ABSTRACT
Glioblastoma multiforme is the most malignant and common brain tumor in adults and is characterized by poor survival and high resistance to chemotherapy and radiotherapy. Among the new chemotherapy drugs, curcumin, a popular dietary supplement, has proven to have a potent anticancer effect on a variety of cancer cell types; however, it remains difficult to achieve a satisfactory therapeutic effect with curcumin using the traditional single-drug treatment. In this study, we found that expression of miR-326, a tumor suppressor microRNA in various tumor types, resulted in a marked increase of curcumin-induced cytotoxicity and apoptosis and a decrease of proliferation and migration in glioma cells. Moreover, we found that combination treatment of miR-326 and curcumin caused significant inhibition of the SHH/GLI1 pathway in glioma cells compared with either treatment alone, independent of p53 status. Furthermore, in vivo, the curcumin-induced increase in miR-326 expression altered the anti-glioma mechanism of this combination treatment, which further reduced tumor volume and prolonged the survival period compared to either treatment alone. Taken together, our data strongly support an important role for miR-326 in enhancing the chemosensitivity of glioma cells to curcumin.
Abbreviations
| miRNA | = | microRNA |
| SHH | = | sonic hedgehog |
| GLI1 | = | glioma-associated oncogene family zinc finger protein 1 |
| GBM | = | glioblastoma multiforme |
| miR-scr | = | miRNA-scramble |
Introduction
Glioma is the most common primary brain tumor derived from glial cells, and glioblastoma multiforme (GBM) is one of the most malignant types of gliomas with a median survival of 15 months.Citation1 In the past 30 y, there has been no improvement in the clinical treatment of gliomas worldwide.Citation2,Citation3 The current standard of care involves combination of maximal surgical resection with subsequent radiotherapy with concomitant and adjuvant temozolomide treatment. Because of its tendency to infiltrate into the extracellular matrix, it is difficult to treat GBM with surgery and radiotherapy.Citation4 Therefore, studies on the molecular mechanism underlying the tumorigenesis of gliomas and alternative non-operative treatment options have received increased attention in recent years.
Chemotherapeutic drugs are fundamental to cancer management and are used as adjuvant treatment for patients with GBMs after surgical procedures in most cases. In recent years, the use of curcumin for treating gliomas has received much attention, because of its strong antitumor effect and hypotoxicity. Curcumin is a pigment extracted from turmeric that shows an antitumor effect in many types of cancer. It plays an effective role in cellular proliferation, growth, survival, apoptosis, migration, invasion, angiogenesis, and autophagy.Citation5-Citation7 The mechanisms of the curcumin antitumor effects are comprehensive and complex, seeming to target cellular processes at many levels of regulation by regulating different signaling pathways such as NF-kB, Notch-1, AKT, and SHH/GLI1.Citation8-Citation10 However, it has thus far been difficult to achieve satisfactory results with the traditional single-drug treatment of curcumin due to its poor bioavailability and inability to cross the blood–brain barrier. Consequently, further studies aiming to enhance the therapeutic effect of curcumin are warranted.
MicroRNAs (miRNAs) are a class of small non-coding RNAs that can identify and interact with the specific 3′-untranslated regions of mRNA (mRNA). MiRNAs have recently emerged as important mediators in the regulation of gene expression by translational repression or degradation of the target mRNAs, and regulate a range of biological processes, including cell differentiation, growth, proliferation, and apoptosis.Citation11,Citation12 Previous studies have shown that miRNA expression profiles provide valuable molecular signatures for different tissues and human cancers. In particular, miR-326 has been reported as a tumor suppressor miRNA and shows low expression in glioma.Citation13 We and other researchers have demonstrated that miR-326 functions as a tumor suppressor in various tumor types, including gliomas, and we have also confirmed the inhibition of the biological behaviors of gliomas by miR-326 via regulation of the Hh/SMO/GLI1 pathway.Citation14 However, little is known about the anticancer effect of the interaction between miR-326 and chemotherapeutic drugs, especially with respect to curcumin.
In this study, we aimed to explore whether up-regulation of miR-326 could enhance the chemotherapeutic effect of curcumin in the human glioblastoma cell lines U251 (p53 mutant) and U87 (p53 wild-type). The cells were treated with curcumin and miR-326 alone and in combination and their inhibitory and anticancer effects were evaluated through cell viability assays and analysis of cellular proliferation, apoptosis, and invasion. Finally, we evaluated the potential mechanism and targeted pathways contributing to the effects of miR-326 on the chemosensitivity of curcumin. Taken together, our findings could provide a new effective therapeutic strategy for suppressing the growth of GBM.
Results
Curcumin promoted the expression of miR-326
miR-326 has been confirmed to show low expression in gliomas and was related to prognosis in our previous work.Citation14 Curcumin has shown an anti-tumor effect, and there is also evidence of various mechanisms by which miR-326 exerts a tumor inhibition effect, including by decreasing the activity of the SHH/GLI1 pathway.Citation10,Citation15,Citation16 To investigate whether there is an interaction between curcumin and miR-326 for inhibiting glioma cell growth and invasive behavior, the cells were transfected with miR-scramble (miR-scr) or miR-326 and treated with different doses of curcumin. The result of qRT-PCR showed that the expression of miR-326 increased significantly with increasing doses of curcumin, and the miR-326 mimics effectively induced the expression of miR-326 ().
Figure 1. Expression of miR-326 in glioblastoma cells treated with miR-326 mimics, curcumin, or both, and the effect of miR-326 on the chemosensitivity of U87 and U251 cells to curcumin. (A) RT-PCR results of the expression of miR-326 in cells treated with different doses of curcumin and/or miR-326 mimics. (B) U87 and U251 cells were treated with miR-326 and/or increasing concentrations of curcumin for 24 h. Viability was analyzed using the MTT assay. The data represent the mean ± SEM of 3 replicates. Statistical significance levels are indicated as: *P < 0.05; **P < 0.01 and ***P < 0.001
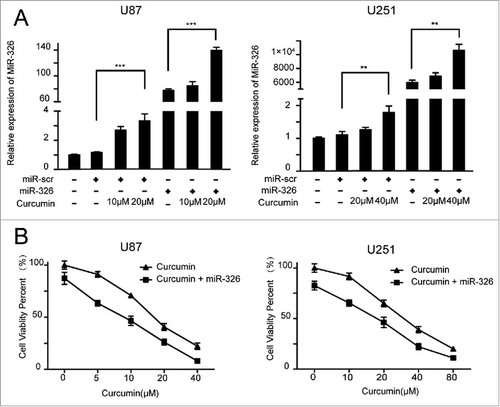
Overexpression of miR-326 increases the cytotoxicity of curcumin in malignant glioma cells
We investigated the effects of miR-326 on curcumin-induced cytotoxicity in glioma cells using the MTT assay. As shown in , the medial half-maximal inhibitory concentration (IC50) of curcumin was 15 μM and 30 μM for U87 and U251 cells transfected with miR-326, respectively, which was much higher than the values observed in cells transfected with miR-326 mimics (9 μM for U87 and 18 μM for U251). These results suggest that overexpression of miR-326 enhanced the curcumin-induced cytotoxicity in a dose-dependent manner. Therefore, we chose 2 doses of curcumin for each cell line for subsequent experiments (10 μM and 20 μM for U87, and 20 μM and 40 μM for U251).
miR-326 and curcumin have an additive effect in U251 cells and a synergistic effect in U87 cells
We used the Jin methodCitation17 to determine whether the combined effect of miR-326 and curcumin on U251 and U87 cells is representative of antagonism, additivity, or synergy. The Q value for U251 cells was 1.11, indicating additive effects of miR-326 and curcumin combination therapy. Moreover, the Q value for U87 cells was 1.27, indicating synergistic effects after combined treatment.
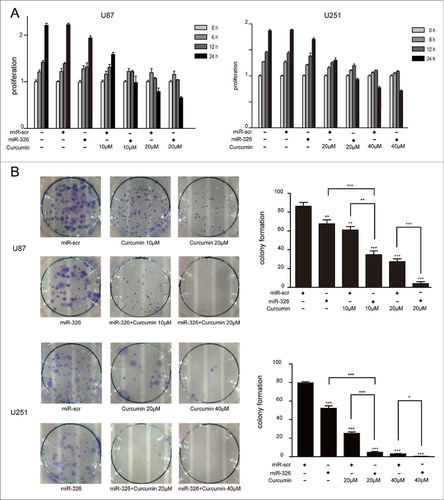
Overexpression of miR-326 combined with curcumin inhibited proliferation, promoted apoptosis, induced cell death and restrained the migration of glioma cells
The results of the MTT assay to evaluate the synergistic inhibitory effects of miR-326 and curcumin on glioma are shown in . At various doses of curcumin (10 and 20 μM in U87 cells, and 20 and 40 μM in U251cells) over different time periods (0, 6, 12, and 24 h), glioma cells that were transfected with miR-326 mimics showed significantly lower survival than those receiving curcumin treatment alone. Similarly, a colony formation assay showed reduced colony formation when U251 and U87 cells were transfected with miR-326 mimics for 6 h followed by treatment with curcumin (). Therefore, the colony formation and MTT assays both indicated that miR-326 enhanced the inhibitory effect of curcumin on proliferation of glioma cells.
Figure 2. miR-326 and curcumin combination treatment suppressed the proliferation of U87 and U251 cells. (A) U87 and U251 cells were treated with miR-326, curcumin, or both for 6 h, 12 h, and 24 h. Cell viability was examined using the MTT assay. (B) Results of the colony formation assay. U87 and U251 cells transfected with or without miR-326 were incubated with the indicated concentrations of curcumin for 24 h and then and allowed to grow into colonies for 2 weeks. The data represent the mean ± SEM of 3 replicates (*P < 0.05; **P < 0.01, and ***P < 0.001).
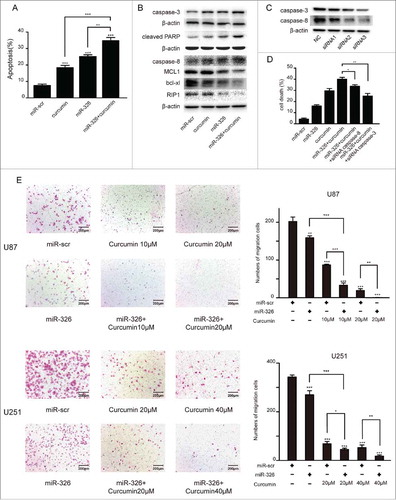
To evaluate the effects of miR-326 upregulation or curcumin treatment on inducing apoptosis, we performed annexin V/PI staining in U87 cells (). Furthermore, we found that the expression levels of the apoptosis markers caspase-3, cleaved anti-poly ADP ribose polymerase 1 (cleaved PARP1), caspase-8 increased and MCL1, bcl-xl, RIP1 decreased significantly after miR-326 and curcumin combination treatment compared with the other groups (). As depicted in the number of dead cells with combination treatment was significantly higher than the other groups and due to the combination treatment increase the expression of csapase-3 and caspase-8, miR-326 and curcumin combination therapy group transfected with siRNA of them had a lower number of dead cells. Moreover, given that SHH/GLI1 signaling can promote the motility and invasiveness of cancer cells,Citation18 a transwell assay was conducted to examine whether this pathway may have been inhibited by miR-326 and curcumin combination treatment. As shown in , the combination treatment attenuated the migration of U87 and U251 cells compared with miR-326 or curcumin treatment alone.
Figure 3. miR-326 and curcumin combination treatment promoted apoptosis and restrained the migration of glioma cells. (A) The annexin V-PI assay revealed increased apoptosis in glioma cells following combination treatment. Percentages of apoptotic cells are shown in the histogram. (B) Western blot assay of the levels of caspase-3, cleaved PARP, caspase-8, MCL1, bcl-xl and RIP1 after miR-326 and curcumin treatment alone and in combination. (C) Western blot of the expression of caspase-3 and caspase-8 after transfected either with negative control or 3 different siRNA sequences againest caspase-3 and caspase-8. (D) Cell death of different treatments was determined by trypan blue staining. (E) Cell migration was analyzed using the transwell assay. Bars represent 200 μm. The data represent the mean ± SEM of 3 replicates (*P < 0.05; **P < 0.01, and ***P < 0.001).
Overexpression of miR-326 combined with curcumin treatment enhanced inhibition of the SHH/GLI1 pathway and regulated the expression of p53 and stemness in glioma cells
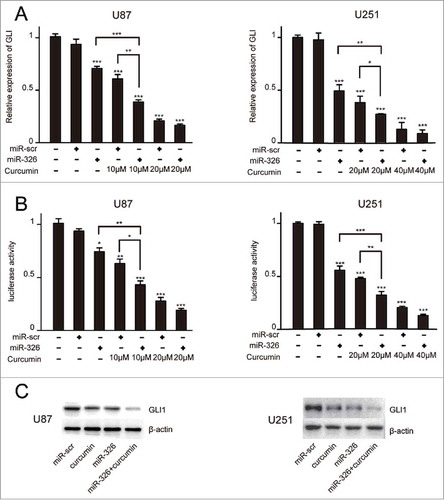
Given that previous reports have found that miR-326 or curcumin could play a role in tumor suppression via inhibition of the SHH/GLI1 signaling pathway,Citation10,Citation19,Citation20 the effect of miR-326 and curcumin combination treatment on the SHH/GLI1 signaling pathway was analyzed by measuring GLI1 expression. qRT-PCR showed that miR-326 upregulation combined with curcumin treatment reduced GLI1 expression more significantly than either single factor (i.e. only transfected with miR-326 or only curcumin treatment) (). Furthermore, the 8×-GLI1 luciferase reporter was transfected into miR-326- or miR-Scr–overexpressing U87 and U251 cell lines. After treatment of curcumin, luciferase activity of the cells transfected with miR-326 also decreased significantly compared with that of the control cells (). Western blot analysis further confirmed that treatment of both U87 and U251 cells with miR-326 and curcumin combination therapy could dramatically downregulate the expression of GLI1 (). Together, these data suggested that miR-326 could enhance the inhibitory effect of curcumin via regulation of the SHH/GLI1 pathway.
Figure 4. Overexpression of miR-326 combined with curcumin treatment decreased GLI1 expression. (A) Real-time PCR analysis of GLI1 expression in U87 and U251 cells following miR-326 transfection and curcumin treatment for 24 h. (B) U87 and U251 cells were transfected with miR-326 or miR-Scr followed by cotransfection of a firefly luciferase reporter construct containing 8 consecutive consensus GLI1-binding sites (8×-GLI). Cells were then treated as indicated with solvent DMSO (control) or curcumin. Both firefly and Renilla luciferase activities were quantified using the Dual-Luciferase Reporter Assay System and normalized with Renilla luciferase activity. The data represent the mean ± SEM of 3 replicates (*P < 0.05; **P < 0.01, and ***P < 0.001). (C) Western blot assay showing protein GLI1 expression in glioma cells treated with miR-326, curcumin, and their combination.
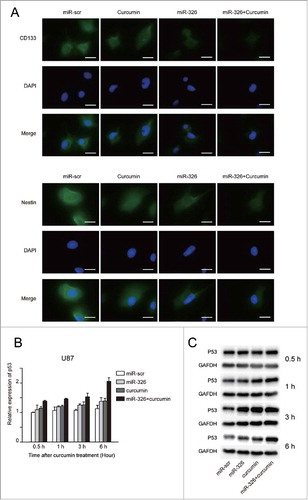
Moreover, the SHH/GLI1 pathway has also been shown to regulate the stemness and invasiveness of cancer cells,Citation21,Citation22 which may be partly regulated by changes in miR-326 expression.Citation14 Therefore, to further investigate whether miR-326 and curcumin treatment could reduce the stemness ability, immunofluorescence was used to examine the expression of stem cells markers (Nestin and CD133) in glioma cells. As shown in , the miR-326 and curcumin combination significantly decreased Nestin and CD133 expression levels compared with either treatment alone. To evaluate the influence on the GLI1-p53 functional network,Citation23 U87 cells (p53 wild-type) transfected with miR-326 or miR-scr were treated with 20 μM curcumin for different time periods (0.5, 1, 3, 6 h) and then p53 mRNA expression was analyzed with RT-PCR. The results showed that p53 mRNA increased significantly in response to the combination treatment compared with other treatment groups in a time-dependent manner (). Western blot analysis further confirmed this result, verifying that combination treatment could significantly upregulate p53 protein expression in a time-dependent manner ().
Figure 5. miR-326 and curcumin combination treatment decreased the stemness ability and expression of p53 in glioma cells. (A) U251 cells with or without transfection of miR-326 and/or curcumin treatment were analyzed with immunofluorescent staining using anti-CD133 and anti-Nestin antibodies. Bars represent 20 μm. (B and C) U87 cells with or without transfection of miR-326 and/or curcumin treatment were harvested after 0.5, 1, 3, and 6 h and subjected to RT-PCR and western blot assay to determine p53 expression.
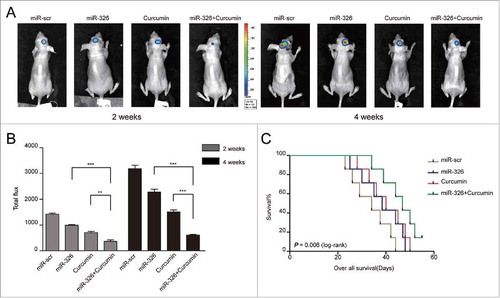
Overexpression of miR-326 combined with curcumin treatment inhibited tumor growth in vivo and prolonged survival
To investigate whether the enhancement of the anti-glioma effect of miR-326 combined with curcumin treatment could also be achieved in vivo, an intracranial glioma model of nude mice was employed and the size of tumors formed was compared between treatment groups using fluorescent images of the whole mouse at 2 time points (2 weeks and 4 weeks). The results indicated that miR-326 overexpression and curcumin combination treatment had a similar effect in vivo as observed in vitro, in that mimic-treated cells with curcumin showed significant reduction in tumor volume compared with other groups (). To further evaluate the potential therapeutic effect of the combined treatment, the survival time of each group was analyzed by a Kaplan–Meier curve. Mice injected with miR-326 mimic-treated cells with curcumin treatment also showed a significant improvement in survival compared with the other treatment groups ().
Discussion
Glioma is currently the most common type of primary malignant brain tumor, and the prognosis of GBM, the most aggressive form of glioma, remains unsatisfactory. Several therapies involving surgery, radiotherapy, and/or chemotherapy are applied in clinical treatment to combat glioma; however, owing to the loss of heterozygosity and heterogeneity of glioma, tumor drug resistance commonly develops during the course of single-drug treatment. In recent years, researchers have begun to pay more attention to the potential of a combination of drug treatments. Furthermore, many studies have shown that correction of altered expression of miRNAs might be an alternative therapeutic strategy to overcome cancer cell resistance.Citation24-Citation26 Thus, in this study, we evaluated the potential of miR-326 and curcumin combination treatment for glioma by evaluating the effects in vitro and in vivo.
We provide the first demonstration that overexpression of miR-326 via transfection of miR-326 mimics contributes to sensitizing human glioma cells to the anticancer drug curcumin. miR-326 was first identified as a tumor suppressor gene in gliomas in 2009.Citation27-Citation29 Despite the well-established role of miR-326 in GBM, the molecular mechanism of its overexpression on the response to chemotherapy remains largely unexplored. In our previous study, we also demonstrated that miR-326 could target the SMO oncogene to inhibit the biological behaviors and stemness of glioma cells.Citation14 providing a potential mechanism of its anti-cancer function. In this study, our dose-response data indicated that increasing the miR-326 level resulted in a 1.6-fold increase in drug sensitivity (based on the IC50 values) between mimics- and curcumin-treated GBM cells. This demonstrated that the miR-326 mimics resulted in increased sensitivity of glioma cells to curcumin.
Therefore, in the present study we sought to comprehensively analyze the combination effect between miR-326 and curcumin. We found additive or synergistic effects in the combination of miR-326 with curcumin. Furthermore, single or combined treatment of miR-326 and curcumin showed different effects on the behaviors of glioma cells, including proliferation, migration, and apoptosis. Specifically, the miR-326 mimics additively and synergistically interacted with curcumin in U251 and U87 cells, respectively, with respect to proliferation and migration, and additively interacted in both cell lines with respect to apoptosis. In addition, the tumor xenograft study illustrated that GLI1 inhibition induced by the miR-326 and curcumin combination treatment had a stronger effect on reducing tumor growth in vivo and significantly prolonged the survival of nude mice compared with miR-326 or curcumin treatment alone.
Several previous studies have demonstrated that the SHH/GLI1 pathway shows abnormal activation in various tumor types, including gliomas.Citation30,Citation31 Other research has shown that relapse and drug resistance might be closely linked with the activated hedgehog signaling pathway.Citation32,Citation33 In addition, miR-326 or curcumin was found to suppress the activity of the hedgehog pathway, which in turn influenced the behaviors of glioma cells and tumor stem cell stemness to relieve the drug resistance.Citation19,Citation20,Citation34 Thus, this is the first study to test the combination of miR-326 with curcumin on the pathway activity and drug resistance. We focused on GLI1 expression, which is regarded as an essential hallmark of aberrant SHH/GLI1 pathway activation.Citation35 The assays verified that miR-326 or curcumin suppressed SHH/GLI1 activity individually, and confirmed that combination treatment resulted in stronger inhibition on pathway activity and stemness. In addition, the observed increase of miR-326 expression in response to curcumin treatment confirmed the presence of a feedback loop between miR-326 and the SHH/GLI1 pathway, which provides a mechanism for the enrichment of the anti-glioma effect due to the combination treatment. Considering the GLI1-p53 functional network,Citation23 it is worth noting that a significant increase in p53 expression was observed only in U87 cells (p53 wild-type) and not in U251 cells (p53 mutant) in response to the miR-326 and curcumin combination treatment. These data suggest that miR-326 and curcumin combination treatment could decrease SHH/GLI1 activity independent of the p53 status. We also found that the IC50 values of curcumin were generally lower in the glioma cell line with the p53 wild-type compared to that with the p53 mutant. We suspect that the observed difference in sensitivity to curcumin between these cell lines may be related to activation of p53, but more evidence is needed to provide support for this hypothesis, which might be verified in our future experiments.
In summary, the results from this study provide new rationales for novel combinational therapies using miR-326 to synergistically cooperate with curcumin for glioma patients, and we first proposed and confirmed a mechanism for this effect, whereby the miRNA could enhance curcumin's anti-glioma effect via the SHH/GLI1 pathway; in turn, curcumin was shown to regulate miRNA expression. These results suggest that miR-326 is a potential candidate for combination therapy of miRNA and curcumin. This study not only provides insight into the pharmacological mechanism of curcumin's anti-tumor effects but also provides evidence for curcumin as a promising therapeutic agent for the treatment of GBM.
Materials and methods
Cell culture
The two human glioma cell lines, U87 and U251, were purchased from the Chinese Academy of Science Cell Bank. All cells were grown in Dulbecco's modified Eagle's medium (Biological Industries, 01-052-1ACS) with 10% fetal bovine serum (FBS; Biological Industries, 04-001-1ACS) and were maintained at 37°C in a humidified atmosphere with 5% CO2 and 95% air.
Cell transfection
Human miR-326 mimics and miR-scramble (miR-scr) were purchased from Gene Pharma (Shanghai, China). Twenty-four hours prior to transfection, U87 and U251 cells were seeded in 6-well plates at 4 × 105 cells per well, respectively. When the cells were 60–80% confluent, they were transfected with miR-326 mimics or miR-scr using Lipofectamine 2000 reagent (Invitrogen, 11668–019) in accordance with the manufacturer protocol; 100 pmol of miRNA was added to each well. Cells transfected with miR-Scr were considered as a control. The miRNA-transfected cells were harvested at 12–24 h post-transfection.
Cytotoxicity and cell proliferation assays
The has-miR-326- or has-miR negative control-transfected cells were plated into 96-well plates at 5000–7000 cells per well and allowed to grow overnight. Fresh medium containing various concentrations of curcumin (Sigma-Aldrich, C1386-5G) was added, and the cells were cultured for an additional 24 h. At this point, 20 μl of 0.5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Beyotime, C0009) was added to each well and the cells were incubated for another 4 h. Subsequently, 150 μl of dimethyl sulfoxide (DMSO; Biotopped, D6371) was added to each well and mixed for 10 min. The amount of formazan was quantified based on the absorbance at 490 nm using an IMARK microplate reader. Six parallel samples were measured in each experiment. Cell proliferation was determined in the same manner except that absorbance was compared among groups cells treated with curcumin over different time intervals (0, 6, 12, and 24 h).
Colony formation assay
Cells were seeded at 1000 cells per well in 6-well plates and transfected with miR-326 mimics. Then, the cells were treated with or without different concentrations of curcumin for another 24 h. Two weeks later, the cells were washed with phosphate-buffered saline, fixed in methanol for 10 min, and stained with Giemsa stain. Each experiment was repeated 3 times.
Apoptosis assay
Twenty-four hours after transfection and treatment of curcumin, the cells were harvested and resuspended in 1X binding buffer. Then, 5 μl of fluorescein isothiocyanate (FITC)-conjugated annexin V and 5 μl of propidium iodide (PtdIns; BD Pharmingen, 556547) were added and the cells were further incubated in the dark for 15 min. Stained cells were detected by flow cytometry (FACSCanto II, BD Biosciences, USA).
Western blot analysis
To investigate the potential molecular mechanisms and pathways involved in the anti-glioma effects, the protein expression level of GLI1, PARP1, caspase-3 and p53 were analyzed by western blot. PARP1 normally functions in the routine repair of DNA damage and becomes cleaved into 2 fragments by caspase during apoptosis.Citation36 Accordingly, PARP1 and caspase-3 are commonly used as biomarkers of apoptosis.Citation37 Twenty-4 hours after transfection and treatment of curcumin, the cells were lysed with RIPA buffer (Pierce, 89900) with protease inhibitors at a dilution of 1%. After centrifugation, total proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore, IPVH00010). Then, the membranes were washed with Tris-buffered saline with Tween (TBST), blocked with a 5% milk–TBST solution, and incubated with primary antibodies, including anti-human caspase-3 (1:300, Proteintech, 19677-1-AP), anti-PARP (1:600, Proteintech, 13371-1-AP), anti-caspase-8 (1:500, Proteintech, 13423-1-AP), anti-Bcl-XL (1:1000, Proteintech, 10783-1-AP), anti-MCL1 (1:600, Proteintech, 16225-1-AP), anti-RIP1 (1:300, Proteintech, 17519-1-AP), anti-GLI1 (1:1000, Cell Signaling Technology, 3538), anti-p53 (1:600, Proteintech, 10442-1-AP), anti-glyceraldehyde 3-phosphatedehydrogenase (GAPDH; 1:800, Proteintech, 10494-1-AP), and anti-β-actin (1:800, ZSGB, TA-09) overnight at 4°C. The cells were then incubated with horseradish peroxidase-conjugated anti-mouse (1:4000, ZSGB, ZB-2305) or anti-rabbit (1:4000, ZSGB, ZB-2301) secondary antibodies, and protein bands were detected with ChemiDoc™ MP Imaging System (BIO-RAD).
RNA interference
Three small interfering RNA specifically against caspase-3 or caspase-8 were purchased from Ribobio (Guangzhou, China) and transfected into glioma cells in 6-well plates using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Then the gene silencing effect was measured by protein gel blotting 48 hours post transfection.
Cell death assay
Cell death was determined by trypan blue staining. Cells were seeded in 12-well plates (2 × 105 cells/well) and incubated for 24 hours at 37 – with different treatment. After 24 hours, cells were suspended with trypsin and stained with trypan blue dye. The cell death was evaluated by the percentage of death relative to the total cell.
Migration assay
The transwell assay was performed using the Transwell Permeable Supports (CORNING, 3422) according to the manufacturer protocol. The cells were resuspended at a concentration of 2.5 × 105 cells/ml in FBS-free medium containing 0.1% bovine serum albumin with different concentrations of curcumin. A cell suspension (0.2 ml) was added to the top of each well, and 0.5 ml of complete medium was added into the bottom wells as a chemoattractant. The cells were incubated for 16 h at 37°C and allowed to invade through a polycarbonate membrane. Subsequently, the cells in the upper chamber were removed, and those that invaded to the lower surface of the membrane were fixed with methanol, stained with hematoxylin and eosin, and counted.
Analysis of the combined effect of miR-326 and curcumin
We used the method described by Jin Citation17 to analyze the combined effect of miR-326 and curcumin on glioma cells. This method provides a “Q” value to classify the combination effect as an antagonistic effect (Q < 0.85), additive effect (0.85 < Q < 1.15), or synergistic effect (Q >1.15), which is calculated as: Q = Ea+b/(Ea+Eb – Ea×Eb), where Ea+b, Ea, and Eb represent the average effect of the combination treatment, miR-326 mimics only, and curcumin only, respectively.
RNA extraction and real-time PCR
Quantitative real-time polymerase chain reaction (qRT-PCR) was carried out as previously described.Citation14 Total RNA was extracted from the U87 and U251 cell lines using TRIzol reagent (Invitrogen, 15596-026). Real-time PCR was performed using LightCycler2.0 (Roche Diagnostics, 04887352001) and GAPDH or U6 snRNA was used as an endogenous control for mRNA or miRNA detection, respectively. All procedures were performed according to the manufacturer instructions. The fold change for miRNA-326 and GLI1 expression was calculated by the 2−ΔΔCT method.
GLI1 reporter assay
As previously describedCitation10, a reporter containing 8 directly repeated copies of a consensus GLI1-binding site (8×-GLI1) downstream of the luciferase gene was used to assess GLI1 expression; it was kindly provided by Gregory J. Gores, M.D. (Division of Liver Pathobiology, Mayo Clinic, Rochester, MN). Cells treated with miR-326 mimics or miR-Scr were co-transfected with the plasmid expressing Renilla luciferase and the above-mentioned luciferase reporter, respectively. Twenty-four hours after treatment with and without curcumin, luciferase activities were quantified using the Dual-Luciferase Reporter Assay System (Promega, E1910) and normalized to Renilla luciferase activity. Each experiment was repeated in triplicate.
Immunofluorescence
U251 cells were transfected with miR-326 or miR-scr, and treated with or without curcumin. The cells were permeabilized in 0.1% Triton X-100 and blocked with 5% bovine serum albumin. All cells were then fixed with 4% paraformaldehyde and incubated with primary antibodies Nestin (Proteintech, 19483-1-AP) or CD133 (Proteintech, 18470-1-AP) overnight at 4°C. FITC-labeled secondary antibody (1:200 dilutions, BOSTER, BA1127) was added for 2 h at 37°C. DAPI reagent was used to stain the U251 cell nuclei. Images were acquired on a fluorescence microscope (Nikon, Japan).
Tumor xenograft study
Five-week–old female BALB/c-nude mice were used for tumor implantation and were randomly distributed into 4 groups (miR-scr, miR-326, miR-scr+curcumin and miR-326+curcumin, n = 7 per group). The mice were intracranially injected with U87 cells transfected with luciferase lentivirus and co-transduced with miR-326 mimics/scramble oligonucleotide according to the grouping above, as previously described.Citation10,Citation14 The next day, the mice were intraperitoneally injected with curcumin (60 mg/kg) or DMSO (control) every day. The tumors developed in the mice were measured by bioluminescence using an IVIS Lumina Imaging System (Xenogen) after 2 and 4 weeks. These procedures were performed following approval by the Harbin Medical University Institutional Animal Care and Use Committee.
Statistical analysis
The data were assessed using the GraphPad Prism software 5.0 and SPSS version 11.0. Comparisons of the data from each treatment group with the control were carried out to assess the statistical significance of the difference by the paired t-test. A P-value of <0.05 was considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Tate MC, Aghi MK. Biology of angiogenesis and invasion in glioma. Neurotherapeutics 2009; 6:447-57; PMID:19560735; https://doi.org/10.1016/j.nurt.2009.04.001
- Kang JH, Adamson C. Novel chemotherapeutics and other therapies for treating high-grade glioma. Expert Opin Invest Drugs 2015:24(10):1361-79; PMID:26289791 https://doi.org/10.1517/13543784.2015.1048332
- Norden AD, Drappatz J, Wen PY. Antiangiogenic therapies for high-grade glioma. Nat Rev Neurol 2009; 5:610-20; PMID:19826401; https://doi.org/10.1038/nrneurol.2009.159
- Mahesparan R, Tysnes BB, Edvardsen K, Haugeland HK, Cabrera IG, Lund-Johansen M, Engebraaten O, Bjerkvig R. Role of high molecular weight extracellular matrix proteins in glioma cell migration. Neuropathol Appl Neurobiol 1997; 23:102-12; PMID:9160895; https://doi.org/10.1111/j.1365-2990.1997.tb01192.x
- Zhuang W, Long L, Zheng B, Ji W, Yang N, Zhang Q, Liang Z. Curcumin promotes differentiation of glioma-initiating cells by inducing autophagy. Cancer Sci 2012; 103:684-90; PMID:22192169; https://doi.org/10.1111/j.1349-7006.2011.02198.x
- Seo JA, Kim B, Dhanasekaran DN, Tsang BK, Song YS. Curcumin induces apoptosis by inhibiting sarco/endoplasmic reticulum ca ATPase activity in ovarian cancer cells. Cancer Lett 2015; 371(1):30-7; PMID:26607901; https://doi.org/10.1016/j.canlet.2015.11.021
- Chen QY, Jiao DM, Wang LF, Wang L, Hu HZ, Song J, Yan J, Wu LJ, Shi JG. Curcumin inhibits proliferation-migration of NSCLC by steering crosstalk between a Wnt signaling pathway and an adherens junction via EGR-1. Mol Biosys 2015; 11:859-68; PMID:25578635; https://doi.org/10.1039/C4MB00336E
- Li Y, Zhang J, Ma D, Zhang L, Si M, Yin H, Li J. Curcumin inhibits proliferation and invasion of osteosarcoma cells through inactivation of Notch-1 signaling. FEBS J 2012; 279:2247-59; PMID:22521131; https://doi.org/10.1111/j.1742-4658.2012.08607.x
- Xu X, Qin J, Liu W. Curcumin inhibits the invasion of thyroid cancer cells via down-regulation of PI3K/Akt signaling pathway. Gene 2014; 546:226-32; PMID:24910117; https://doi.org/10.1016/j.gene.2014.06.006
- Du WZ, Feng Y, Wang XF, Piao XY, Cui YQ, Chen LC, Lei XH, Sun X, Liu X, Wang HB, et al. Curcumin suppresses malignant glioma cells growth and induces apoptosis by inhibition of SHH/GLI1 signaling pathway in vitro and vivo. CNS Neurosci Ther 2013; 19:926-36; PMID:24165291; https://doi.org/10.1111/cns.12163
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006; 6:857-66; PMID:17060945; https://doi.org/10.1038/nrc1997
- Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 2003; 113:25-36; PMID:12679032; https://doi.org/10.1016/S0092-8674(03)00231-9
- Wang S, Lu S, Geng S, Ma S, Liang Z, Jiao B. Expression and clinical significance of microRNA-326 in human glioma miR-326 expression in glioma. Med Oncol 2013; 30:373; PMID:23292865; https://doi.org/10.1007/s12032-012-0373-y
- Du W, Liu X, Chen L, Dou Z, Lei X, Chang L, Cai J, Cui Y, Yang D, Sun Y, et al. Targeting the SMO oncogene by miR-326 inhibits glioma biological behaviors and stemness. Neuro Oncol 2015; 17:243-53; PMID:25173582; https://doi.org/10.1093/neuonc/nou217
- Babashah S, Sadeghizadeh M, Hajifathali A, Tavirani MR, Zomorod MS, Ghadiani M, Soleimani M. Targeting of the signal transducer Smo links microRNA-326 to the oncogenic Hedgehog pathway in CD34+ CML stem/progenitor cells. Int J Cancer 2013; 133:579-89; PMID:23341351; https://doi.org/10.1002/ijc.28043
- Lian N, Jiang Y, Zhang F, Jin H, Lu C, Wu X, Lu Y, Zheng S. Curcumin regulates cell fate and metabolism by inhibiting hedgehog signaling in hepatic stellate cells. Lab Invest 2015; 95:790-803; PMID:25938627; https://doi.org/10.1038/labinvest.2015.59
- Jin ZJ. About the evaluation of drug combination. Acta Pharmacol Sin 2004; 25:146-7; PMID:14769200
- Onishi H, Morisaki T, Nakao F, Odate S, Morisaki T, Katano M. Protein-bound polysaccharide decreases invasiveness and proliferation in pancreatic cancer by inhibition of hedgehog signaling and HIF-1alpha pathways under hypoxia. Cancer Lett 2013; 335:289-98; PMID:23485726; https://doi.org/10.1016/j.canlet.2013.02.041
- Jiang Z, Cushing L, Ai X, Lu J. miR-326 is downstream of Sonic hedgehog signaling and regulates the expression of Gli2 and smoothened. Am J Respir Cell Mol Biol 2014; 51:273-83; PMID:24617895; https://doi.org/10.1165/rcmb.2013-0127OC
- Sun XD, Liu XE, Huang DS. Curcumin reverses the epithelial-mesenchymal transition of pancreatic cancer cells by inhibiting the Hedgehog signaling pathway. Oncol Rep 2013; 29:2401-7; PMID:23563640; https://doi.org/10.3892/or.2013.2385
- Sun Y, Wang Y, Fan C, Gao P, Wang X, Wei G, Wei J. Estrogen promotes stemness and invasiveness of ER-positive breast cancer cells through Gli1 activation. Mol Cancer 2014; 13:137; PMID:24889938; https://doi.org/10.1186/1476-4598-13-137
- Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol 2007; 17:165-72; PMID:17196391; https://doi.org/10.1016/j.cub.2006.11.033
- Zbinden M, Duquet A, Lorente-Trigos A, Ngwabyt SN, Borges I, Ruiz i Altaba A. NANOG regulates glioma stem cells and is essential in vivo acting in a cross-functional network with GLI1 and p53. EMBO J 2010; 29:2659-74; PMID:20581802; https://doi.org/10.1038/emboj.2010.137
- He X, Xiao X, Dong L, Wan N, Zhou Z, Deng H, Zhang X. MiR-218 regulates cisplatin chemosensitivity in breast cancer by targeting BRCA1. Tumour Biol 2015; 36:2065-75; PMID:25394901; https://doi.org/10.1007/s13277-014-2814-z
- Chen J, Fu X, Wan Y, Wang Z, Jiang D, Shi L. miR-125b inhibitor enhance the chemosensitivity of glioblastoma stem cells to temozolomide by targeting Bak1. Tumour Biol 2014; 35:6293-302; PMID:24643683; https://doi.org/10.1007/s13277-014-1821-4
- Huang N, Wu J, Qiu W, Lyu Q, He J, Xie W, Xu N, Zhang Y. MiR-15a and miR-16 induce autophagy and enhance chemosensitivity of Camptothecin. Cancer Biol Ther 2015; 16:941-8; PMID:25945419; https://doi.org/10.1080/15384047.2015.1040963
- Qiu S, Lin S, Hu D, Feng Y, Tan Y, Peng Y. Interactions of miR-323/miR-326/miR-329 and miR-130a/miR-155/miR-210 as prognostic indicators for clinical outcome of glioblastoma patients. J Transl Med 2013; 11:10; PMID:23302469; https://doi.org/10.1186/1479-5876-11-10
- Li Y, Gao Y, Xu Y, Ma H, Yang M. Down-regulation of miR-326 is associated with poor prognosis and promotes growth and metastasis by targeting FSCN1 in gastric cancer. Growth Factors 2015; 33:267-74; PMID:26359764; https://doi.org/10.3109/08977194.2015.1076406
- Yu S, Huang H, Deng G, Xie Z, Ye Y, Guo R, Cai X, Hong J, Qian D, Zhou X, et al. miR-326 targets antiapoptotic Bcl-xL and mediates apoptosis in human platelets. PloS One 2015; 10:e0122784; PMID:25875481; https://doi.org/10.1371/journal.pone.0122784
- Jeng KS, Sheen IS, Jeng WJ, Lin CC, Lin CK, Su JC, Yu MC, Fang HY. High expression of patched homolog-1 messenger RNA and glioma-associated oncogene-1 messenger RNA of sonic hedgehog signaling pathway indicates a risk of postresection recurrence of hepatocellular carcinoma. Ann Surg Oncol 2013; 20:464-73; PMID:22911366; https://doi.org/10.1245/s10434-012-2593-y
- Yan GN, Yang L, Lv YF, Shi Y, Shen LL, Yao XH, Guo QN, Zhang P, Cui YH, Zhang X, et al. Endothelial cells promote stem-like phenotype of glioma cells through activating the Hedgehog pathway. J Pathol 2014; 234:11-22; PMID:24604164; https://doi.org/10.1002/path.4349
- Xu M, Gong A, Yang H, George SK, Jiao Z, Huang H, Jiang X, Zhang Y. Sonic hedgehog-glioma associated oncogene homolog 1 signaling enhances drug resistance in CD44(+)/Musashi-1(+) gastric cancer stem cells. Cancer Lett 2015; 369:124-33; PMID:26276718; https://doi.org/10.1016/j.canlet.2015.08.005
- Zahreddine HA, Culjkovic-Kraljacic B, Assouline S, Gendron P, Romeo AA, Morris SJ, Cormack G, Jaquith JB, Cerchietti L, Cocolakis E, et al. The sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation. Nature 2014; 511:90-3; PMID:24870236; https://doi.org/10.1038/nature13283
- Kim Y, Kim H, Park H, Park D, Lee H, Lee YS, Choe J, Kim YM, Jeoung D. miR-326-histone deacetylase-3 feedback loop regulates the invasion and tumorigenic and angiogenic response to anti-cancer drugs. J Biol Chem 2014; 289:28019-39; PMID:25138213; https://doi.org/10.1074/jbc.M114.578229
- Infante P, Alfonsi R, Botta B, Mori M, Di Marcotullio L. Targeting GLI factors to inhibit the Hedgehog pathway. Trends Pharmacol Sci 2015; 36:547-58; PMID:26072120; https://doi.org/10.1016/j.tips.2015.05.006
- Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol 2006; 7:517-28; PMID:16829982; https://doi.org/10.1038/nrm1963
- Bressenot A, Marchal S, Bezdetnaya L, Garrier J, Guillemin F, Plenat F. Assessment of apoptosis by immunohistochemistry to active caspase-3, active caspase-7, or cleaved PARP in monolayer cells and spheroid and subcutaneous xenografts of human carcinoma. J Histochem Cytochem 2009; 57:289-300; PMID:19029405; https://doi.org/10.1369/jhc.2008.952044