ABSTRACT
Background: Peripheral primitive neuroectodermal tumor isolated in the heart, presenting as a primary cardiac tumor is considered as extremely rare.
Methods: We present a 53-year-old Chinese female with a cardiac tumor which was discovered by CT.
Results: A hypo-intense tumorous mass was shown extending from the left ventricle by Cardiac CT, and fused FDG positron emission tomography demonstrated no other abnormal FDG active lesions in the body. We performed a total resection surgery of the tumor subsequently and the patient recovered well and discharged from hospital 6 d after surgery.
Conclusion: The pathological diagnosis was primary cardiac peripheral primitive neuroectodermal tumor. No tumor recurrence was shown by echocardiography during the 24 months follow-up visits.
Introduction
Cardiac tumors are not common and they are often diagnosed postmortem.Citation1 Peripheral primitive neuroectodermal tumor (pPNET) usually occurs in peripheral nervous system or soft tissue and bone,Citation2 while isolated in the heart is considered as extremely rare. Here we present a case of pPNET isolated in the heart.
Case report
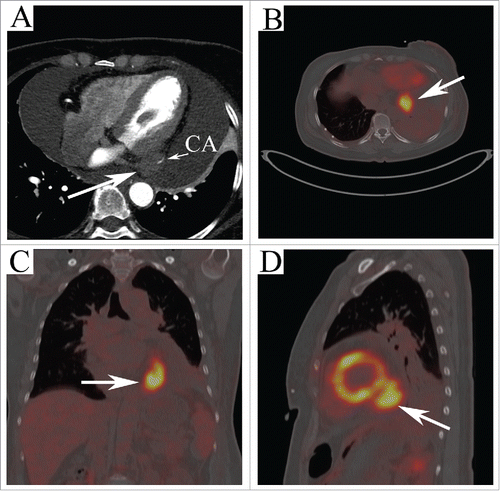
A 53-year-old woman presented with 2 weeks history of progressive shortness of breath was referred to our cardiovascular department to remove pericardial effusion being detected with trans-thoracic echocardiography. The pulse and blood pressure of her was 95/min and 112/82mmHg, respectively on the physical examination. Murmurs or pericardiac friction sounds were not audible on cardiac examination. Cytological examination of the fluid showed various blood constituents but no atypical cells. Computed tomography was obtained and 4 chamber view () depicting a hypo-intense tumorous mass extending from the left ventricle. In order to detect whether the tumor extend outside the heart, fused FDG positron emission tomography (PET)-CT imaging () was performed and demonstrated a hypermetabolic soft tissue mass (40 mm × 30 mm) located in the posterior-inferior of the left ventricle with severe focal FDG activity (SUV max 9.0). Three-dimensional reconstruction of PET-CT conformed the diagnosis and demonstrated no other abnormal FDG active lesions in the body (see supplementary video).
Figure 1. (A) Four chamber view of CT showing a hypo-intense tumorous mass extending from the left ventricle with the coronary artery infiltrating (arrow). FDG PET-CT shows a high uptake lesion (40 mm × 30 mm) located in the posterior-inferior of the left ventricle (arrows in B, (C) and D) with severe focal FDG activity (SUV max 9.0).
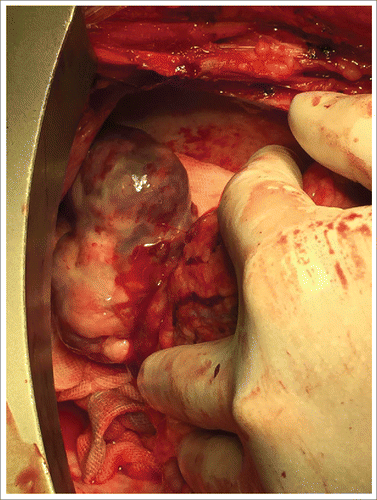
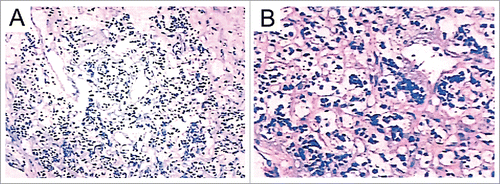
Subsequently, a totally resection of the mass was performed () via a median sternotomy with cardiopulmonary bypass (CPB). In order to close the hollow of the tumor, a ‘sandwich’ method with 2 sheets of felt and 3–0 prolene was used. CPB and aortic cross-clamp times were 55 and 30 minutes, respectively. The patient was sent to intensive care unit (ICU), he had recovered well after surgery and discharged from hospital 6 d after. Pathology showed small cell malignant tumor and further confirmed the diagnosis of pPNET with CD99 and NSE, 2 markers for pPNET, intense positive identification ().
Discussion
Primary malignant cardiac tumors is rare but seldom show invasive or metastasis behavior.Citation3 Symptoms depending on the cardiac structures involved. Pericardial involvement can lead to effusion and tamponade.Citation4 Primitive neuroectodermal tumor usually occurs in central and peripheral nervous system or soft tissue and bone,Citation2 while isolated in the heart is considered as extremely rare. Kath R et al.Citation5 first reported a case of cardiac pPNET, and since then there are no more reports of such cases.
Echocardiography is usually the first choice to discover cardiac tumors. Computed tomography, which can clearly show the size, location, modality, amount and the pericardical structure, was widely used. While PET was the most useful imaging modality in monitoring disease response.Citation6 The final diagnoses was depending on the results of pathology and CD99 accompanied by NSE is the currently most accepted immunohistochemical markers for pPNET.Citation5,7
Routine surgical pathology allow the diagnosis of most cases of PNET, but molecular analysis can be helpful when morphology is not conclusive or tumors present in unusual clinical settings. Molecular assays for specific chromosomal changes, such as those found in PNET, are attractive for several reasons. A particularly appealing advantage for diagnostic molecular studies is that, despite distortions of histologic patterns or the absence of histologic or immunohistochemical evidence of differentiation, the basic molecular genetic alterations are present in tumor cells and molecular analysis may permit greater confidence in a specific diagnosis.Citation8
The great majority of PNET express CD99 (MIC2), a 32-kDa membrane glycoprotein, is encoded by a pseudoautosomal gene on the X and Y chromosomes involved in cell adhesion, migration, and apoptosis. Although immunohistochemical detection of CD99 expression localized on membrane is a sensitive marker for the PNET, many other tumors, even many normal tissues, are also immunoreactive with anti-CD99 antibodies in some cases which make it lack of specificity.Citation9 The diagnosis of PNET has been largely a process of exclusion.
Molecular heterogeneity in the PNET may contribute to the prognosis. Gene fusions in PNET show molecular variability, with more than 18 different structural possibilities. There are 2 types of variability: the fusion partner and the break point locate within the genes. Patients with localized tumors expressing the most common chimeric transcript compared with other fusion types has been reported to had a better outcome. This increases the chance that heterogeneity in chimeric transcript structure may reliably define clinically distinct risk groups.Citation8
Around 85% of tumors diagnosed as PNET harbor the translocation t(11;22)(q24;q12). At the molecular genetic level, the chromosome 22q12 breakpoints are clustered within a single gene designated EWS and the chromosome 11q24 breakpoints are within a gene called FLI1 (a gene homologous to the Friend leukemia virus integration site 1). In this rearrangement, the distal portion of FLI1 is juxtaposed to the proximal portion of EWS, thereby creating a functional EWS-FLI1 fusion gene. The encoded protein contains the amino-terminal domain of EWS and the C-terminal region of FLI1, forming a totally new protein with a distinct function. In the remaining PNET, other chromosomal translocations are present that result in an analogous fusion of EWS to genes with structural homology to FLI1. These genetic changes are now considered a specific diagnostic feature of these tumors, and the fusion gene products are believed to play an important role in PNET development and biology. The EWS gene was identified as the site disrupted by the chromosomal translocation t(11;22)(q24;q12) found in PNET. It encodes a widely expressed 656-amino acid protein of unknown function. The C-terminal domain, which is replaced by tumor-specific translocations, contains an RNA recognition motif.Citation10
The FLI1 gene on 11q24 was the first EWS translocation partner identified in the PNET. It is a member of a large family of DNA-binding transcription factors which play an important role in cellular development, proliferation, and tumorigenesis. The family members are all presented with a highly conserved 85-amino acid domain termed the erythroblastosis virus-transforming sequence (ETS) domain. In vertebrates, deregulation of ETS-domain protein activity often leads to tumorigenesis. ETS family members have been shown to interact with other nuclear proteins and often act as part of a larger protein complex that helps to enhance promoter specificity, modulates transcriptional regulation, and establish linkage to signal transduction pathways.
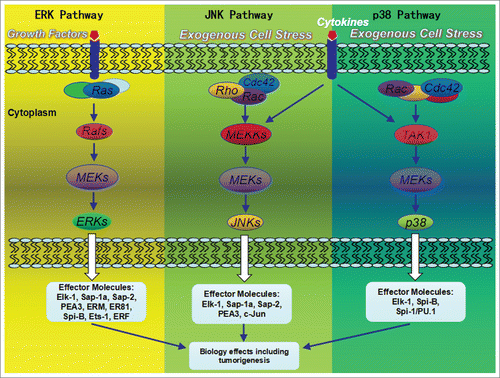
Differential activation of transcription factors by signal transduction pathways such as the mitogen activated protein kinase (MAPK) pathways play a major role in the regulation of gene expression.Citation11 Mitogenic stimuli are transduced through pathways that terminate in the phosphorylations of ERK-1 and ERK-2 MAPKs, whereas the exogenous cellular stresses such as IJV irradiation may lead to the activation of the stress activated kinases SAPK/JNK and p38.Citation11 A combination of biochemical, molecular biological and genetic approaches have recently demonstrated that the activation of MAPK cascades leads to changes in the activity of many ETS-domain transcription factors ()Citation12 several of which have been demonstrated to be direct MAPK targets.
Figure 4. MAP kinase pathways and their mammalian ETS-domain transcription factor targets. ERK, p38 and JNK pathways are activated by distinct upstream kinases in response to a wide variety of extracellular signals such as growth factors, cytokine factors, and stress. Various substrates in the cytoplasma and the nucleus of the cell were activated, including transcription factors. These downstream targets control cellular responses.
There are at present no adequate data on optimal treatment of pPNET in the heart.Citation5 Surgery is required in symptomatic cases and is a satisfactory method for the treatment. To date, there have been no evidence-based studies on whether post-operative radiotherapy and/or chemotherapy is necessary for the treatment of primary pericardial sarcomas.Citation13 Follow-up is recommended to identify any recurrence, even though the rate of recurrence is unknown.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementarymaterials.zip
Download Zip (2.7 MB)References
- Fan J, Liao X, Zhou X. A case report of primary cardiac capillary hemangioma. Cancer Biol Ther 2016; 17(1):11-3; PMID:26650115; http://dx.doi.org/10.1080/15384047.2015.1109391
- Wang J, Wang W, Li Y, Jin B, Yu M, Liu W, Yao S, Liao Y, Ouyang C. A Primary Primitive Neuroectodermal Tumor Arising from Left Subclavian Vein and Extending along Left Brachiocephalic Vein and Superior Vena Cava into Right Atrium. Ann Vasc Surg 2015; 29(4):839.e13-20; PMID:25725277; http://dx.doi.org/10.1016/j.avsg.2014.12.007
- Bekkers SC, van Garsse LA, Jansen RL, van Suylen RJ. Primary monophasic mediastinal, cardiac and pericardial synovial sarcoma: a young man in distress. Neth Heart J 2007; 15(6):226-8; PMID:17612689; http://dx.doi.org/10.1007/BF03085986
- van der Zee PM, van Schuppen J, van de Brink RB. Right sided invasive metastatic thymoma of the heart. Neth Heart J 2011; 19(9):392-4; PMID:21487745; http://dx.doi.org/10.1007/s12471-011-0114-4
- Kath R, Krack A, Schneider C, Katenkamp D, Höffken K. Cardiac manifestations of peripheral primitive neuroectodermal tumor (pPNET): a rare case. Dtsch Med Wochenschr 2000; 125(40):1192-4; PMID:11075251; http://dx.doi.org/10.1055/s-2000-7698
- Mato AR, Morgans AK, Roullet MR, Bagg A, Glatstein E, Litt HI, Downs LH, Chong EA, Olson ER, Andreadis C, Schuster SJ. Primary cardiac lymphoma: Utility of multimodality imaging in diagnosis and management. Cancer Biol Ther 2007; 6(12):1867-70; PMID:18075298; http://dx.doi.org/10.4161/cbt.6.12.5166
- Tsokos M, Alaggio RD, Dehner LP, Dickman PS. Ewing sarcoma/peripheral primitive neuroectodermal tumor and related tumors. Pediatr Dev Pathol 2012; 15(1 Suppl):108-26; PMID:22420726; http://dx.doi.org/10.2350/11-08-1078-PB.1
- de Alava E, Gerald WL. Molecular biology of the Ewing's sarcoma/primitive neuroectodermal tumor family. J Clin Oncol 2000; 18:204-13; PMID:10623711; http://dx.doi.org/10.1200/jco.2000.18.1.204
- Tsokos M, Alaggio RD, Dehner LP, Dickman PS. Ewing sarcoma/peripheral primitive neuroectodermal tumor and related tumors. Pediatr Dev Pathol 2012; 15:108-26; PMID:22420726; http://dx.doi.org/10.2350/11-08-1078-PB.1
- Ohno T, Ouchida M, Lee L, Gatalica Z, Rao VN, Reddy ES. The EWS gene, involved in Ewing family of tumors, malignant melanoma of soft parts and desmoplastic small round cell tumors, codes for an RNA binding protein with novel regulatory domains. Oncogene 1994; 9:3087-97; PMID:8084618
- Sharrocks AD. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol 2001; 2:827-37; PMID:11715049; http://dx.doi.org/10.1038/35099076
- Zhang Y, Pizzute T, Pei M. A review of crosstalk between MAPK and Wnt signals and its impact on cartilage regeneration. Cell Tissue Res 2014; 358:633-49; PMID:25312291; http://dx.doi.org/10.1007/s00441-014-2010-x
- Neragi-Miandoab S, Kim J, Vlahakes GJ. Malignant tumours of the heart: A review of tumour type, diagnosis and therapy. Clin Oncol (R Coll Radiol) 2007; 19(10):748-56; PMID:17693068; http://dx.doi.org/10.1016/j.clon.2007.06.009