ABSTRACT
Thymic carcinomas are rare tumors that arise in the anterior mediastinum. Most of these malignancies develop local metastases limited in the thorax. Splenic metastases from thymic carcinomas are extremely rare. Here we report a case of isolated splenic metastasis from a 38-year-old female patient with Stage IV thymic carcinoma, who was treated with chemoradiotherapy. At twenty-2 months follow-up, the patient was found to have an isolated spleen metastasis, which was treated by Cyberknife with a reduced size of the metastasis, representing a partial response. Although splenic metastasis is a rare phenomenon, physicians need to be aware of the possibility of such metastases.
Abbreviations
| CT | = | computed tomography |
| FDG-PET | = | fluorodeoxyglucose positron emission tomography |
| SUVmax | = | maximum standardized uptake value |
| SBRT | = | stereotactic body radiation therapy |
| OS | = | overall survival |
Introduction
Thymic carcinomas originate from thymic epithelial cells, which are significantly different from thymoma in biological characteristics and prognosis. It is mainly metastasized by regional intrusion and hematogenous spread. Extrathoracic metastases are seen in less than 7 percent of patients at presentation, most commonly to the kidney, extrathoracic lymph nodes, liver, brain, adrenals, thyroid, and bone.Citation1 Rare but not exceptional is metastasis occurring on spleen.
Case presentation
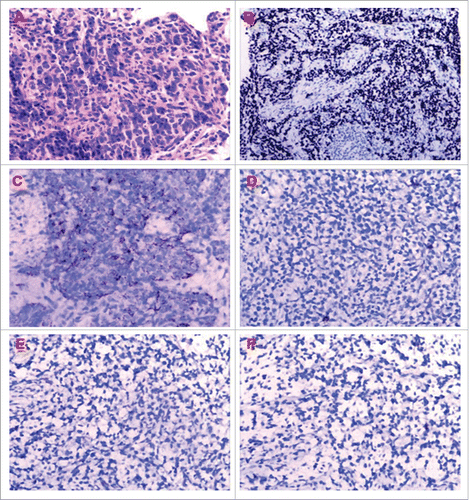
A 38-year-old female patient presented with the symptoms of chest tightness and dyspnea. The chest CT (CT) scan revealed a voluminous mass in the left anterior mediastinum that measured 8.9 cm × 8.0 cm, with pericardial and pleural effusion. The fluorodeoxyglucose positron emission tomography (FDG-PET)/CT confirmed involvement in left anterior mediastinum, hilar lymph nodes, pericardium, pulmonary artery and aorta. Distant metastasis was not detected at that time. Pericardiocentesis and drainage cytology analysis showed a small amount of atypical cells. The biopsy of thymic mass revealed a poorly differentiated squamous carcinoma with expression of cytokeratins 5/6, P63 and absence of neuroendocrine and adrenal markers such as CD56, synaptophysin and NapsinA (). The patient was diagnosed as thymic squamous carcinoma in Stage IVb according to Masaoka staging system and received 7 cycles of chemotherapy with gemcitabine, cisplatin and angiogenesis inhibitor endostar. Follow-up imaging showed that the size of primary thymic carcinoma was reduced by 26% after 6 cycles of treatment. The patients received concurrent chemoradiotherapy with taxane following the completion of the chemotherapy. The radiation therapy was delivered by 6 MV external beams with a dose of 66 Gy in 33 fractions for the primary tumor. She achieved complete response after radiotherapy and was followed with routine physical exam, abdominal ultrasound and chest CT scan every 3 months afterwards.
Figure 1. (A) Hematoxylin-Eosin staining of the thymic mass showed tumor cells grew diffusely. Immunohistochemical analysis demonstrated the tumor cells were strongly positive for (B) P63, (C) cytokeratins 5/6, immunostaining revealed negative for (D) CD56, (E) synaptophysin and (F) NapsinA respectively.
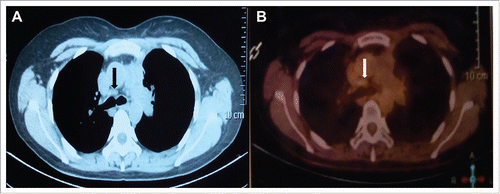
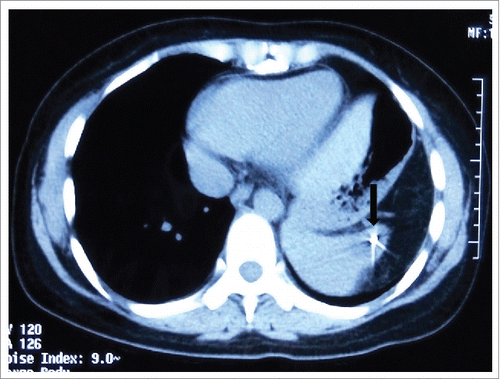
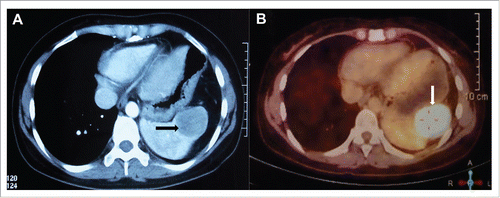
Abdominal CT scan revealed a moderate contrast enhanced lesion that measured 4.5 cm × 3.5 cm in her spleen at 22-month follow up visit. This finding was confirmed by PET/CT, with the maximum standardized uptake value (SUVmax) of 30.67 (). Whereas the thymic mass was stable with an SUVmax of 1.8 (). No metastatic lesions were detected in the liver, adrenal gland and abdominal lymph nodes. Biopsy of spleen metastasis was not performed due to patient's refusal. Patient also refused surgery resection for this splenic mass. She was then treated with stereotactic body radiation therapy (SBRT) on Cyberknife (30Gy in 5 fractions) as alternative treatment. Abdominal CT () showed that the volume of spleen metastasis reduced to 2.6 cm × 3.5 cm at one month follow up, which indicates a partial response.
Figure 2. Abdominal computed tomography (CT) scan revealed a moderate contrast enhanced lesion that measured 4.5 cm × 3.5 cm in the spleen (A), which was confirmed by fluorodeoxyglucose positron emission tomography (PET/CT), with the SUVmax of 30.67 (B).
Discussion
Thymic carcinomas are rare and aggressive thymic epithelial tumors, accounting for only 0.06% of all thymic neoplasms.Citation2 Thymic carcinomas are usually moderately differentiated squamous carcinomas, which account for 40.0%–77.5%.Citation3-5 Some authors agreed that squamous carcinomas have more favorable prognosis than other histologic subtypes, such as lymphoepithelioma, sarcomatoid carcinoma and undifferentiated carcinoma.Citation6 There are several systems to stage thymic carcinomas. Currently the WHO system is widely used and well correlates with Masaoka staging. About 65% thymic carcinomas, in WHO type C, had stage III or IV diseases with Masaoka staging.Citation7 The early stage thymic carcinomas occur locally and may require surgical resection with favorable prognosis. By contrast, stage III and IV thymic carcinomas appear to be more aggressive with distant metastases. The recommended therapy for invasive thymoma has been radical surgical resection. For the patients with inoperable or recurrent diseases, chemotherapy is the alternative treatment.Citation8 If the tumor is not resectable after neoadjuvant chemotherapy, additional chemotherapy and/or radiotherapy can be used. Standard chemotherapy for thymic carcinomas remains undefined. Yusuke Okumaf and his colleagues believed that cisplatin-based chemotherapy might be superior to carboplatin-based chemotherapy for advanced thymic carcinomas, with the response rates of 53.6% and 32.8% respectively.Citation9 Radiotherapy is necessary and important for this type of patients despite resection is complete or incomplete.Citation10 The median survival time is 48 months in patients with thymic carcinomasCitation11 and 5-year survival rates ranges from 28% to 60%.Citation12,13
Splenic metastases present with an incidence of only 0.3%–7.3% in autopsy reports.Citation14 The most common primary sites of splenic metastases originate from lung, breast, ovary, and colon. Rare metastatic sites reported are kidney, prostate, skin, endometrium.Citation15 Most patients with splenic involvement usually present with multi-visceral disseminated diseases. Isolated splenic metastasis is exceedingly rare. There are some hypotheses that might explain this phenomenon. Anatomically, it is lack of afferent lymphatics and the acute angle of the splenic artery from the celiac artery, which may prevent large clumps of tumor cells from getting access to the spleen. Rhythmic contractions of the spleen force the blood flow from the sinusoids to the splenic veins, which, in case of constant blood flow, could prevent tumor fixation.Citation16 In addition, the spleen, which includes amount of lymphocytes and macrophagocytes, serves the immune function to inhibit the induction and growth of tumor cells.Citation17
Splenic metastasis primary from thymic carcinoma is quite uncommon. According to literature, only 2 cases have been reported so far.Citation18,19 The patient discussed in the paper revealed a limited disease in advanced carcinoma. As the patient refused to perform surgical resection for this mass, we performed localized therapy with Cyberknife instead of chemotherapy for this isolated metastasis. Hellmanand and WeichselbaumCitation20 proposed a theory that oligometastatic state was that for many cancers, a few metastases exist at first, before the malignant cells acquire widespread metastatic potential. Localized therapies including surgery and SBRT are radical treatment options to achieve control of metastatic sites. One recent studyCitation21 of SBRT for oligometastases reported that local control rates was 84.4%, the overall survival (OS) was 84.4% at 1 y and 63.2% at 2 y. A partial response was observed in our case with local Cyberknife therapy and patient is still followed by our group closely.
In summary, although it is extremely rare, splenic metastasis should be taken into consideration in patients with thymic carcinomas. Radiotherapy is radical treatment option for isolated metastasis. Long-term follow-up is crucial to detect disease progression since recurrences are frequent.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Lewis JE, Wick MR, Scheithauer BW, Bernatz PE, Taylor WF. Thymoma. A clinicopathologic review. Cancer 1987; 60:2727-43; PMID:3677008; http://dx.doi.org/10.1002/1097-0142(19871201)60:11<2727::AID-CNCR2820601125>3.0.CO;2-D
- Greene MA, Malias MA. Aggressive multimodality treatment of invasive thymic carcinoma. J Thorac Cardiovasc Surg 2003; 125:434-6; PMID:12579125; http://dx.doi.org/10.1067/mtc.2003.133
- Detterbeck FC, Parsons AM. Thymic tumors. Ann Thorac Surg 2004; 77:1860-9; PMID:15111216; http://dx.doi.org/10.1016/j.athoracsur.2003.10.001
- Suster S, Rosai J. Thymic carcinoma: A clinicopathologic study 60 cases. Cancer 1991; 67:1025-32; PMID:1991250; http://dx.doi.org/10.1002/1097-0142(19910215)67:4<1025::AID-CNCR2820670427>3.0.CO;2-F
- Filosso PL, Guerrera F, Rendina AE, Bora G, Ruffini E, Novero D, Ruco L, Vitolo D, Anile M, Ibrahim M, et al. Outcome of surgically resected thymic carcinoma: a multicenter experience. Lung cancer 2014; 83:205-10; PMID:24370198; http://dx.doi.org/10.1016/j.lungcan.2013.11.015
- Daisuke N, David K, Juan R. Thymic mucoepidermoid carcinomas: a clinicopathologic study of 10 cases and review of the literature. Am J Surg Pathol 2004; 28:1526-31; PMID:15489658
- Strobel P, Bauer A, Puppe B, Kraushaar T, Krein A, Toyka K, Gold R, Semik M, Kiefer R, Nix W, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol 2004; 22:1501-9; PMID:15084623; http://dx.doi.org/10.1200/JCO.2004.10.113
- Kelly RJ, Petrini I, Rajan A, Wang Y, Giaccone G. Thymic malignancies: from clinical management to targeted therapies. J Clin Oncol 2011; 29:4820-7; PMID:22105817; http://dx.doi.org/10.1200/JCO.2011.36.0487
- Okuma Y, Saito M, Hosomi Y, Sakuyama T, Okamura T. Key components of chemotherapy for thymic malignancies: a systematic review and pooled analysis for anthracycline-, carboplatin- or cisplatin-based chemotherapy. J Cancer Res Clin Oncol 2015; 141:323-31; PMID:25146529; http://dx.doi.org/10.1007/s00432-014-1800-6
- Wang J, Zhang S. Advances on diagnosis and treatment of malignant thymic tumors. Chin J Lung cancer 2010; 13:985-91; PMID:20959073; http://dx.doi.org/10.3779/j.issn.1009-3419.2010.10.10
- Lanting G, Changlu W, Wentao F, Jie Z, Changxing L, Shen F. Outcome of multimodality treatment for 188 cases of type B3 thymoma. J Thorac Oncol 2013; 8:1329-34; PMID:24457243; http://dx.doi.org/10.1097/JTO.0b013e31829ceb50
- Yau-Lin T, Shan-Tair W, Ming-Ho W, Mu-Yen L, Wu-Wei L, Fen-Fen C. Thymic carcinoma: involvement of great vessels indicates poor prognosis. Ann Thorac Surg 2003; 76:1041-5; PMID:14529981; http://dx.doi.org/10.1016/S0003-4975(03)00831-2
- Kazuya K, Yasumasa M. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003; 76:878-84; PMID:12963221; http://dx.doi.org/10.1016/S0003-4975(03)00555-1
- Lam KY, Tang V. Metastatic tumors to the spleen: a 25-year clinicopathologic study. Arch Pathol Lab Med 2000; 124:526-30; PMID:10747308; http://dx.doi.org/10.1043/0003-9985(2000)124<0526:MTTTS>2.0.CO;2
- Gupta T, Nair N, Fuke P, Bedre G, Basu S, Shrivastava SK. Splenic metastases from cervical carcinoma: a case report. Int J Gynecol Cancer 2006; 16:911-4; PMID:16681784; http://dx.doi.org/10.1111/j.1525-1438.2006.00220.x
- NisaldaMartins, SandraLamelas, Javier. Isolated splenic metastasis of colon cancer: a case report and literature review. Jcoloproctol 2012; 32:88-93; http://dx.doi.org/10.1590/S2237-93632012000100014
- Capizzi PJ, Allen KB, Amerson JR, Skandalakis JE. Isolated splenic metastasis from rectal carcinoma. South Med J 1992; 85; PMID:1411716; http://dx.doi.org/ 10.1097/00007611-199210000-00017
- Guillan RA, Zelman S, Smalley RL, Iglesias PA. Malignant thymoma associated with myasthenia gravis, and evidence of extrathoracic metastases. An analysis of published cases and report of a case. Cancer 1971; 27:823-30; PMID:5574073; http://dx.doi.org/10.1002/1097-0142(197104)27:4<823::AID-CNCR2820270411>3.0.CO;2-X
- Kao H, Lin W, Peng Y, Chen C. Unusual isolated splenic metastasis secondary to thymic carcinoma. Chin J Radiol 2005; 30:189-192
- Rr HS, Weichselbaum. Oligometastases. J Clin Oncol 1995; 13:8-10; PMID:7799047
- Bhattacharya IS, Woolf DK, Hughes RJ, Shah N, Harrison M, Ostler PJ, Hoskin PJ. Stereotactic body radiotherapy (SBRT) in the management of extracranial oligometastatic (OM) disease. Br J Radiol 2015; 88:20140712; PMID:25679321; http://dx.doi.org/10.1259/bjr.20140712