ABSTRACT
Although the molecular therapeutics targeting key biomarkers such as epithelial growth factor receptor (EGFR), PI3K/AKT/mTOR, and vascular endothelial growth factor (VEGF) shows some success in clinical trials, some internally existing challenges in endothelial cancer biology hinder the drug effects. One of the major challenges stems from cancer stem cell-derived drug resistance. CD133 positive cells are well believed as cancer stem cells (CSC) in endometrial cancers and NOTCH pathway plays a critical role in retaining CD133+ cells by promoting CSC self-renewal and chemoresistance. Here, we initiated a therapeutic strategy to improve effects of EGFR inhibition by targeting NOTCH pathway of CD133+ cells in endometrial cancers. We first detected and purified the CD133+ cell fraction in endometrial cancer cell line Ishikawa (IK), and validated activation of NOTCH pathway in the CD133+ cells that have higher proliferation rate and lower apoptosis rate, comparing to CD133- cells. Results of nude mouse xenograft experiments further demonstrated CD133+ cells retain higher tumorigenesis capacity than CD133- cells, indicating their tumor-initiating property. Last, we applied both NOTCH inhibitor DAPT and EGFR inhibitor AG1478 treatment on endometrial cancer lines IK and HEC-1A and the results suggested improvement effects of the combination therapy compared to the treatments of DAPT or AG1478 alone. These findings indicated targeting NOTCH pathway in CD133+ cells, combining with EGFR inhibition, which provides a novel therapeutic strategy for endometrial cancer diseases.
Introduction
Endometrial cancer (EC) is the sixth most common cancer in women worldwide and one of the major mortality sources of gynecological malignancies in Chinese women. Currently, the molecular therapeutics has not been well established inEC.Citation1 Although the clinical trials of the molecular therapies in EC made some achievements, one of the major limitations in current therapeutics is primary/acquired drug resistance that is partially attributed to a small population of cancer-initiating cells, referred as cancer stem cells (CSCs) in malignant tumors. CSCs possess some common properties of normal stem cells such as a great advantage of cell proliferation, self-renewal and differentiation and give rise to heterogeneous tumor clones.Citation2 Another important property of CSCs is that they are believed to be the cell sources developing drug resistance due to high expression levels of specific ABC drug transporters.Citation3 CD133 is a widely accepted bio-marker for tumor-initiating cells in various cancer types including EC and CD133+ cells exhibited significant resistance to chemotherapy.Citation4
Studies indicate that activation of NOTCH signaling pathway is a common event in CD133+ cells and inactivating NOTCH pathway can sensitize CD133+ cells to chemotherapy.Citation5 In mammals, NOTCH (1-4) signals are activated through binding to 5 ligands (Jagged-1, -2, and Delta-like-1, -3, and -4), and this leads to sequential proteolytic cleavages and the nuclear translocation of the intracellular domains of Notch receptors (NICDs) that regulates downstream NOTCH-dependent transcription. Disordered NOTCH signaling can promote tumorigenesis in variant types of cancers by increasing cancer stem cell activities. Therefore, NOTCH pathway appears to be a promising therapeutic target for CSCs. The γ-secretase complex is critically necessary for NOTCH pathway activation and inhibitors of γ-secretase such as DAPT have been used to successfully block NOTCH signaling both in vitro and in vivo .Citation6
We report CD133+ cells can be the tumor-initiating cells in EC by testing this special groups in lines IK and HEC-1A. Our data further indicated combining EGFR inhibition with NOTCH pathway suppression to target both CD133+ and CD133- cells can be a novel therapeutic strategy for EC.
Materials and methods
Cell lines and cell culture
Endometrial cancer HEC-1A and IK cell line were used in the present study. Both cell lines were obtained from Shanghai Institute of Cell Biology, Chinese Academy of Sciences and were cultured in DMEM medium supplemented with 10% fetal bovine serum (Life Technologies Corporation, Carlsbad, CA, USA), and culture condition was 5% CO2 at 37°C.
Isolation and culture of CD133-expressing cells
CD133+ IK cells were evaluated using the FACS. Totally 100 μl 10 mg/L FITC-conjugated CD133 antibodies (Miltenyi Biotec, German) were used to incubate IK cells at 4°C for 2 h, than FACS were used to calculate the CD133+ IK cells after washed by PBS twice. Finally, CD133+ IK cells were obtained using the CD133 MicroBead Kit (Miltenyi Biotec, German) in combination with the autoMACSR Separator. FITC-conjugated CD133 antibodies were used to evaluate the efficiency of magnetic separation by flow cytometry. CD133+ and CD133– cell populations were re-suspended in DMEM supplemented with 10% fetal bovine serum and used for the following assays.
Cell proliferation assay
The cell proliferation assay was done by the MTT method, which was performed by plating CD133+ and CD133– cells in 96-well plate at a density of 4000 cell/well. Each group contained 5 wells. Then cells were stained with 100 μl sterile MTT dye (0.5 mg/ml, Sigma) for 4h at 37°C, dissolved with 150μl DMSO. Spectrometric absorbance at a wavelength of 570nm was measured on a microplate reader (Bio-Rad) and the cell growth curve was obtained using OD570 as ordinate axis on days 3, 5 and 7.
Limiting dilution assay
The Limiting dilution assay was conducted as previously described,Citation7 with minor modifications. CD133+ and CD133–cells were plated at a final density of one cell/well in 96-well plates .Citation8 Colonies (each one comprising more than 30 cells) were counted at day 21 and expressed as the percentage of the number of wells plated.
Flow cytometric apoptosis assays
Apoptosis was detected by using Annexin V-FITC apoptosis detection kit (Biosea, Beijing, China) following the procedure of the manufacturer. Cells incubated with Annexin V-FITC and propidium iodide (PI) for 15 min at room temperature were subjected to Flow Cytometry (Ex = 488 nm, Em=635 nm) in an hour and analyzed using BD FACS Diva 8.0 software. Annexin V-positive cells were regarded as apoptosis.
Immunofluorescence
Immunofluorescence was performed as previously described .Citation9 In brief, coverslip cell cultures were fixed in 3.7% paraformaldehyde, permeabilized, and blocked with 1% BSA. Cell slides were incubated with anti-Notch1 antibody (1:200, C-20; Santa Cruz Biotechnology), then with FITC-conjugated secondary antibodies (Invitrogen). The nuclei were detected with DAPI. Coverslip cultures were mounted in inverted positions on glass slides and analyzed in confocal microscope.
Western blotting
Protein was extracted by RIPA buffer (20 mM Tris PH7.5, 150 mM NaCl, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM EDTA, 1%Na3VO4, 0.5 μg/ml leupeptin, 1 mM phenylmethanesulfonylfluoride (PMSF)) and Western blot was performed according to the procedure of Towbin et al. (1979). In brief, after SDS-PAGE was performed, the PVDF membrane was blocked and incubated with antibodies followed by horseradish peroxidase-conjugated antibodies. Detection was performed by enhanced chemiluminescence (ECL) using a Western blotting luminological reagent (Santa Cruz Biotechnology) according to the manufacturer's instructions. GAPDH was used as a reference protein.
Effect of EGFR and Notch1 inhibitor on IK-CD133+ cells and Notch1 pathway
IK-CD133+ cells and HEC-1A cells were cultured as normal and pre-treating both cells with 20ng/ml EGF for 2 h, then treated the cells in the following manners. The first group treated the cells by EGFR inhibitor AG 1478 at 5.0 μmol/L, 10 μmol/L, 15 μmol/L for 24 h. The second group treated the cells with Notch1 inhibitor DAPT at 1.0 μmol/L, 5 μmol/L, 10 μmol/L for 24 h. MTT was used the pick the optimal conditions. The third group, treated the cells with the optimal concentration of AG 1478 together with DAPT. Western blot was used to detect the expression of notch1 pathway associated proteins. Cell apoptosis assay was done as described before.
For the cell cycle assay, 1×106 cells were harvested and fixed by 75% ethanol for 20 minutes at 4°C. After a thorough rinse, the cells were digested with RNase (20 µg/ml) and stained with propidium iodide (50 µg/ml) for 40 minutes a`t 37°C. Rate of cell apoptosis and DNA content at each cell cycle stage was determined by flow cytometry within an hour.
For the cell invasion assay, 2 × 105 cells were seeded at the upper compartment of the Transwell chambers (Coster, USA. 0.5ml supernatant of human NIH3T3 was added to the bottom chamber. After incubation for 12h at 37°C, cells invading the lower surface of the membrane were fixed with methanol and stained with haematoxylin and eosine. The mean of cell number in 5 fields randomly selected was used to represent cell invasion ability.
Xenograft tumor studies in nude mice
Female BALB/c nude mice (∼20 gram in weight, 4-6 weeks old) were obtained from Vitalriver (Beijing, China) and housed in a pathogen-free facility according to the guidelines. IK-CD133+ and IK-CD133- cells were injected subcutaneously into the left and right dorsal region of the mice. The tumor size and mice body weight were monitored every week. The longest and shortest diameter of the tumor was noted as L and W. The tumor volume was calculated using the equation: V= (LW2)/2. After 4 weeks, the mice were killed and the tumors were observed with whether there is a distant metastasis.
Statistical analysis
Statistical analysis was completed with software SPSS 13.0 (IBM, IL). The data were presented as mean ± SD of 3 independent experiments and compared using Student's t-test and twoone-way ANOVA. P < 0.05 means significant difference.
Results
IK-CD133+ cell isolation and purification
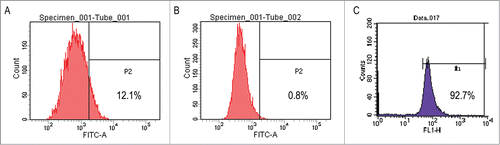
Single-cell suspensions from IK cells were flow cytometrically analyzed for the expression of the CD133 antigen. The frequency of CD133-expressing cells with FITC-labeled group was 12.1% and was significantly different from the values obtained in control group which was 0.80% (P < 0.05) (). The data showed that IK cells could be used for the isolation of CD133 positive cells. depicts the analysis of CD133+ cells after MACSR Cell Separation of the preparation shown in using the CD133 MicroBead Kit, yielding a high purity of CD133+ cells up to 92.7%, which was significant different from the control, which could be used for the following cell signal pathway study.
IK-CD133+ cell culture and identification
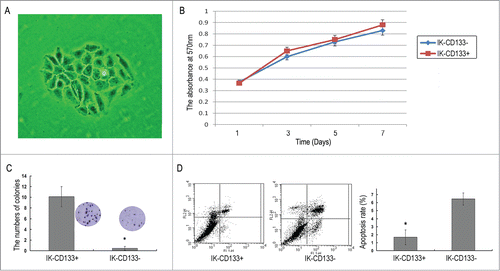
The CD133+ cells were separated by FACS from the CD133- cells and both fractions were seeded in a DMEM medium containing 10% FBS on collagen-coated 24-well plates (2 cm2). After cultured for 2 to 4 weeks, a great number of suspended cell masses gradually grew larger and became sphere-shaped in the medium (). The MTT assay showed that the proliferation ability of CD133+ cells and CD133- cells were increased in a day dependent manner (p < 0.05), but there was no statistic difference between the CD133+ and CD133- groups (). The limiting dilution experiments were performed in order to document the ability of isolated CD133+ cells to form colonies. CD133+ cells colonies gave up to (10.13 ± 1.89)%, while the colonies of CD133- population was (0.43 ± 0.35)% after cultured for 21 d, which had significant difference(P < 0.05) (). Also, FACS showed the difference of apoptosis between the CD133+ cells and CD133- cells, the apoptosis rate of them are (1.67 ± 0.92) % and (6.45 ± 0.75) %, respectively(P<0.05) ().
Location and expression of Notch1 in IK-CD133+ cells
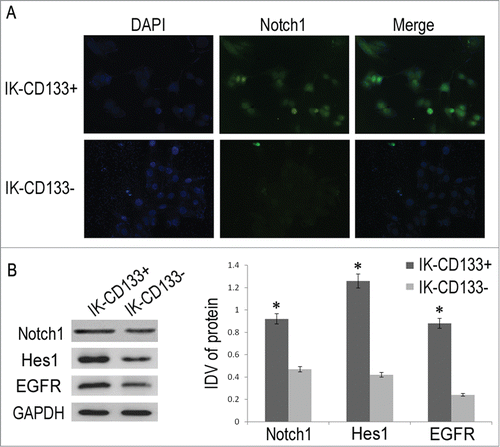
Immunofluorescence result showed green fluorescence signal in both IK-CD133+ and IK-CD133- cells, with notch1 expressed mainly in the cytoplasm and the nucleus (). However, the signal in the IK-CD133+ cells was stronger than that in the IK-CD133- cells. These results indicated that IK-CD133+ cell expressed higher notch1 protein than in the IK-CD133- cells. Following the western blot confirmed the result of immunofluorescence that notch1 expression was higher in the IK-CD133+ cells than that in the IK-CD133- cells, which was significant difference with p < 0.05, although both bands were shown in the results ().
AG1478 and DAPT inhibit IK-CD133+ cells proliferation
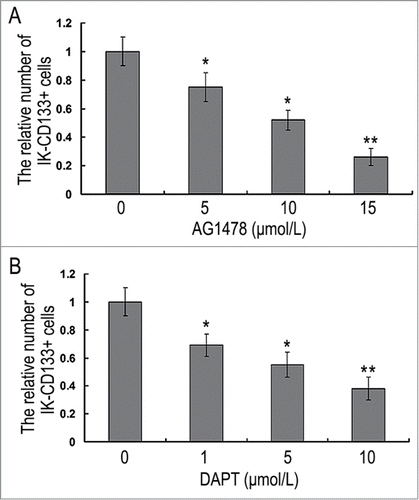
MTT assay showed significant proliferation ability inhibition in IK-CD133+ cells and HEC-1A cells when treated with 5 μmol/L, 10 μmol/L, 15 μmol/L EGFR inhibitor AG1478 for 24h. The absorbance at a wavelength of 570 nm in IK-CD133+ cells were 0.75 ± 0.10, 0.52 ± 0.07 and 0.26 ± 0.06 when compared with the control groups, which was significant difference (P < 0.05). As for the absorbance of 15μmol/L AG1478 treatment group was less than 0.3, we chose 10 μmol/L as an optimal condition (). Also, notch1 inhibitor DAPT treatment IK-CD133+ cells and HEC-1A cells showed the same tendency with concentration of 1 μmol/L, 5 μmol/L and 10 μmol/L. Finally, 10 μmol/L was chosen to be the optimal condition ().
AG1478 and DAPT effects the Notch1 pathway and IK-CD133+ cells behaviors
After treatment with AG1478 and DAPT, flow cytometry was used to identify changes in apoptosis and cell cycle profiles of IK-CD133+ cells and HEC-1A cells. With comparison of control groups to 10 μmol/L AG1478, 10 μmol/L DAPT, and 10 μmol/L AG1478+ 10μmol/L DAPT treatment ones, the apoptotic rate of both IK-CD133+ cells and HEC-1A cells increased with G1 phase cell cycle block. The proportion of IK-CD133+ cells apoptosis rate increased from (18.38 ± 1.52)%, (26.25 ± 1.75)% to (38.76 ± 2.13)%, the proportion of G1 stage cells increased from (48.38 ± 2.82)%, (59.25 ± 3.17)% to (83.69 ± 3.13)%, which were significant difference with P < 0.05 ().
Figure 5. The apoptotic rate (A), the proportion of G1 stage cells (B), the numbers of trans-membrane cells (C) and the expression of HES-1, NICD, Notch1, pMAPK and pAKT protein (D) in IK-CD133+ cells and HEC-1A cells treated with 10 μmol/L AG1478, 10 μmol/L DAPT and 10μmol/L AG1478+ 10 μmol/L DAPT. * P < 0.05, ** P < 0.01.
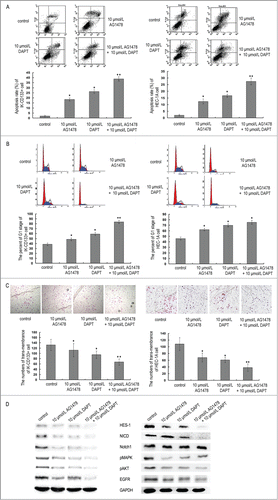
Meanwhile, Transwell (Coster) results showed a decrease of trans-membrane IK-CD133+ cells and HEC-1A cells undergoing 10 μmol/L AG1478, 10 μmol/L DAPT, and 10 μmol/L AG1478 + 10 μmol/L DAPT treatment compared to control groups after 24 h. The trans-membrane IK-CD133+ cells numbers were 112 ± 25, 95 ± 14 and 67 ± 12 to 132 ± 21 after treatment (). ANOVA analysis showed that there was a statistically significant difference between the treatment groups and control groups (P < 0.05).
Using non-treatment IK-CD133+ cells and HEC-1A cells as normal controls, the results of Notch1, NICD, HES-1, pMAPK and pAKT gene expression level of protein were normalized to the housekeeper gene (GAPDH), and we found that all these genes expressed down-regulated in 10 μmol/L AG1478, 10 μmol/L DAPT, and 10 μmol/L AG1478 + 10 μmol/L DAPT treatment groups when compared with control groups (P < 0.05). Furthermore, only a faint band of almost all the genes could be seen in the 10 μmol/L AG1478 + 10 μmol/L DAPT treatment group in which these genes were downregulated significantly with p < 0.01. These results showed that the EGFR inhibitor AG1478 and Notch1 inhibitor DAPT could block the Notch1 signal pathway, and this effect was significant when treated IK-CD133+ cells together with AG1478 and DAPT ().
IK-CD133+ cells promote tumor growth in vivo
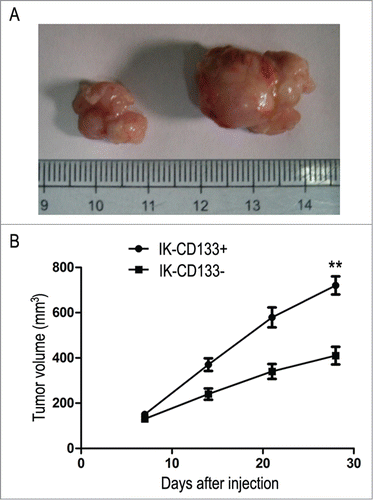
Subsequently, we evaluated the therapeutic effect of IK-CD133+ cells in vivo by monitoring the tumor formation in established IK-CD133+ cell BALB/c mouse model. As a result, most mice in IK-CD133+ cell inoculation group had much bigger tumor formation, but without any distant metastasis (P < 0.05) ().
Figure 6. (A) The nude mice transplanted tumor established from IK-CD133+ cells and IK-CD133- cells in vivo. Left tumor is derived from IK-CD133+ cells, right tumor IK-CD133- cells. (B) Tumor growth curves were measured after the injection of IK-CD133+ cells and IK-CD133- cells. Tumor volume was calculated every 5 d. **P < 0.01 vs. IK-CD133- cells (n = 8).
Discussion
Cancer stem cells (CSC) are increasingly becoming the focus of many tumor biomedical researches. They are rare, undifferentiated cells contributing to tumor growth and presumably the inevitable recurrence, by virtue of their malignant proliferation, self-renew and resistance to therapy .Citation10 Compared with normal cancer cells, CSCs are considered to be more tumorigenic by causing relapse and metastasis of tumor cells .Citation11 Thus, it is critical for cancer therapy to target this special population of cancer cells. CD133 is one of the common useful markers for isolation of CSC cells, in ovarian, breast, pancreas, brain, colon, and prostate tumors, et al ,Citation12 responsible for initiation, maintenance, and progression of tumors. In this study, we successfully isolated and purified the CD133+ cells by MACS, together with the flow cytometry technologies. Cancers stem cell properties, including clonogenic and proliferative potentials were confirmed by in vitro studies. As for in vitro studies can alter the cellular physiology in some circumstances which forcing non-stem cells to behave like stem cells, we identified the CSCs characterization in vivo which made the isolation and following assays more considerable.
As we all know, CSCs behave like normal stem cells and are capable of comprising many specific characteristics, such as drug transporter expression, apoptosis inhibition and DNA methylation like the stem cells in normal tissues .Citation13,14 These features lead to the prevalence of CSCs in chemotherapy resistance, the major cause of treatment failure .Citation15 In this study, we observed that IK CD133+ cells were more resistant to chemotherapy than CD133- cells in the same AG1478 and DAPT concentrations. The combined treatment of AG1478 and DAPT on the IK CD133+ cells were more effective than that of their individual monotherapies. Moreover, the improvement effects could be seen in the HEC1A cells.
The Notch pathway is a well-established mediator of cell–cell communication that plays a critical role in stem cell survival, self-renewal, cell fate decisions, tumorigenesis, invasion, metastasis, and drug resistance in a variety of cancers .Citation16 It has been referred to as the Notch-survival pathway, as it promotes cell growth and proliferation and inactivating NOTCH pathway can sensitize CD133+ cells to chemotherapy .Citation17 As CSCs exhibit similar properties to normal stem cells, we suggest that Notch pathway may also play an important role in EC tumorigenesis. Notch signaling is altered in many cancers, and the up-regulation of the Notch pathway may be a frequent event in EC cancers, which may enhance the invasive properties of endometrial carcinomas .Citation18,19 However, some research has demonstrated contradictory results, which suggested that the Notch pathway has tumor-suppressive function rather than oncogenic role in human EC cells .Citation20 As an example, the downregulation of Notch signaling inhibits the growth, migration, and invasiveness of cancer cells and induces cell apoptosis .Citation21 In the current study, we found that Notch signaling was expressed in both CD133+ and CD133- cells. However, compared with CD133- cells, the expression in CD133+ cells was higher. We blockaded the Notch pathway in bath CD133- and CD133+ cells using both AG1478 and DAPT, as anticipated, the Notch1, NICD, HES-1, pMAPK and pAKT proteins were significantly deregulated and the IK CD133+ cells showed observed chemo-resistance with promoted apoptosis and significant G1 phase cell arrest. As well, this effect could be seen in HEC1A cells.
All these results dedicated that cancer treatment can be made more effective by using a combination of drugs in targeting cancer stem cells.
Disclosure of potential conflicts of interest
All authors announce that there are not competing interests between them.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Funding
Reference
- Dong P, Kaneuchi M, Konno Y, Watari H, Sudo S, Sakuragi N. Emerging Therapeutic Biomarkers in Endometrial Cancer. Bio Med Res Int 2013; 2013:1-11; PMID:23819113; https://doi.org/10.1155/2013/130362
- Nakamura M, Kyo S, Zhang B, Zhang X, Mizumoto Y, Takakura M, Maida Y, Mori N, Hashimoto M, Ohno S, Inoue M. Prognostic impact of CD133 expression as a tumor-initiating cell marker in endometrial cancer. Hum Pathol 2010; 41(11):1516-29; PMID:20800872; https://doi.org/10.1016/j.humpath.2010.05.006
- Zhao D, Mo Y, Li MT, Zou SW, Cheng ZL, Sun YP, Xiong Y, Guan KL, Lei QY. NOTCH-induced aldehyde dehydrogenase 1A1 deacetylation promotes breast cancer stem cells. J Clin Investig 2014; 124(12):5453-65; PMID:25384215; https://doi.org/10.1172/JCI76611
- Dean M, Fojo T, Bates S. Tumor stem cells and drug resistance. Nat Rev Cancer. 2005; 5 (4): 275-84; PMID:15803154; https://doi.org/10.1038/nrc1590
- Friel AM, Zhang L, Curley MD, Therrien VA, Sergent PA, Belden SE, Borger DR, Mohapatra G, Zukerberg LR, Foster R, Rueda BR. Epigenetic regulation of CD133 and tumorigenicity of CD133 positive and negative endometrial cancer cells. Reproductive Biol Endocrinol 2010; 8:147-60; PMID:21122138; https://doi.org/10.1186/1477-7827-8-147
- Liu J, Mao Z, Huang J, Xie S, Liu T, Mao Z. Blocking the NOTCH pathway can inhibit the growth of CD133-positive A549 cells and sensitize to chemotherapy. Biochem Biophys Res Commun 2014;444(4):670-5; PMID:24502949; https://doi.org/10.1016/j.bbrc.2014.01.164
- Grenman R1, Burk D, Virolainen E, Buick RN, Church J, Schwartz DR, Carey TE. Clonogenic cell assay for anchorage-dependent squamous carcinoma cell lines using limiting dilution. Int J Cancer 1989; 44(1):131-6; PMID:2744882; https://doi.org/10.1002/ijc.2910440123
- Ferrandina G1, Bonanno G, Pierelli L, Perillo A, Procoli A, Mariotti A, Corallo M, Martinelli E, Rutella S, Paglia A, et al. Expression of CD133-1 and CD133-2 in ovarian cancer. Int J Gynecol Cancer 2008; 18(3):506-14; PMID:17868344; https://doi.org/10.1111/j.1525-1438.2007.01056.x
- Fu WN, Guo Y, Huang DF, Shang C, Sun KL. Novel partners of S100A8 identified in laryngeal cancer cell lines. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2007; 24(3): 266-70; PMID:17557234
- Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer 2003; 3(12):895-902; PMID:14737120; https://doi.org/10.1038/nrc1232
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003; 100(7):3983-8; PMID:12629218; https://doi.org/10.1073/pnas.0530291100
- Hubbard SA, Friel AM, Kumar B, Zhang L, Rueda BR, Gargett CE. Evidence for cancer stem cells in human endometrial carcinoma. Cancer Res 2009; 69(21):8241-8; PMID:19843861; https://doi.org/10.1158/0008-5472.CAN-08-4808
- Besançon R, Valsesia-Wittmann S, Puisieux A, Caron de Fromentel C, Maguer-Satta V. Cancer stem cells: the emerging challenge of drug targeting. Curr Med Chem 2009; 16(4):394-416; PMID:19199913;https://doi.org/10.2174/092986709787315531
- Zhou J, Zhang Y. Preclinical development of cancer stem cell drugs. Expert Opin Dryg Discov 2009; 4(7):741-52; PMID:23489167; https://doi.org/10.1517/17460440903002059
- Nadal R1, Ortega FG, Salido M, Lorente JA, Rodríguez-Rivera M, Delgado-Rodríguez M, Macià M, Fernández A, Corominas JM, García-Puche JL, et al. CD133 expression in circulating tumor cells from breast cancer patients: potential role in resistance to chemotherapy. Int J Cancer 2013; 133(10):2398-407; PMID:23661576; https://doi.org/10.1002/ijc.28263
- Liu J, Mao Z, Huang J, Xie S, Liu T, Mao Z. Blocking the Notch Pathways can inhibit the growth of CD133- positive A549 cells and sensitize to chemotherapy. Biochem Biophys Res Commun 2014; 444(4):670-5; PMID:24502949; https://doi.org/10.1016/j.bbrc.2014.01.164
- Mitsuhashi Y1, Horiuchi A, Miyamoto T, Kashima H, Suzuki A, Shiozawa T. Prognostic significance of Notch signalling molecules and their involvement in the invasiveness of endometrial carcinoma cells. Histopathology 2012; 60(5):826-37; PMID:22348356; https://doi.org/10.1111/j.1365-2559.2011.04158.x
- Chen Y1, Li D, Liu H, Xu H, Zheng H, Qian F, Li W, Zhao C, Wang Z, Wang X. Notch-1 signaling facilitates survivin expression in human non-small cell lung cancercells. Cancer Biol Ther 2011; 11(1):14-21; PMID:20962575; https://doi.org/10.4161/cbt.11.1.13730
- Sasnauskienė A, Jonušienė V, Krikštaponienė A, Butkytė S, Dabkevičienė D, Kanopienė D, Kazbarienė B, Didžiapetrienė J. NOTCH1, NOTCH3, NOTCH4 and JAG2 protein levels in humanendometrial cancer. Medicina (Kaunas) 2014; 50(1):14-8; PMID:25060200; https://doi.org/10.1016/j.medici.2014.05.002
- Ji X, Wang Z, Geamanu A, Sarkar FH, Gupta SV. Inhibition of cell growth and induction of apoptosis in non-small cell lung cancer cells by delta-tocotrienol is associated with notch-1 down-regulation. J Cell Biochem 2011; 112(10):2773-83; PMID:21598300; https://doi.org/10.1002/jcb.23184
- Jonusiene V1, Sasnauskiene A, Lachej N, Kanopiene D, Dabkeviciene D, Sasnauskiene S, Kazbariene B, Didziapetriene J. Down-regulated expression of Notch signaling molecules in human endometrial cancer. Med Oncol 2013; 30(1):438; PMID:23315219; https://doi.org/10.1007/s12032-012-0438-y