdU incorporation, effects of the UV dose and the EdU-incubation period were examined. Cells were cultured on coverslips and maintained at confluent density. Cells were washed with PBS, which was followed by irradiation with different doses (5–20 J/m2) of UVC (254 nm). After UV irradiation, cells were immediately incubated with serum-free DMEM supplemented with 10 μM EdU for different periods (0.5, 1, 2 and 4 h). Serum-free medium was used because serum often contains thymidine, which competes with EdU for incorporation into DNA. Cells were then washed with PBS, followed by fixation and permeabilization with PBS containing 2% formaldehyde, 0.5% triton X-100 and 300 mM sucrose for 20 min. After extensive washing with PBS, cells were blocked with 10% FBS in PBS for 30 min. Inc. EdU was detected by a fluorescent-azide coupling reaction. Briefly, cells were incubated for 30 min with azide-conjugated Alexa Fluor 488 dye in TBS supplemented with 4 mM CuSO4. Cells were then washed 3 times with PBS. Coverslips were soaked in PBS, fixed with 3.7% formaldehyde in PBS for 20 min, and mounted on glass slides with Aquapolymount (Polysciences). Photographs of the cells were captured with a fluorescent microscope, and captured images were processed and analyzed with ImageJ software (NIH). At least 50 non-S-phase cells were randomly selected from a single captured field, and the average nuclear fluorescent intensity was calculated. Data points presented in the text are the averages calculated from 5 different fields.
Measurement of intracellular ROS
The level of intracellular reactive oxygen species was quantified using the Reactive Oxygen Species Assay Kit. DCFH-DA is oxidized by reactive oxygen species in viable cells to 2′,7′-dichlorofluorescein (DCF), which is highly fluorescent at 530 nm. The cells were washed 3 times with PBS. DCFH-DA, diluted to a final concentration of 10 μM, was added and incubated for 30 min at 37°C in the dark. After being washed 3 times with PBS, the relative levels of fluorescence were quantified using a flow cytometer (530 nm).
Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM) from at least 3 separate experiments. Two treatment groups were compared using Student's t test. For statistical analysis, Graph Pad Prism version 5 was used. The results were considered statistically significant when P < 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by the National Natural Science Foundation of China (81202091, 81372390 and 81402186).
References
- Jeffrey SS, Lonning PE, Hillner BE. Genomics-based prognosis and therapeutic prediction in breast cancer. J Natl Compr Canc Net 2005; 3:291-300; PMID:16002001
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989; 244:707-12; PMID:2470152; http://dx.doi.org/10.1126/science.2470152
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001; 2:127-37; PMID:11252954; http://dx.doi.org/10.1038/35052073
- Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol 2012; 9:16-32; PMID:22124364; http://dx.doi.org/10.1038/nrclinonc.2011.177
- Higa GM, Abraham J. Lapatinib in the treatment of breast cancer. Exp Rev Anticancer Ther 2007; 7:1183-92; PMID:17892419; http://dx.doi.org/10.1586/14737140.7.9.1183
- Wood ER, Truesdale AT, McDonald OB, Yuan D, Hassell A, Dickerson SH, Ellis B, Pennisi C, Horne E, Lackey K, et al. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res 2004; 64:6652-9; PMID:15374980; http://dx.doi.org/10.1158/0008-5472.CAN-04-1168
- Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR, Liu DG, Ashby CR Jr, Huang Y, Robey RW, Liang YJ, et al. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res 2008; 68:7905-14; PMID:18829547; http://dx.doi.org/10.1158/0008-5472.CAN-08-0499
- Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, Untch M, Rusnak DW, Spehar G, Mullin RJ, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res 2006; 66:1630-9; PMID:16452222; http://dx.doi.org/10.1158/0008-5472.CAN-05-1182
- Xia W, Mullin RJ, Keith BR, Liu LH, Ma H, Rusnak DW, Owens G, Alligood KJ, Spector NL. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 2002; 21:6255-63; PMID:12214266; http://dx.doi.org/10.1038/sj.onc.1205794
- Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, Keith BR, Murray DM, Knight WB, Mullin RJ, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther 2001; 1:85-94; PMID:12467226
- Campone M, Juin P, Andre F, Bachelot T. Resistance to HER2 inhibitors: is addition better than substitution? Rationale for the hypothetical concept of drug sedimentation. Crit Rev Oncol/Hematol 2011; 78:195-205; PMID:20684884; http://dx.doi.org/10.1016/j.critrevonc.2010.04.012
- Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res 2008; 22:999-1012; PMID:18618524; http://dx.doi.org/10.1002/ptr.2399
- Singhal KC. Anthelmintic activity of berberine hydrochloride against Syphacia obvelata in mice. Indian J Exp Biol 1976; 14:345-7; PMID:1033150
- Tang J, Feng Y, Tsao S, Wang N, Curtain R, Wang Y. Berberine and Coptidis rhizoma as novel antineoplastic agents: a review of traditional use and biomedical investigations. J Ethnopharmacol 2009; 126:5-17; PMID:19686830; http://dx.doi.org/10.1016/j.jep.2009.08.009
- Kim S, Han J, Lee SK, Choi MY, Kim J, Lee J, Jung SP, Kim JS, Kim JH, Choe JH, et al. Berberine suppresses the TPA-induced MMP-1 and MMP-9 expressions through the inhibition of PKC-alpha in breast cancer cells. J Surg Res 2012; 176:e21-9; PMID:22381172; http://dx.doi.org/10.1016/j.jss.2011.11.1041
- Kim JB, Ko E, Han W, Shin I, Park SY, Noh DY. Berberine diminishes the side population and ABCG2 transporter expression in MCF-7 breast cancer cells. Planta medica 2008; 74:1693-700; PMID:18951337; http://dx.doi.org/10.1055/s-0028-1088313
- Kim DW, Ahan SH, Kim TY. Enhancement of Arsenic Trioxide (As(2)O(3))- Mediated Apoptosis Using Berberine in Human Neuroblastoma SH-SY5Y Cells. J Korean Neurosurg Soc 2007; 42:392-9; PMID:19096576; http://dx.doi.org/10.3340/jkns.2007.42.5.392
- Liu J, He C, Zhou K, Wang J, Kang JX. Coptis extracts enhance the anticancer effect of estrogen receptor antagonists on human breast cancer cells. Biochem Biophys Res Commun 2009; 378:174-8; PMID:19000652; http://dx.doi.org/10.1016/j.bbrc.2008.10.169
- Formentini L, Sanchez-Arago M, Sanchez-Cenizo L, Cuezva JM. The mitochondrial ATPase inhibitory factor 1 triggers a ROS-mediated retrograde prosurvival and proliferative response. Mol Cell 2012; 45:731-42; PMID:22342343; http://dx.doi.org/10.1016/j.molcel.2012.01.008
- Gnocchi D, Leoni S, Incerpi S, Bruscalupi G. 3,5,3′-triiodothyronine (T3) stimulates cell proliferation through the activation of the PI3K/Akt pathway and reactive oxygen species (ROS) production in chick embryo hepatocytes. Steroids 2012; 77:589-95; PMID:22366194; http://dx.doi.org/10.1016/j.steroids.2012.01.022
- Lenaz G. Mitochondria and reactive oxygen species. Which role in physiology and pathology? Adv Exp Med Biol 2012; 942:93-136; PMID:22399420; http://dx.doi.org/10.1007/978-94-007-2869-1_5
- Kuo PL, Chen CY, Hsu YL. Isoobtusilactone A induces cell cycle arrest and apoptosis through reactive oxygen species/apoptosis signal-regulating kinase 1 signaling pathway in human breast cancer cells. Cancer Res 2007; 67:7406-20; PMID:17671211; http://dx.doi.org/10.1158/0008-5472.CAN-07-1089
- Xie J, Xu Y, Huang X, Chen Y, Fu J, Xi M, Wang L. Berberine-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species generation and mitochondrial-related apoptotic pathway. Tumour Biol 2015; 36:1279-88; PMID:25352028; http://dx.doi.org/10.1007/s13277-014-2754-7
- Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev 2006; 38:769-89; PMID:17145701; http://dx.doi.org/10.1080/03602530600971974
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A 1996; 93:14960-5; PMID:8962164; http://dx.doi.org/10.1073/pnas.93.25.14960
- Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O'Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci 2001; 59:169-77; PMID:11134556; http://dx.doi.org/10.1093/toxsci/59.1.169
- Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci U S A 2001; 98:4611-6; PMID:11287661; http://dx.doi.org/10.1073/pnas.081082098
- Li YJ, Takizawa H, Azuma A, Kohyama T, Yamauchi Y, Takahashi S, Yamamoto M, Kawada T, Kudoh S, Sugawara I. Disruption of Nrf2 enhances susceptibility to airway inflammatory responses induced by low-dose diesel exhaust particles in mice. Clin Immunol 2008; 128:366-73; PMID:18614404; http://dx.doi.org/10.1016/j.clim.2008.05.005
- Iizuka T, Ishii Y, Itoh K, Kiwamoto T, Kimura T, Matsuno Y, Morishima Y, Hegab AE, Homma S, Nomura A, et al. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Genes Cells 2005; 10:1113-25; PMID:16324149; http://dx.doi.org/10.1111/j.1365-2443.2005.00905.x
- Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl Pharmacol 2001; 173:154-60; PMID:11437637; http://dx.doi.org/10.1006/taap.2001.9176
- Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci U S A 2001; 98:3410-5; PMID:11248092; http://dx.doi.org/10.1073/pnas.051618798
- Iida K, Itoh K, Kumagai Y, Oyasu R, Hattori K, Kawai K, Shimazui T, Akaza H, Yamamoto M. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res 2004; 64:6424-31; PMID:15374950; http://dx.doi.org/10.1158/0008-5472.CAN-04-1906
- Mao L, Wang H, Qiao L, Wang X. Disruption of Nrf2 enhances the upregulation of nuclear factor-kappaB activity, tumor necrosis factor-alpha, and matrix metalloproteinase-9 after spinal cord injury in mice. Mediators of inflammation 2010; 2010:238321; PMID:20862369; http://dx.doi.org/10.1155/2010/238321
- Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 2008; 29:1235-43; PMID:18413364; http://dx.doi.org/10.1093/carcin/bgn095
- Li Z, Geng YN, Jiang JD, Kong WJ. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid-Based Complement Alternat Med 2014; 2014:289264; PMID:24669227; http://dx.doi.org/10.1155/2014/289264
- Robidoux A, Tang G, Rastogi P, Geyer CE, Jr., Azar CA, Atkins JN, Fehrenbacher L, Bear HD, Baez-Diaz L, Sarwar S, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. Lancet Oncol 2013; 14:1183-92; PMID:24095300; http://dx.doi.org/10.1016/S1470-2045(13)70411-X
- Liu L, Greger J, Shi H, Liu Y, Greshock J, Annan R, Halsey W, Sathe GM, Martin AM, Gilmer TM. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res 2009; 69:6871-8; PMID:19671800; http://dx.doi.org/10.1158/0008-5472.CAN-08-4490
- Wang Q, Quan H, Zhao J, Xie C, Wang L, Lou L. RON confers lapatinib resistance in HER2-positive breast cancer cells. Cancer Lett 2013; 340:43-50; PMID:23811285; http://dx.doi.org/10.1016/j.canlet.2013.06.022
- Brady SW, Zhang J, Seok D, Wang H, Yu D. Enhanced PI3K p110alpha signaling confers acquired lapatinib resistance that can be effectively reversed by a p110alpha-selective PI3K inhibitor. Mol Cancer Ther 2014; 13:60-70; PMID:24249715; http://dx.doi.org/10.1158/1535-7163.MCT-13-0518
- Chen S, Zhu X, Qiao H, Ye M, Lai X, Yu S, Ding L, Wen A, Zhang J. Protective autophagy promotes the resistance of HER2-positive breast cancer cells to lapatinib. Tumour Biol 2016 Feb; 37(2):2321-31; PMID:26369543; http://dx.doi.org/10.1007/s13277-015-3800-9
- Tan W, Zhong Z, Wang S, Suo Z, Yang X, Hu X, Wang Y. Berberine Regulated Lipid Metabolism in the Presence of C75, Compound C, and TOFA in Breast Cancer Cell Line MCF-7. Evid-Based Complement Alternative Med 2015; 2015:396035; PMID:26351511; http://dx.doi.org/10.1155/2015/396035
- Mohanty S, Saha S, Md SHD, Adhikary A, Mukherjee S, Manna A, Chakraborty S, Mazumdar M, Ray P, Das K, et al. ROS-PIASgamma cross talk channelizes ATM signaling from resistance to apoptosis during chemosensitization of resistant tumors. Cell Death Dis 2014; 5:e1021; PMID:24457965; http://dx.doi.org/10.1038/cddis.2013.534
- Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med 2000; 29:323-33; PMID:11035261; http://dx.doi.org/10.1016/S0891-5849(00)00302-6
- Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat 2004; 7:97-110; PMID:15158766; http://dx.doi.org/10.1016/j.drup.2004.01.004
- Zhang M, Wang A, Xia T, He P. Effects of fluoride on DNA damage, S-phase cell-cycle arrest and the expression of NF-kappaB in primary cultured rat hippocampal neurons. Toxicol Lett 2008; 179:1-5; PMID:18485627; http://dx.doi.org/10.1016/j.toxlet.2008.03.002
- Tkachev VO, Menshchikova EB, Zenkov NK. Mechanism of the Nrf2/Keap1/ARE signaling system. Biochem Biokhimiia 2011; 76:407-22; PMID:21585316; http://dx.doi.org/10.1134/S0006297911040031
- Rada P, Rojo AI, Evrard-Todeschi N, Innamorato NG, Cotte A, Jaworski T, Tobón-Velasco JC, Devijver H, García-Mayoral MF, Van Leuven F, et al. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/beta-TrCP axis. Mol Cell Biol 2012; 32:3486-99; PMID:22751928; http://dx.doi.org/10.1128/MCB.00180-12
- DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011; 475:106-9; PMID:21734707; http://dx.doi.org/10.1038/nature10189
- Matkar S, Sharma P, Gao S, Gurung B, Katona BW, Liao J, Muhammad AB, Kong XC, Wang L, Jin G, et al. An Epigenetic Pathway Regulates Sensitivity of Breast Cancer Cells to HER2 Inhibition via FOXO/c-Myc Axis. Cancer Cell 2015; 28:472-85; PMID:26461093; http://dx.doi.org/10.1016/j.ccell.2015.09.005
- Zhang J, Cao H, Zhang B, Cao H, Xu X, Ruan H, Yi T, Tan L, Qu R, Song G, et al. Berberine potently attenuates intestinal polyps growth in ApcMin mice and familial adenomatous polyposis patients through inhibition of Wnt signalling. J Cell Mol Med 2013; 17:1484-93; PMID:24015932; http://dx.doi.org/10.1111/jcmm.12119