ABSTRACT
Background: ALK, ROS1 and RET rearrangements represent 3 most frequent fusion genes in non-small cell lung cancer (NSCLC). Rearrangements of these 3 genes exist predominantly in lung adenocarcinoma while rarely in non-adenocarcinoma. Our objective was to explore the frequency, clinicopathological characteristics and survival of ALK, ROS1 and RET rearrangements in non-adenocarcinoma NSCLC patients.
Methods: ALK, ROS1 and RET rearrangements were screened by reverse transcriptase polymerase chain reaction (RT-PCR) in patients with completely resected non-adenocarcinoma NSCLC. All positive samples were confirmed with fluorescence in situ hybridization (FISH). Survival analysis was performed with Kaplan-Meier method and log-rank for comparison.
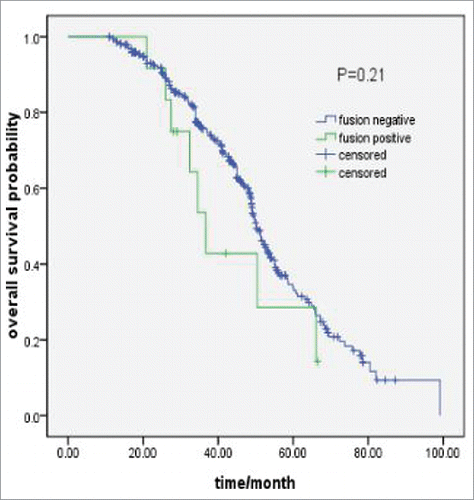
Results: A total of 385 patients underwent complete resection, including squamous cell carcinoma (n = 245), adenosquamous carcinoma (n = 85) and large cell carcinoma (n = 55). Twelve of them were identified as harboring fusion genes, including ALK (n = 7), ROS1 (n = 3) and RET (n = 2) rearrangements. The fusion frequencies of adenosquamous, squamous cell and large cell carcinomas were 8.2%, 1.6% and 1.8% respectively. Their median age was 49.5 y and 3 of them had a smoking history. No survival difference existed between fusion gene positive and negative patients (36.7 vs.50.2 months, P = 0.21).
Conclusion: The frequencies of ALK, ROS1 and RET rearrangements are low in non-adenocarcinoma NSCLC patients. And their clinical characteristics are similar to those in lung adenocarcinoma. Fusions of the above 3 genes are not prognostic factor for non-adnocarcinoma NSCLC patients.
Abbreviations
| NSCLC | = | Non-Small Cell Lung Cancer |
| PCR | = | Polymerase Chain Reaction |
Introduction
Lung cancer is the leading cause of cancer-related death in China.Citation1 Most patients have reached an advanced stage at diagnosis. For patients with advanced and metastatic disease, chemotherapy is a major treatment and the efficacy has already approached a progression-free survival (PFS) of under 6 months.Citation2 With the rapid advances of biotechnology, more and more oncogenes have emerged and the inhibitors of targeting these driver genes demonstrated encouraging efficacies.Citation3-9
Approximately 80% of Asian patients with lung adenocarcinoma harbored at least one genetic abnormality.Citation10-12 The most frequent genes included epidermal growth factor receptor (EGFR), Kirsten rat sarcoma viral oncogene homolog (KRAS) and echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK). Two major types of driver genes are mutation and rearrangements. Regardless of mutation or rearrangements, genetic abnormality was prevalent in lung adenocarcinoma.Citation13-14 The prevalence of gene abnormality, especially gene rearrangements, is currently unknown for squamous cell carcinoma (SCC), adenosquamous and large cell carcinoma. There were only limited studies of gene rearrangements in non-adenocarcinoma.Citation15-17 No definitive clinicopathological characteristics were reported in non-adenocarcinoma NSCLC patients. Moreover, non-adenocarcinoma patients were excluded from most clinical trials so that clinical efficacy of targeted treatment remained unclear.
Here the frequencies of ALK, ROS1 and RET rearrangements were examined in non-adenocarcinoma NSCLC patients to assess their prevalence, clinicopathological characteristics, treatment and prognosis.
Patients and methods
Study populations
Patients with non-adenocarcinoma NSCLC and complete resection between January 2008 and July 2014 at our hospital were screened for ALK, ROS1 and RET genes. Histological classification was based upon the World Health Organization criteria (2004 version). Lung cancer staging was performed according to the seventh TNM classification scheme. The present study was approved by our Institutional Review Board.
Detections of ALK, ROS1 and RET
The detection kits of ALK, ROS1 and RET (Amoy Diagnostics, Xiamen, China) are based on the reverse transcriptase polymerase chain reaction (RT-PCR) technology. All experiments were performed according to the instructions of user manuals. The procedural details were previously described.Citation18
RET FISH
The Vysis PathVysion RNA Probe Kit (Abbott Laboratories) was used for detecting ALK, ROS1 and RET fusions according to the manufacturer's instructions. At least 50 cells were analyzed for each case by 2 independent pathologists. The bi-color FISH probe of detecting ALK, ROS1 and RET break-apart fusions was obtained from Amoy Diagnostics (Xiamen, China). Samples were judged as positive for rearrangement if >15% of tumor cells exhibited split red-green signals or touching golden-green signals respectively.
Statistical processing
Wilcoxon's rank-sum test was used for assessing the clinical characteristics of different groups. And log-rank test was employed for comparing the differences among the groups. Overall survival (OS) was defined as from the start of confirmed pathology to date of death or the last follow-up. Data analysis was performed with Statistics 18.0 (SPSS Inc., Chicago, IL, USA). And the last follow-up date was December 1, 2015.
Results
Clinical characteristics
A total of 385 patients were stratified into SCC (n = 245), adenosquamous carcinoma (n = 85) and large cell carcinoma (n = 55). Among 385 patients, 141 (26.6%) were non-smokers and 244 (63.4%) former or current smokers. There were 167 females and 218 males with a median age of 58 y. The pathologic stages were I (n = 147, 38.2%), II(n = 56, 14.5%) and IIIA (n=182, 47.3%). And their clinicopathologic characteristics were summarized in .
Table 1. Clinical characteristics of fusion genes in non-adenocarcinoma NSCLC patients.
Molecular analysis
ALK, ROS1 and RET genes were successfully detected for all samples. For 12 patients, ALK, ROS1 or RET rearrangement had a detection frequency of 3.1%. The most common gene rearrangements in a declining order were ALK (n = 7), ROS1 (n = 3) and RET (n = 2). The frequencies of adenosquamous carcinoma, SCC and large cell carcinoma were 8.2%, 1.6% and 1.8% respectively.
Among 385 patients, 212 had EGFR mutation detection, including SCC (n = 105), adenosquamous carcinoma (n = 72) and large cell carcinoma (n = 35). Totally, EGFR mutations were found in 26 patients. The frequencies of EGFR mutations in SCC, adenosquamous carcinoma and large cell carcinoma were 27.8%(20/72), 3.8%(4/105) and 5.7%(2/35) respectively. For 26 EGFR-mutated patients, no rearrangements of ALK, ROS1 and RET were found.
For 12 patients, the types were adenosquamous (n = 7), SCC (n = 4) and large cell carcinoma (n = 1). There were 8 males and 4 females with a median age of 49.5 y. Three patients had a smoking history and there were 9 non-smokers. More patients were younger and never-smokers in fusion positive patients than negative counterparts. However, no inter-group difference existed in gender. The comparisons of clinicopathologic characteristics were summarized in .
Table 2. Comparison of clinical characteristics between fusion gene positive and negative subjects.
Treatment and survival
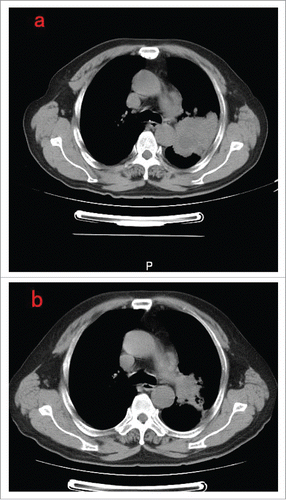
Seven patients became recurrent or metastatic, including 4 ALK rearrangements (adenosquamous, n = 3; SCC, n = 1), ROS1 (adenosquamous, n = 1; SCC, n = 1) and RET rearrangements (SCC, n = 1). Six patients received palliative chemotherapy and one had supportive treatment. Three patients of ALK rearrangements, including adenosquamous (n = 2) and SCC (n = 1), received crizotinib treatment. All three patients achieved disease control, including partial response (n = 2) and stable disease (n = 1). Notably, one patient of SCC achieved partial response (). The median PFS of crizotinib was 8.0 months for 3 patients.
Figure 1. (a) Thoracic computed tomography before crizotinib treatment (b) 2 months after crizotinib treatment.
The median OS was 50.2 months (95%CI: 44.0-56.5) for all patients and 36.7 months (95%CI:30.5-42.9) for 12 patients. No survival difference existed between patients with and without fusion gene (36.7 vs.50.2 months, P = 0.21) ().
Discussion
Our results demonstrated that the frequency of ALK, ROS1 and RET rearrangements was 3.1% in non-adenocarcinoma. And the clinicopathological characteristics of patients with 3 fusion genes were similar to patients of lung adenocarcinoma.With a limited number of patients treated with targeted inhibitor, we showed that patients of non-adenocarcinoma with positive rearrangements may benefit from targeted treatment.
It is well-known that patients of lung cancer with different histologies showed different gene abnormalities. More than 80% of patients with lung adenocarcinoma harbored known gene abnormalities.Citation10-13 However, there are even fewer studies of non-adenocarcinoma lung cancer. Limited number studies have demonstrated gene spectrum was similar to adenocarcinoma in adenosquamous carcinoma.Citation19-20 However, large differences existed in SCC and large cell carcinoma.Citation14-15 As reported by Wang et al, 3/207 (1.4%) cases of lung SCC were ALK positive.Citation21 However, no positive samples of ALK were observed in 214 patients by Zhao et al.Citation22 The frequencies of ALK, ROS1 and RET rearrangements in SCC were 0.8%, 0.8% and 0% in the present study respectively. Totally, fusion genes are rare in SCC. The diverging results may partially contribute to an imbalance of patients with different clinicopathological characteristics. In the present study, more patients were at a young age and never-smoking as compared with the study of Zhao et al.
For patients with known driver genes in lung adenocarcinoma, the fusion proportion of ALK, ROS1 and RET was around 10%.Citation10-13 Fusion proportion was not thoroughly studied in non-adenocarcinoma for its rarity. In the present study, 3 fusion genes were detected for a larger number of patients. We found that 3.1% of non-adenocarcinoma patients had ALK, ROS1 and RET abnormalities. The overall frequency was lower than that of lung adenocarcinoma. Thus the clinicopathological characteristics of fusion gene patients with non-adenocarcinoma were unknown. Previous studies showed that fusion genes were predominant in younger, adenocarcinoma and non-smokers.Citation10-15 Our study revealed similar clinicopathological characteristics of fusion genes in non-adenocarcinoma versus adenocarcinoma. In the NCCN guideline, testing for ALK rearrangements and EGFR mutations can be considered in patients with squamous cell histology who never smokerd and those whose histology was determined using small biopsy specimens or mixed histology specimens. However, patients with pure squamous cell carcinoma was not recommended for EGFR and ALK detections. Our results indicated that younger and never-smoking patients with pure non-adenocarcinoma histology after surgery should be screened routinely for fusion genes in Asian populations.
For rarity of fusion genes in non-adenocarcinoma NSCLC, no clinical study has examined the clinical efficacy of targeted treatment. Only several cases were reported and all of them focused on ALK positive patients.Citation23-24 However, nearly all patient histologies were based on biopsy in previous reports. There is always the problem of verifying whether or not it is a true non-adenocarcinoma. In the present study, 3 ALK positive patients received crizotinib and all achieved disease control, including one patient of SCC with partial response. This patient underwent complete resection and no adenocarcinoma component was found. Our study provided evidence with limited number patients that non-adenocarcinoma patients with positive fusion gene may benefit from inhibitor treatment.
Due to a relatively low frequency of fusion in lung cancer, the role of fusion genes as a prognostic marker is not well-established. Some studies suggested a worse prognosis for fusion in lung adenocarcinoma while others contradicted such a finding.Citation25 Our study revealed no survival difference existed between fusion positive and negative groups. The prognostic role of fusion genes should be further explored with a larger number of non-adenocarcinoma NSCLC patients.
Our study has some inherent limitations. Firstly, a small number of fusion genes might affect our conclusion. Secondly, only 3 patients received targeted treatment and clinical efficacy was not fully validated. However, our study was the first one of focusing upon fusion genes in non-adenocarcinoma lung cancer. The results may provide some guidance for clinical practices.
In conclusion, we have firstly demonstrated the proportions of ALK, ROS1 and RET genes in non-adenocarcinoma lung cancer. Gene abnormality in non-adenocarcinoma lung cancer is rare with a frequency of 3.1%. The clinicopathological characteristics of patients with fusion genes were similar for patients of lung adenocarcinoma.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66:115-32; PMID:26808342; https://doi.org/10.3322/caac.21338
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH. Eastern cooperative oncology group: comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002; 346:92-8; PMID:11784875; https://doi.org/10.1056/NEJMoa011954
- Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361:947-57; PMID:19692680; https://doi.org/10.1056/NEJMoa0810699
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, et al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med 2010; 362:2380-88; PMID:20573926; https://doi.org/10.1056/NEJMoa0909530
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010; 11:121-8; PMID:20022809; https://doi.org/10.1016/S1470-2045(09)70364-X
- Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011; 12:735-42; PMID:21783417; https://doi.org/10.1016/S1470-2045(11)70184-X
- Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13:239-46; PMID:22285168; https://doi.org/10.1016/S1470-2045(11)70393-X
- Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31:3327-34; PMID:23816960; https://doi.org/10.1200/JCO.2012.44.2806
- Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, Li W, Hou M, Shi JH, Lee KY, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014; 15:213-22; PMID:24439929; https://doi.org/10.1016/S1470-2045(13)70604-1
- Wu C, Zhao C, Yang Y, He Y, Hou L, Li X, Gao G, Shi J, Ren S, Chu H, et al. High Discrepancy of Driver Mutations in Patients with NSCLC and Synchronous Multiple Lung Ground-Glass Nodules. J Thorac Oncol. 2015; 10:778-83; PMID:25629635; https://doi.org/10.1097/JTO.0000000000000487
- Zhang Y, Sun Y, Pan Y, Li C, Shen L, Li Y, Luo X, Ye T, Wang R, Hu H, et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res 2012; 18:1947-53; PMID:22317764; https://doi.org/10.1158/1078-0432.CCR-11-2511
- Sun Y, Ren Y, Fang Z, Li C, Fang R, Gao B, Han X, Tian W, Pao W, Chen H, Ji H. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol. 2010; 28:4616-20; PMID:20855837; https://doi.org/10.1200/JCO.2010.29.6038
- Li H, Pan Y, Li Y, Li C, Wang R, Hu H, Zhang Y, Ye T, Wang L, Shen L, et al. Frequency of well-identified oncogenic driver mutations in lung adenocarcinoma of smokers varies with histological subtypes and graduated smoking dose. Lung Cancer 2013; 79:8-13; PMID:23098378; https://doi.org/10.1016/j.lungcan.2012.09.018
- An SJ, Chen ZH, Su J, Zhang XC, Zhong WZ, Yang JJ, Zhou Q, Yang XN, Huang L, Guan JL, et al. Identification of Enriched Driver Gene Alterations in Subgroups of Non-Small Cell Lung Cancer Patients Based on Histology and Smoking Status. PLoS One 2012; 7:e40109; PMID:22768234; https://doi.org/10.1371/journal.pone.0040109
- Qiong Z, Na WY, Bo W, Zhu Z, Ling P, Bo MH, Min TY, Lei Z, Na HD, Bo Z, Fang LJ, Seng ZS. Alterations of a spectrum of driver genes in female Chinese patients with advanced or metastatic squamous cell carcinoma of the lung. Lung Cancer 2015; 87:117-21; PMID:25488863; https://doi.org/10.1016/j.lungcan.2014.11.011
- Drilon A, Rekhtman N, Ladanyi M, Paik P. Squamous-cell carcinomas of the lung: emerging biology, controversies, and the promise of targeted therapy. Lancet Oncol 2012; 13:e418-26; PMID:23026827; https://doi.org/10.1016/S1470-2045(12)70291-7
- Pan Y, Wang R, Ye T, Li C, Hu H, Yu Y, Zhang Y, Wang L, Luo X, Li H, et al. Comprehensive analysis of oncogenic mutations in lung squamous cell carcinoma with minor glandular component. Chest 2014; 145:473-9; PMID:24158231; https://doi.org/10.1378/chest.12-2679
- Wu C, Zhao C, Yang Y, He Y, Hou L, Li X, Gao G, Shi J, Ren S, Chu H, et al. High Discrepancy of Driver Mutations in Patients with NSCLC and Synchronous Multiple Lung Ground-Glass Nodules. J Thorac Oncol 2015; 10:778-83; PMID:25629635; https://doi.org/10.1097/JTO.0000000000000487
- Wang R, Pan Y, Li C, Zhang H, Garfield D, Li Y, Ye T, Hu H, Luo X, Li H, et al. Analysis of major known driver mutations and prognosis in resected adenosquamous lung carcinomas. J Thorac Oncol 2014; 9:760-8; PMID:24481316; https://doi.org/10.1097/JTO.0b013e3182a406d1
- Toyooka S, Yatabe Y, Tokumo M, Ichimura K, Asano H, Tomii K, Aoe M, Yanai H, Date H, Mitsudomi T, Shimizu N. Mutations of epidermal growth factor receptor and K-ras genes in adenosquamous carcinoma of the lung. Int J Cancer 2006; 118:1588-90; PMID:16187277; https://doi.org/10.1002/ijc.21500
- Wang J, Shen Q, Shi Q, Yu B, Wang X, Cheng K, Lu G, Zhou X. Detection of ALK protein expression in lung squamous cell carcinomas by immunohistochemistry. J Exp Clin Cancer Res 2014; 33:109; PMID:25527865; https://doi.org/10.1186/s13046-014-0109-2
- Zhao W, Choi YL, Song JY, Zhu Y, Xu Q, Zhang F, Jiang L, Cheng J, Zheng G, Mao M.ALK, ROS1 and RET rearrangements in lung squamous cell carcinoma are very rare. Lung Cancer 2016; 94:22-7; PMID:26973202; https://doi.org/10.1016/j.lungcan.2016.01.011
- Mikes RE, Jordan F, Hutarew G, Studnicka M. First line crizotinib in anaplastic lymphoma kinase (ALK) rearranged squamous cell lung cancer. Lung Cancer. 2015; 90:614-6; PMID:26519123; https://doi.org/10.1016/j.lungcan.2015.10.013
- Schwab R, Petak I, Kollar M, Pinter F, Varkondi E, Kohanka A, Barti-Juhasz H, Schönleber J, Brauswetter D, Kopper L, Urban L. Major partial response to crizotinib, a dual MET/ALK inhibitor, in a squamous cell lung (SCC) carcinoma patient with de novo c-MET amplification in the absence of ALK rearrangement. Lung Cancer 2014; 83:109-11; PMID:24192513; https://doi.org/10.1016/j.lungcan.2013.10.006
- Lin C, Wang S, Xie W, Chang, Gan Y. The RET Fusion Gene and Its Correlation with Demographic and Clinicopathological Features of Non-Small Cell Lung Cancer: A Meta-Analysis. Cancer Biol Ther 2015; 16:1019-28; PMID:25975578; https://doi.org/10.1080/15384047.2015.1046649