ABSTRACT
Selenium (Se) is an essential dietary micronutrient that has been examined for protection against different types of cancers including colon cancer. Despite an established inverse association between Se and chronic inflammation induced colon cancer (CICC), the mechanistic understanding of Se's protective effects requires additional in-vivo studies using preclinical animal models of CICC. Adiponectin (APN) is an adipocytokine that is protective against CICC as well. However, its role in the anti-mutagenic effects of the Se-diet remains unknown. To address this knowledge gap, here we examine the ability of dietary Se in reducing CICC in APN knockout mice (KO) and its wild-type C57BL/6. CICC was induced with the colon cancer agent 1,2 dimethyl hydrazine (DMH) along with dextran sodium sulfate (DSS). Se-enhanced diet increased selenoproteins, Gpx-1 and Gpx-2, in the colon tissues, thereby reducing oxidative stress. Se-mediated reduction of CICC was evident from the histopathological studies in both mouse models. In both mice, reduction in inflammation and tumorigenesis associated well with reduced p65 phosphorylation and elevated 53 phosphorylation. Finally, we show that in both models Se-administration promotes goblet cell differentiation with a concomitant increase in the levels of associated proteins, Muc-2 and Math-1. Our findings suggest that Se's protection against CICC involves both colonic epithelial protection and anti-tumor effects that are independent of APN.
Introduction
Colon cancer is the second leading cause of cancer deaths and is ranked third in the number of new cases occurring every year in both sexes in the United States.Citation1 Although treatment strategies such as chemotherapy and radiotherapy exist, they can cause severe side effects that increase morbidity. Hence, complementary medicine that manipulate micronutrients and phytochemicals are becoming increasingly popular preventive options for combating this disease.Citation2
Selenium (Se) is an essential micronutrient obtained through several dietary sources including Brazil nut, fish, liver, chicken, certain vegetables and meatsCitation3 and is widely accepted as an anti-oxidant element.Citation3 It integrates into proteins to form 25 selenoproteins,Citation4 and have protective roles against several human diseases such as, cardiovascular disorders, immune dysfunction, neurologic disorders, type 2 diabetes and several types of cancer.Citation5,6 Several lines of evidence suggest that Se is protective against colorectal carcinoma. For example, a recent observational epidemiological study with a nested case-control design (involving 966 colorectal cancer patients and the matched controls) reported an inverse association between higher serum Se (and selenoproteins) and colorectal cancer risk.Citation7 Cell-culture studies performed with human colorectal carcinoma cells HCT 116 and SW620Citation8 demonstrated that different dosages (1 μM, 5 μM and 10 μM) of Se lead to apoptosis of colon cancer cells through a Bax dependent pathwayCitation8 with 10 μM being the most effective. Also animal studies with colon xenograft modelCitation9 showed that supranutritional dosage of sodium selenite (10 μM) lead to apoptosis of colon cancer cells by ROS dependent modulation of phosphatase and tensin homolog (PTEN) mediated PI3K/AKT/FoxO3a signaling pathway.Citation9
Most biologic effects of the ingested elemental Se are mediated by the selenoproteins.Citation6 Glutathione peroxidases, Gpx-1 and Gpx-2, are the most abundant selenoproteins present and mediate most of the anti-oxidant properties by neutralizing the formation of reactive oxygen species (ROS).Citation10 Gpx-1, Gpx-2 knockout and double knockout mice exhibit severe pathology of colorectal cancer,Citation11 suggesting that these selenoproteins contribute significantly to the antioxidant and anti-cancer effects of dietary selenium.
Chronic inflammation occurring in inflammatory bowel disease, especially Crohn's disease (CD), is one significant causal factor in the development of colon cancer.Citation12 A recent study in which selenoproteins in the serum of 37 patients with CD were compared with 20 healthy individuals (controls), demonstrated that several selenoproteins are downregulated in CD.Citation13 Also animal (Sprague Dawley rats) studies support the role sodium selenite in reducing gastrointestinal inflammation by downregulating the expression of nuclear factor kappa light-chain-enhancer of activated B cells (NFκB) and related cytokines,Citation14 suggesting that dietary Se and selenoproteins have anti-inflammatory properties that are key in prevention against chronic inflammation induced colon cancer (CICC).
Despite these findings, the molecular mechanism underlying Se's protection against CICC still remains unclear, in part due to the absence of knowledge on whether and/or how other intrinsic anti-inflammatory mediators influence this protection mechanism. Adiponectin (APN) is one such anti-inflammatory and anti-cancer adipocytokine.Citation15,16 secreted by adipose tissues, and is an important chemokine known to inhibit tumorigenesis in preclinical mouse models for colorectal cancer.Citation17 Whether APN is needed for Se's protective effects against colorectal cancer is currently unclear. Understanding the regulatory influence of APN on Se's protective effects against CICC is essential in establishing appropriate doses for Se supplementation during reduced APN secretion, a common pathophysiological condition that contributes to increased risk of CICC.Citation18
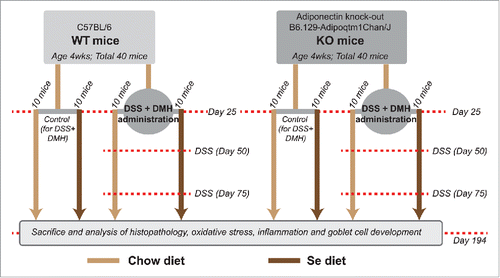
A recent pilot study by Rayman et al.Citation19 comprising of a randomized, double-blind, placebo-controlled trial involving 501 elderly volunteers, randomly assigned with either a 6-month selenium supplementation or placebo yeast, show that Se supplementation had no effect on APN concentration. This prompted us to hypothesize that the protective effects of Se against CICC can be APN independent. In the described study, we test this hypothesis using 2 established preclinical murine models: APN knockout mice (KO) and its wild-type C57BL/6 (WT), in which CICC was induced with the colon cancer agent 1,2 dimethyl hydrazine (DMH) along with dextran sodium sulfate (DSS), that we denote in this study as DSS + DMH. The design of the study is illustrated as a flowchart in . Both WT and KO have been used as pre-clinical models by others and us in the study of CICC.Citation18,20 As reported earlier by Saxena et al.,Citation18 KO mice develop more tumors and show increased pathology of CICC. This makes KO an ideal model to examine Se's protection against CICC in an APN independent setting. Here we demonstrate the effect of dietary supplementation of Se on the primary outcomes of CICC, tumor numbers and tumor area, in presence and absence of APN, and elucidate the possible mechanisms underlying Se's effect on CICC.
Figure 1. Schematic of the study design. This study was designed to test the central hypothesis that the protective effects of Se against CICC are APN independent. Flowchart illustrates the time-points at which DSS and DMH were administered in the DSS+DMH group, the time period during which se-diet was administered and the time-point at which the animals were killed and analyzed for CICC (see results).
Results
Se- diet reduced CICC in both WT and KO mice
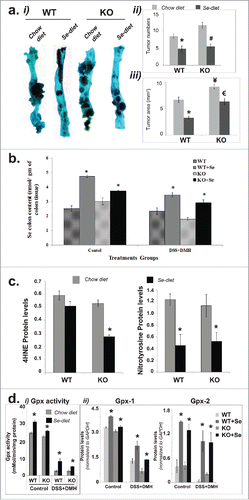
The recommended dietary allowance for Se is 55μg per day for human adults and the upper safe limit is 400 μg/day. Beneficial effects of Se can be obtained at a dosage of 200 μg per day; this dose can decrease the total incidence of cancer by 25%.Citation21 This is also the dosage that has been extensively used in clinical trials and correspond to 0.75 ppm of Se mouse diet. To study the effect of Se enhanced diet in our animal model we increased the selenium content in the regular chow diet from 0.02 ppm to 0.75 ppm (see methods). As shown in , both WT and KO mice administered with DSS + DMH showed significant reduction in tumor number upon Se-supplementation compared with the regular chow diet (). This reduction was more prominent in KO mice. As shown in , although KO mice under regular chow diet displayed significantly higher tumor numbers compared with the WT, there was no significant difference in the tumor numbers were between KO and WT mice on Se diet (). Se- diet also significantly reduced compared with the regular chow diet, the tumor area in WT as well as KO mice ().
Figure 2. Association of Se mediated reduction of CICC with Se and Selenoprotein accumulation and reduction of oxidative stress in colon tissues. (a) Reduction in tumor numbers and tumor area. (i) Representative methylene blue stained tissues. (ii) Comparison of colon tumor numbers in KO and WT mice on Se and chow diets (N = 10 per group). (iii) Comparison of colon tumor area (mm2) in KO and WT mice on Se and chow diets (N = 10 per group). Significance in difference was calculated using one way ANOVA. *p < 0.05 between WT and WT+Se, #p < 0.01 between KO and KO+Se, €p < 0.05 between WT+Se and KO+Se, ¥p < 0.05 between WT mice on chow diet and KO mice on chow diet. (b) Increased Se content in colon tissues of mice on Se diet. Mass spectrometry data showing Se content of the colon tissues in mice on control and Se diet. *p < 0.05, significance in difference in Se content in colon tissues of WT or KO mice (either control group or DSS+DMH administered group) on chow diet vs. Se diet was calculated using one-way ANOVA, N = 10 per group (c) Se-mediated reduction in oxidative stress in colon tissues. Bars showing a quantitative comparison between 4HNE and Nitrotyrosine levels. *p < 0.05, significance in difference between mice on control vs. Se diet was determined using one way ANOVA, N = 10 per group (d) Effect of Se diet on selenoproteins activity and Gpx1 and Gpx2 protein levels: i. Bar graph representing the Gpx activity (nmol/min/mg protein) in the colon tissue sections in control group mice and DSS+DMH administered mice. *p < 0.05 between mice on Se and regular chow diet within same treatment group and genotype, N = 10 per group ii. Bar graphs derived from Western Blot data (SI- 2) showing a quantitative comparison of Gpx-1 and Gpx-2 protein levels in control group mice and DSS+DMH administered mice, N = 10 per group. One way ANOVA was used to determine the significance in difference between mice on Se and regular chow diet within the same treatment group with same genotype.
Since common clinical symptoms of colon cancer include fecal hemocult, diarrhea and weight loss along with nausea and constant fatigue,Citation22 we also monitored whether our results corresponded with the clinical manifestation of the disease. Using a described previouslyCitation18 protocol we used an integrated score (denoted as ‘clinical score’) to quantify the extent of the clinical symptoms of CICC (combination of weight-loss, fecal hemoccult and diarrhea). As shown in Fig. S1, KO mice always displayed higher clinical scores compared with the WT feeding a similar diet. Both KO and WT mice feeding regular chow diets showed higher clinical score as compared with mice given Se enhanced diets. The reduction of clinical scores upon Se-diet was significant after day 85 and continued until day 194 (day of sacrifice).
Se-diet resulted in selenium enrichment in colon
To investigate whether the decreased CICC upon dietary Se supplementation was associated with enrichment of Se in colon tissues. We compared the Se-content in mice colon of DSS + DMH administered WT and KO mice fed with regular chow diet versus Se-diet () and performed same analyses for the WT and KO mice (labeled as control groups in ). Our results demonstrated a significant increase in the Se-content in colon tissues for both WT and KO mice fed with Se-diet, suggesting that the ingested Se accumulates in colon tissues for both mice genotypes.
Dietary Se reduced oxidative stress in colon tissues
Since previous studiesCitation10 have attributed antioxidant property to Se, we hypothesized that the significant reduction of CICC with Se diet is associated with the ability of Se to reduce oxidative stress in colon tissues. To test this, we examined the degree of oxidative stress in colon tissues by Western Blot using antibodies against nitrotyrosine and 4-hydroxynonenal (4-HNE), which are oxidation products from proteins and lipids respectively.Citation23 As shown in and Fig. S2, colon tissues of both WT and KO mice demonstrated a significant decrease in oxidative stress in presence of dietary Se. In the WT mice, the expression of Nitrotyrosine (and not 4HNE) was reduced significantly by Se diet compared with the regular chow diet. However, in KO mice Se-diet was able to significantly reduce the expression of both Nitrotyrosine and 4HNE compared with the regular chow diet.
Se-diet increased accumulation and activity of selenoproteins
Since antioxidant properties of Se are commonly attributed to selenoproteins, Gpx 1 and 2, we investigated whether colon tissues demonstrated any increase in the levels of proteins Gpx1 and 2 and the total Gpx activity in snap frozen colon tissues. As shown in , total Gpx activity decreased with induction of CICC both in WT and KO mice; however, in both mice this activity increased significantly with Se diet. Also as shown in and Fig. S3, induction of CICC decreased Gpx-1 and Gpx-2 levels for both KO and WT and Se diet was able to significantly increase these levels. Our results therefore demonstrate that Se-mediated reduction of oxidative stress colon tissues described earlier in is associated with an increase in total Gpx activity and Gpx proteins (Gpx 1 and 2).
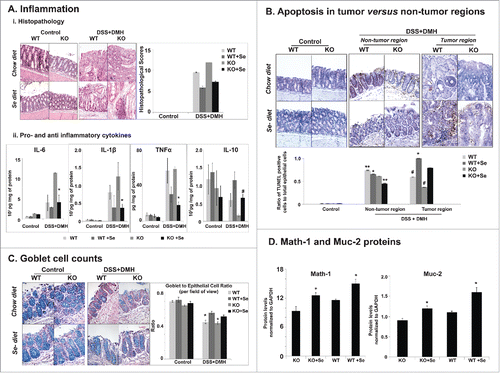
Figure 3. Effect of Se diet on inflammation, apoptosis and goblet cell differentiation. (A) Effect on inflammation i) Histopathology Staining and Scores. Left panel. Representative hematoxylin and eosin stained colon tissue sections of control and DSS+DMH treated mice. Right panel. Histopathology scores representing a culmination of inflammation, immune cell infiltration and degree of tumor, derived from HE stained images. One way ANOVA was used to determine significance in difference (N = 10 per group). *p < 0.05 between mice on Se and regular chow diets with in the same genotype and treatment group, #p < 0.03 between WT mice and KO mice on regular chow diet, **p < 0.04 between WT mice on regular chow diet and Se diet, ***p < 0.01 KO mice on control and Se diet. ii) Effect on markers of inflammation. Bar graphs representing the levels of secreted cytokines from the colon tissue section. *p < 0.05, #p < 0.01, One way ANOVA was used to determine significance in the difference between mice of a particular genotype and treatment on Se and regular chow diet (N = 10 per group). (B) Effect of Se diet on apoptosis. Upper panel. Images of representative colon tissues showing the TUNEL positive epithelial cells (brown color) in the control group and the non-tumor and tumor regions of colon tissue sections of DSS+DMH administered KO and WT mice on control or Se diet. The tissues were counterstained with methyl green. Lower panel, Graph generated from the TUNEL stained images representing ratio of the number of TUNEL positive cells to the total epithelial cells (degree of apoptosis). One way ANOVA was used to determine significance in difference; N = 10 per group. #p < 0.04 and **p < 0.05 between mice same genotype and treatment group on Se and regular chow diet, *p < 0.05 between WT+Se and KO+Se, (C) Effect of Se diet on goblet cell counts. Left panel, Representative Alcian Blue and Nuclear Fast Red stained colon tissue images. Right panel. Quantification of goblet and epithelial cells in colon tissues in mice on control or Se diet. One way ANOVA (N = 10 per group) was used to determine significance in difference, *p < 0.05 between mice same genotype and treatment group on Se and regular chow diet. (D) Quantitative comparison of Math-1 and Muc-2 protein levels generated from Western Blots (representative images shown in SI- 4). *p < 0.05, significance of difference between mice on regular chow diet and Se diet was determined using one way ANOVA.
Dietary Se reduced inflammation, immune cell filtration and cancer pathology of CICC
Next we investigated whether dietary Se has anti-inflammatory effects in colon. To do this we performed histopathological examination of colon tissues stained with Hematoxylin and Eosin (H&E). The representative H&E stains are shown in . Colon tissues in the control group showed normal appearing histology with well-formed crypts in mucosal layer without any sign of immune cell infiltration or inflammation. The DSS + DMH administered mice on regular chow diet showed highest degree of inflammation, immune cell infiltration and tumor growth compared with the other groups. In these animals, the lamina propria was fully infiltrated with immune cells resulting in a complete change of the morphology of the muscularis and submucosal layers. Se-diet was able to reduce this severity of inflammation in the DSS + DMH administered animals.
To quantify our observations, we adopted a histopathological scoring system (See methods) that would indicate the severity of the disease, constituting inflammation, immune cell infiltration and degree of tumor. In agreement with our visual observations, the DSS + DMH group showed the highest histopathological scores. For both WT and KO mice, the Se- diet was able to significantly reduce the histopathological scores obtained on regular chow diet. Under regular chow diet, the DSS + DMH administered KO mice showed a significantly higher histopathological score when compared with WT; however, the Se-diet was able to reduce this score to the extent that no significance was observed between DSS + DMH groups on Se diet ().
In DSS + DMH administered KO mice, Se-diet also resulted in a significant reduction in the levels of pro-inflammatory cytokines IL-6, TNF-α and IL-1β and a significant increase in the anti-inflammatory cytokine IL-10 compared with the regular chow diet (). We observed similar trends in the levels of IL-6, TNF-α, IL-1β and IL-10 in the DSS + DMH administered WT mice; however, these changes were not statistically significant by ANOVA, possibly due to the lesser severity of CICC in WT (compared with KO mice).
Dietary Se increased apoptosis in tumor regions of the colon but decreased apoptosis in non-tumor regions
Colonic tissue sections (both tumor bearing and non-tumor colon regions) were prepared to perform TUNEL assay for quantification of apoptosis. In both DSS + DMH administered KO and WT mice, Se-diet resulted in a significant increase in the TUNEL positive cells in the colon tumor tissue sections as compared with regular chow diet (). However, we also discovered to our surprise, that Se-diet also reduced the DSS + DMH induced apoptosis of the colonic epithelial cells in the non-tumor areas.
We further investigated whether Se-diet could restore the differentiation of epithelial to goblet cells. Goblet cells are known for secreting mucins (highly glycosylated proteins) that comprise the mucus layer of the gastrointestinal tract and are associated with protection against CICC.Citation24,18 Deficiency of mucin increases colorectal carcinoma in murine models.Citation25 To assess the effect of Se-diet on goblet cell differentiation, we initially performed a comparative quantification of goblet to epithelial cell ratio using Alcian blue staining on the colon tissue sections counterstained with nuclearfast solution. As shown in , the control group showed the highest ratio of goblet to epithelial cells with no significant difference between WT and KO mice ether on regular chow diet or Se-diet. The ratios dropped significantly compared with the control upon inducing CICC with DSS + DMH. However, for both the WT and KO mice, the Se-diet increased the goblet cells to epithelial cell ratios significantly. Based on these results we then proceeded to compare protein levels of Math-1, a protein that regulates the differentiation of epithelial cells to goblet cells and Muc-2, a protein that drives mucin secretion by goblet cells. As shown in and Fig. S3, in line with our observations for goblet cell to epithelial cell ratios, the Se-diet demonstrated a significant increase of both protein levels. These results strongly suggest that Se's protects against CICC through upregulation of tumor cell apoptosis and downregulation of epithelial cell apoptosis, thereby aiding the epithelial to goblet cell differentiation. This protection is independent of APN.
Dietary Se decreased NFκB p65 activation and increased p53 activation
It is well established that activation of the transcription factor NFκB p65, one global regulator in inflammation, is critical in the spontaneous secretion of pro-inflammatory cytokines in colon cancer.Citation26-28 Here we examined whether Se-diet mediated reduction of inflammation was associated with p65 phosphorylation at serine-536. Our choice to assess the effect of Se enhanced diet on the expression of phosphor-Ser536-p65 was based on a recent study on colon cancer patients,Citation29 which demonstrated that NF- κB p65 subunit phosphorylated at serine-536 is an independent prognostic factor in the patients. Our results ( and Fig. S3,) demonstrate that while DSS + DMH significantly increased p65 activation (as demonstrated by the increased levels of its phosphorylation), the Se-diet significantly reduced phosphorylated p65 levels both in DSS + DMH administered WT and in KO mice.
Figure 4. Selenium's effects on p65 and p53 phosphorylation and the protection model against CICC. (a) i) Comparisons in p65 phosphorylation in DSS+DMH administered mice on regular chow diet and Se diet. Ratios of phospho p65 to total p65 levels were derived from the Western Blot data (representative image shown in SI-5). ii) Comparisons in p53 phosphorylation in DSS+DMH administered mice on regular chow diet and Se diet. Ratios of phospho p53 to GAPDH levels were derived from the Western Blot data (representative image shown in SI-6). *p < 0.05 and #p < 0.01, significance in difference between mice on control and Se diets were determined using one way ANOVA, (b) Selenium protection model: Se-mediated reduction of inflammation and increase in tumor apoptosis in colon tissues of DSS+DMH administered mice. According to this model, one mode of Se's protection involves the downregulation of inflammation that is driven by decreased phosphorylation of p65 global transcription factor and which results in decrease of pro-inflammatory cytokines IL-6, TNFα and IL-1β and increase in anti-inflammatory cytokine IL-10. The decrease in inflammation reduces epithelial cell apoptosis in non-tumor regions and thereby supports the epithelial to goblet cell differentiation (mediated by increase in Math-1) resulting in higher production of mucin (mediated by increase in Muc-2), a glycoprotein that in turn has protective effects against colon cancer. In the second mode, Se simultaneously results in increased tumor apoptosis possibly by Bax pathway (suggested by increase in cleaved caspase 9 levels), which in turn mediated by increase in phosphorylation of the tumor suppressor p53. Gray solid arrows represent mechanisms that have been established previously (and cited in the text). Red arrows (upward, increase and downward, decrease) are results reported in the current study. Our results suggest that Se mediated drop in oxidative stress regulates the cross-talk between p65 and p53; this molecular mechanism underlying this regulation remains unknown (dashed arrows) and will be elucidated in our future studies.
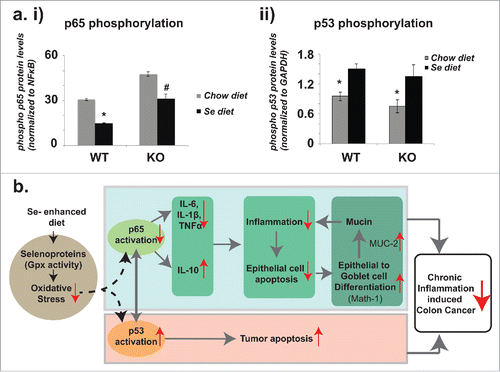
The cross-talk of NFκB p65 and the tumor apoptosis inducing global transcription factor, tumor suppressor p53, has recently been established for CICC.Citation30 Hence we investigated whether Se displays a regulatory role on the phosphorylation of p53 at Serine 15 based on a previous study on LnCaP human prostrate cancer cell lines,Citation31 which indicted that upregulation of p53 Ser15P resulted in caspase activation and apoptosis of the cancer cells. As shown in and Fig. S3, for both KO and WT mice, the Se-diet resulted in a significant increase in p53 activation (demonstrated as increase in phosphorylation levels). These results were in agreement with immunofluorescent staining of cleaved caspase 9, a downstream product of the Bax apoptotic pathway activated by p53.Citation32 As demonstrated in Fig. S4, significant increase in cleaved caspase 9 protein levels in the tumor tissue was observed for mice on Se-diet in comparison to those on regular chow diet.
Hence, our results demonstrate that Se-diet inhibits CICC at least in part, by regulating the activation of 2 important global regulators of CICC, p53 and p65. We show here that Se reverses 2 critical events connected with the pathology of CICC: (a) upregulation of p65 activation and (b) downregulation of p53 activation.
Discussion
The central and novel aspect of this study was the demonstration that Se's inhibition of CICC is APN independent. We have shown that in absence of APN, dietary selenium reduced tumor size and numbers and resulted in decreased clinical symptoms as well, almost similar to the WT. The molecular mechanisms that we describe in the current study can now explain the recent reports from Krehl et al,Citation33 which demonstrated the inverse association of CICC with selenium and selenoproteins. Our findings are also in line with previous reports from Li et alCitation8 and Yang et al.Citation34 that provide evidence of increased Bax pathway dependent apoptosis of HCT116 and SW620 colorectal cancer cells together with increased levels of Bax, Bad, Bim and activated caspase-9 and the downregulation of Bcl#xL in xenografted human colorectal carcinoma SW480 cell lines upon administration of sodium selenite.
Previous reports by Saxena et al.Citation20 indicated that APN demonstrates anti-inflammatory properties that protect against colon cancer by preventing apoptosis of colon goblet cells and promoting the differentiation of epithelial to goblet cells. In that study, APN's anti-inflammatory properties were also demonstrated in its ability to suppress NFκB p65 activation along with reduction of pro-inflammatory markers including TNF-α, IL-8 and IL-6. In this study we establish that Se supplementation offers similar anti-tumor effects and colonic epithelial protection as offered by APN. We emphasize here that, while reduced secretion of APN by adipose tissues has been linked with increased obesity and severity of colorectal cancer,Citation20 an increase in serum APN also increases the risk of type-2 diabetes.Citation35 Recent clinical studies by Rayman et al.Citation19 suggest that Se-supplementation to patients with low selenium did not increase type-2 diabetes. Hence, the protective effect of selenium-enhanced diet against CICC similar to APN but independent of APN, is extremely significant to CICC patients with obesity and type-2 diabetes. We reason here that the differences in the outcomes of the study were not a contribution of body weights. Similar to a previous study by Saxena et al.,Citation18 based on which the experimental protocols for this study was designed, here we did not observe differences in body weight between C57BL6 and the adiponectin knockout mice (data not shown). It is possible that the absence of such body weight differences, as would be expected when mice are fed ad libitum, was due to the fact that we did not feed the animals with high-fat diet. As suggested in a previous report by Guo et al.,Citation36 adiponectin deficiency aggravates obesity only when fed with a high fat diet. We also did not observe any significant differences in body weight between mice (of same genotypes) fed with regular chow diet vs. selenium-enhanced diet.
Significant reduction in the oxidative stress was detected upon Se enrichment in colon tissues, which were consistent with previous reports showing that Se diet reduced F2-isoprostanes (marker of lipid peroxidation and oxidative stress) and DNA damage in response to DSS induced inflammation in AOM/DSS murine modelsCitation37 and DMH treated rats.Citation38 In addition, here we link this reduction of oxidative stress to 2 selenoproteins, Gpx-1 and GPx-2 and the total Gpx activity. Gpx proteins are well known antioxidants and considered as an anti-inflammatory markers that are known to drop with increase in inflammation and cancer.Citation39,33 Based on the current study and the previous reports, we propose a Se- protection model (). In the first mode of this model, Selenium reduces oxidative stress by increasing synthesis of selenoproteins Gpx-1 and Gpx-2, reduces inflammation through the downregulation of the pro-inflammatory cytokines IL-6, TNF-α and IL-1β and upregulation of at least one anti-inflammatory cytokine IL-10. In the second mode Se provides protection to the colonic epithelium while increasing tumor apoptosis. Protection to the colonic epithelium is facilitated through a decrease in epithelial cell apoptosis with a concomitant increase in goblet cell differentiation through upregulation of Math-1, and an increase of Muc-2 (a protein that drives mucin secretion). Our data showing a significant increase in cleaved caspase 9 in the tumor regions are in line with the previous reports of upregulation of Bax apoptotic pathway by Selenium.Citation34
Finally, the current study links dietary Se to p65 activation, thereby tethering its link to one of the most complex and dynamic signaling networks that are central to the regulation of variety of genes involved in inflammation, cell differentiation and proliferations and stress response.Citation40 The interplay between the tumor suppressor p53 on NFκB activation (including p65) is now well-established,Citation41,42 which prompted us to investigate Se's effect on p53 activation as well. Surprisingly, while Se reduced p65 activation, it increased p53 activation, which suggests that Se at least in part, exercises its protection against CICC (of reducing inflammation and protecting the colonic epithelium) by regulating the cross-talk between the global regulators of CICC, p65 and p53. Since these regulators are also central to many other diseases (including other cancer types), our results can now at least in part, explain Se's protective role in multiple cancers, inflammatory and autoimmune diseases.
In conclusion, we have conducted the first in-vivo studies using pre-clinical models of CICC to demonstrate that Se offers protection against CICC independent of APN. Our study used APN knockout, KO and its wild type C57BL/6 mice in which CICC was induced with the application of CICC induced by DMH+DSS, an established chemical combination that generates CICC pathology very similar to that observed in human colorectal cancer. Our study has indicated a dual nature of Se as a pro-apoptotic molecule for cancer cells, while at the same time providing protection to normal colon epithelial cells leading to faster epithelial cell renewal which lead to further reduction in the inflammation, immune cell infiltration and colon insult. We simultaneously point out here that further studies are needed to understand exactly why/how in our animal models, DSS + DMH mediated the reduction in Gpx-1/2 protein expression and the Gpx activity (that associated with increase in oxidative stress in colon tissues). It is possible that end products of lipid peroxidation such as malondialdehyde may be inhibit the expression of Gpx-1/2 and other protective enzymes as claimed in earlier studies by Seven et al.,Citation43 thereby resulting in increased oxidative stress.
Our future studies will harness the established experimental setting of this current study and reveal more mechanistic details of the cross-talk between selenoproteins, the components of the NFκB signaling pathway and p53 to understand their collective contribution in oxidative stress, inflammation, apoptosis and carcinogenesis.
Material and methods
Animal groups and diet
Four week-old C57BL/6 and KO mice (B6.129‐Adipoqtm1Chan/J; stock number 008195) were obtained from Jackson Laboratories and bred in the animal facility at the University of South Carolina under the Institutional Animal Care and Use Committee guidelines. KO mice were homozygous with the absence of APN expression confirmed by serum protein expression. Animals used for the study were randomized into 8 different treatment groups with n = 10/group as follows: C57BL/6 (WT) + regular chow diet, WT + Se-diet, KO + regular chow diet, KO + Se-diet, WT + DSS + DMH+ regular chow diet, WT + DSS + DMH+Se-diet, KO + DSS + DMH+ regular chow diet and KO + DSS + DMH+Se-diet. The experimental protocols for inducing CICC in mice with DSS and DMH were adopted in accordance with a previous study by Saxena et al.Citation18 Mice were fed ad libitum. Before any chemical administration, the mice were acclimatized to the animal facility for 24 d in a low stress environment (22°C, 50% humidity and low noise) with 12:12 hours of light-dark cycle. The first DSS or DMH administration was performed on day 25 of the start of the study. Mice were killed by cervical dislocation under anesthesia on day 194. We also ensured that there was no significant difference in the body weight of KO and WT mice at the beginning of the study. Selenium enhanced diet (regular chow diet with 0.75 ppm of added Se) was administered to 8 groups on day 25 simultaneously with the administration of DSS, DMH, or both or to the control group. The diet was administered till the day of sacrifice (day 194). The increase of Se levels in the diet was done by mixing sodium selenite with the regular chow diet and confirming the final Se concentration flourometrically as described previously.Citation44
Induction of CICC
CICC was induced in WT and KO mice using a combination of DSS and DMH as described previously.Citation18
Colon tissue and serum collection for analysis of histopathology, proteins and cytokines
Blood was collected before sacrifice through retro-orbital puncture and was spun down at 10,000 rpm for 18 minutes and serum was obtained and stored at −20°C to measure APN. Mice colon was excised and flushed clean with PBS. 2 mm2 colon tissue section with tumor and non-tumor area was fixed in 10% formalin. 24 hours later, tissues were submerged with 70% ethanol followed by paraffin embedding and sectioning to obtain 5 µm thin section on glass slide. Sections with tumor and non-tumor areas were snap frozen on dry ice and stored at −80°C for protein analysis. Another 2 mm2 colon tissue section was incubated in RPMI medium containing 5000 IU/mL and 5000 IU/mL penicillin and streptomycin (CELLGRO) respectively at 37°C and 5% CO2 for 24 hours. This was followed by centrifugation at 2500 rpm for 15 minutes. Supernatant was obtained and stored at −20°C to measure secreted cytokine levels.
Tumor number and tumor area and histopathology
Mice colon was excised and flushed with PBS. Tumor number and area were counted under the microscope in all mice of different groups and any statistical significance in difference between different groups was calculated using student's t-test.
Hematoxylin and Eosin staining was used to determine the morphology of mice colon. Histopathology was quantified based on the scoring system indicating the severity of disease and constituting inflammation, immune cell infiltration and degree of tumor. This was on the scale of 12 where highest score of 4 was given for each parameter, where 0 = no infiltration or no inflammation or no cancer; 2 = moderate infiltration or inflammation or pre-cancerous lesions; and 4 = severe inflammation with distorted crypts or infiltration and formation of lymphatic follicles or visible tumors. All the images were taken in 20X magnification with Nikon e600 microscope. Two investigators in blinded fashion measured the scores independently.
Determination of clinical scores
Clinical score was measured for each mouse in each group from day 25 to day 194. Mice were killed after the last clinical score measurement. Score for the weight loss is based on the following published scale, where 0 = 0–5% weight loss; 1 = 6–10% weight loss; 2 = 11–15% weight loss; 3 = 16–20% weight loss; and 4 = >20% weight loss. Scoring of diarrhea is as follows: 0 = well-formed pellets, 2 = pasty and semi-formed stools that do not adhere to the anus, 4 = liquid stools that adhere to the anus. Detection of blood in the stools was determined using hemoccult kit (Beckmann Coulter). The higher intensity of blue color indicates greater bleeding. The followings are the score rates for the fecal hemoccult: 0 = no blood, 2 = positive hemoccult, 4 = gross bleeding. The total clinical score was the summation of the individual score of weight loss, diarrhea and fecal hemoccult. The maximum score a mouse could get is 12. Hence, higher clinical score indicates more severity of colon cancer symptoms.
TUNEL assay
Degree of apoptosis was measured in the tumor and non-tumor tissue sections of the colon by TUNEL assay. TUNEL assay (EMD Millipore) was used to determine the number of TUNEL positive cells and total number of epithelial cells of the colon in 2 mm2 tissue cross-sectional tissue area. 5 sections were randomly selected from each tissue section and 10 tissue sections were randomly selected from each group. The ratios of TUNEL positive cells to total epithelial cells were used to determine the ratio of apoptosis and were plotted for different treatment groups. All the images were taken in 20X magnification with Nikon e600 microscope.
Alcian blue staining and quantification of goblet to epithelial cell ratio
Tissues were deparaffinized using xylene and gradation of ethanol. Tissues were stained with Alcian blue (1% in 3% acetic acid; pH 2.5) and counterstained with nuclear fast red solution. Goblet to epithelial cell ratio was counted per crypt with 10 crypts per section and 5 sections per group.
Protein determination using Western Blot
Colon tissue frozen at −80°C was homogenized in RIPA buffer added to protease and phosphatase inhibitors (SIGMA). It was then centrifuged at 10,000 rpm for 15 minutes and supernatant was obtained for protein analysis. Protein concentration in the supernatant was determined by using Bradford protein assay. This was followed by loading equal amounts of protein (50 µg) in each well for a 10% Sodium Dodecyl Sulfate (SDS) gel electrophoresis. The protein from the gel was then transferred to a nitrocellulose membrane (Pall Scientific) and blocked with 5% non-fat dry milk (Biorad) in phosphate buffer saline (PBS) (cellgro) with 0.1% Tween 20. The membrane was incubated overnight with the primary antibody. The primary antibodies against Gpx-1, Gpx-2 and GAPDH obtained from Genetex and those against phosphor-Ser536-p65, p65, p53 Ser15P and Nitrotyrosine were obtained from cell signaling technology. Membrane was washed by PBS containing 0.1% Tween 20 (Biorad). The membrane was then incubated with secondary antibody (Santa Cruz) followed by another washing step and finally incubated in ECL substrate (Western Bright, Advansta). The film was developed using by using the developer, SRX-101A, Konica Minolta Medical & Graphic, Inc. in the dark room. Finally, the film was scanned and the densities of the protein bands obtained were analyzed using Image J software.
Enzyme linked immunosorbent assay (ELISA)
Spontaneous secreted cytokines were measured from the tissue incubated in the RPMI medium for 24 hours at 37°C. The media were collected and centrifuged at 2500 rpm for 16 minutes. Pellet was discarded and the supernatant was isolated. Cytokines IL-6, TNF-α, IL-1β and IL-10 levels were measured using BD OptEIA ELISA kit (BD biosciences) and normalized by total protein content estimated by using standard Bradford assay procedure.
Immunofluorescence
Colon tumor tissue sections were deparaffinzed by xylene and dehydrated with different concentrations of ethanol. The sections then underwent heat mediated antigen retrieval step with 10 nM citrate buffer (Prohisto), 0.05% Tween 20, pH 6.0 in an autoclave at 121°C, 15 psi for 20 minutes. The tissues were then blocked with 10% goat anti-rabbit serum in PBS. Tissues were then incubated in a primary antibody that is cleaved caspase 9 (Cell Signaling Technology) for overnight incubation. This was followed by 5 washing steps and 2 hour- incubation with anti-rabbit secondary antibody (Aexa Flour 488) (Cell Signaling Technology). Finally the tissue sections were mounted with DAPI based mounting media (Genetex) before imaging. Ten random 20X magnification, 2×2 images of the 8 slides per group belonging to different mice were obtained. Quantitative image analyses of these images were performed Image J software.
Mass Spectrometry for selenium quantification
Colon tissues were freeze-dried using lyophilizer and weighed before use for mass spectrometry. The tissues were then digested using aqua regia (1 mL of optima grade nitric acid plus 3 mL of optima grade hydrochloric acid) at 140°C and the final volumes were brought to 10 ml. The samples were analyzed for Selenium using the Finnigan ELEMENT2 double focusing magnetic sector field inductively coupled plasma-mass spectrometer (ICP-MS). Iridium was used as the internal standard. The instrument was calibrated for element 82Se. The calibration range was from 1.0 to 60 ppb. The R squared value for the initial calibration curve was greater than 0.99. The samples were analyzed immediately after the initial calibration, and the target element results were within the calibration range. The samples were then diluted (5X) and the instrument blank contained 0.31 ppb of Se. The ending continuing calibration verification recovery for Se standard was 112%. The results were then converted to the amount in nanomolar per gram of the colon tissue and plotted on a graph.
Statistical analysis
All statistical analyses were performed using the GrpahPad Prism software (Graphpad, CA, USA). Multiple comparisons among animal groups were performed using one-way ANOVA and the Student-Newman-Keuls test for post hoc comparisons. P value <0.05 was considered statistically significant.
Author contributions
AS, RF, KK, JAC and AC formulated the overall research goals and aims and developed the experimental design, AS, KK, ST, JG performed the experiments, AS, RF, KK, ST, JG, JAC and AC analyzed overall data, AS, RF, KK, JAC and AC wrote the manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_Data.zip
Download Zip (1.7 MB)Funding
This work was funded and supported by Grant P20 RR-017698, the National Center for Research Resources, Center for Colon Cancer Research, Center of Biomedical Research Excellence (COBRE) Program, University of South Carolina, Columbia SC.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66:7-30; PMID:26742998; http://dx.doi.org/10.3322/caac.21332
- Lee WL, Huang JY, Shyur LF. Phytoagents for cancer management: regulation of nucleic acid oxidation, ROS, and related mechanisms. Oxidative Med Cell Longevity 2013; 2013:925804; PMID:24454991; http://dx.doi.org/10.1155/2013/925804
- Maseko T, Howell K, Dunshea FR, Ng K. Selenium-enriched Agaricus bisporus increases expression and activity of glutathione peroxidase-1 and expression of glutathione peroxidase-2 in rat colon. Food Chem 2014; 146:327-33; PMID:24176350; http://dx.doi.org/10.1016/j.foodchem.2013.09.074
- Metanis N, Hilvert D. Natural and synthetic selenoproteins. Curr Opin Chem Biol 2014; 22C:27-34; PMID:25261915; http://dx.doi.org/10.1016/j.cbpa.2014.09.010
- Fairweather-Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, et al. Selenium in human health and disease. Antioxidants Redox Signal 2011; 14:1337-83; PMID:20812787; http://dx.doi.org/10.1089/ars.2010.3275
- Bellinger FP, Raman AV, Reeves MA, Berry MJ. Regulation and function of selenoproteins in human disease. Biochem J 2009; 422:11-22; PMID:19627257; http://dx.doi.org/10.1042/BJ20090219
- Hughes DJ, Fedirko V, Jenab M, Schomburg L, Meplan C, Freisling H, et al. Selenium status is associated with colorectal cancer risk in the European prospective investigation of cancer and nutrition cohort. Int J Cancer 2015; 136:1149-61; PMID:25042282; http://dx.doi.org/10.1002/ijc.29071
- Li Z, Meng J, Xu TJ, Qin XY, Zhou XD. Sodium selenite induces apoptosis in colon cancer cells via Bax-dependent mitochondrial pathway. Eur Rev Medical And Pharmacol Sci 2013; 17:2166-71; PMID:23893182
- Luo H, Yang Y, Duan J, Wu P, Jiang Q, Xu C. PTEN-regulated AKT/FoxO3a/Bim signaling contributes to reactive oxygen species-mediated apoptosis in selenite-treated colorectal cancer cells. Cell Death Dis 2013; 4:e481; PMID:23392169; http://dx.doi.org/10.1038/cddis.2013.3
- Kosaric JV, Cvetkovic DM, Zivanovic MN, Curcic MG, Seklic DS, Bugarcic ZM, Markovic SD. Antioxidative and antiproliferative evaluation of 2-(phenylselenomethyl)tetrahydrofuran and 2-(phenylselenomethyl)tetrahydropyran. J BUON 2014; 19:283-90; PMID:24659677
- Lee DH, Esworthy RS, Chu C, Pfeifer GP, Chu FF. Mutation accumulation in the intestine and colon of mice deficient in two intracellular glutathione peroxidases. Cancer Res 2006; 66:9845-51; PMID:17047045; http://dx.doi.org/10.1158/0008-5472.CAN-06-0732
- Rubin DC, Shaker A, Levin MS. Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Frontiers Immunol 2012; 3:107; PMID:22586430; http://dx.doi.org/10.3389/fimmu.2012.00107
- Andoh A, Hirashima M, Maeda H, Hata K, Inatomi O, Tsujikawa T, Sasaki M, Takahashi K, Fujiyama Y. Serum selenoprotein-P levels in patients with inflammatory bowel disease. Nutrition 2005; 21:574-9; PMID:15850963; http://dx.doi.org/10.1016/j.nut.2004.08.025
- Pillai SS, Sugathan JK, Indira M. Selenium downregulates RAGE and NFkappaB expression in diabetic rats. Biol Trace Element Res 2012; 149:71-7; PMID:22476978; http://dx.doi.org/10.1007/s12011-012-9401-1
- Nagaraju GP, Aliya S, Alese OB. Role of adiponectin in obesity related gastrointestinal carcinogenesis. Cytokine Growth Factor Rev 2015; 26:83-93; PMID:25007742; http://dx.doi.org/10.1016/j.cytogfr.2014.06.007
- Tae CH, Kim SE, Jung SA, Joo YH, Shim KN, Jung HK, Kim TH, Cho MS, Kim KH, Kim JS. Involvement of adiponectin in early stage of colorectal carcinogenesis. BMC Cancer 2014; 14:811; PMID:25370174; http://dx.doi.org/10.1186/1471-2407-14-811
- Otani K, Kitayama J, Yasuda K, Nio Y, Iwabu M, Okudaira S, Aoki J, Yamauchi T, Kadowaki T, Nagawa H. Adiponectin suppresses tumorigenesis in Apc(Min)(/+) mice. Cancer Letters 2010; 288:177-82; PMID:19646806; http://dx.doi.org/10.1016/j.canlet.2009.06.037
- Saxena A, Chumanevich A, Fletcher E, Larsen B, Lattwein K, Kaur K, Fayad R. Adiponectin deficiency: role in chronic inflammation induced colon cancer. Biochim Et Biophys Acta 2012; 1822:527-36; PMID:22198319; http://dx.doi.org/10.1016/j.bbadis.2011.12.006
- Rayman MP, Blundell-Pound G, Pastor-Barriuso R, Guallar E, Steinbrenner H, Stranges S. A randomized trial of selenium supplementation and risk of type-2 diabetes, as assessed by plasma adiponectin. PLoS One 2012; 7:e45269; PMID:23028897; http://dx.doi.org/10.1371/journal.pone.0045269
- Saxena A, Baliga MS, Ponemone V, Kaur K, Larsen B, Fletcher E, Greene J, Fayad R. Mucus and adiponectin deficiency: role in chronic inflammation-induced colon cancer. International J Colorectal Dis 2013; 28:1267-79; PMID:23474825; http://dx.doi.org/10.1007/s00384-013-1664-2
- Reid ME, Duffield-Lillico AJ, Slate E, Natarajan N, Turnbull B, Jacobs E, Combs GF, Jr, Alberts DS, Clark LC, Marshall JR. The nutritional prevention of cancer: 400 mg per day selenium treatment. Nutr Cancer 2008; 60:155-63; PMID:18444146; http://dx.doi.org/10.1080/01635580701684856
- Korsgaard M, Pedersen L, Sorensen HT, Laurberg S. Reported symptoms, diagnostic delay and stage of colorectal cancer: a population-based study in Denmark. Colorectal Dis 2006; 8:688-95; PMID:16970580; http://dx.doi.org/10.1111/j.1463-1318.2006.01014.x
- Zigdon H, Kogot-Levin A, Park JW, Goldschmidt R, Kelly S, Merrill AH, Jr, Scherz A, Pewzner-Jung Y, Saada A, Futerman AH. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J Biol Chem 2013; 288:4947-56; PMID:23283968; http://dx.doi.org/10.1074/jbc.M112.402719
- Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev 2004; 23:77-99; PMID:15000151; http://dx.doi.org/10.1023/A:1025815113599
- Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002; 295:1726-9; PMID:11872843; http://dx.doi.org/10.1126/science.1069094
- Kim HY, Kim SL, Park YR, Liu YC, Seo SY, Kim SH, Kim IH, Lee SO, Lee ST, Kim SW. Balsalazide potentiates parthenolide-mediated inhibition of nuclear factor-κB signaling in HCT116 human colorectal cancer cells. Intest Res 2015; 13:233-41; PMID:26130998; http://dx.doi.org/10.5217/ir.2015.13.3.233
- Müller-Edenborn K, Léger K, Glaus Garzon JF, Oertli C, Mirsaidi A, Richards PJ, Rehrauer H, Spielmann P, Hoogewijs D, Borsig L, et al. Hypoxia attenuates the proinflammatory response in colon cancer cells by regulating IκB. Oncotarget 2015; 6:20288-301; PMID:25978030; http://dx.doi.org/10.18632/oncotarget.3961
- Mladenova D, Pangon L, Currey N, Ng I, Musgrove EA, Grey ST, Grey ST, Kohonen-Corish MR. Sulindac activates NF-κB signaling in colon cancer cells. Cell Commun Signal 2013; 11:73; PMID:24083678; http://dx.doi.org/10.1186/1478-811X-11-73
- Lewander A, Gao J, Carstensen J, Arbman G, Zhang H, Sun XF. NF-kappaB p65 phosphorylated at serine-536 is an independent prognostic factor in Swedish colorectal cancer patients. Int J Colorectal Dis 2012; 27:447-52; PMID:22102084; http://dx.doi.org/10.1007/s00384-011-1356-8
- Cooks T, Pateras IS, Tarcic O, Solomon H, Schetter AJ, Wilder S, Lozano G, Pikarsky E, Forshew T, Rosenfeld N, et al. Mutant p53 prolongs NF-kappaB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell 2013; 23:634-46; PMID:23680148; http://dx.doi.org/10.1016/j.ccr.2013.03.022
- Jiang C, Hu H, Malewicz B, Wang Z, Lu J. Selenite-induced p53 Ser-15 phosphorylation and caspase-mediated apoptosis in LNCaP human prostate cancer cells. Mol Cancer Ther 2004; 3:877-84; PMID:15252149
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004; 303:1010-4; PMID:14963330; http://dx.doi.org/10.1126/science.1092734
- Krehl S, Loewinger M, Florian S, Kipp AP, Banning A, Wessjohann LA, Brauer MN, Iori R, Esworthy RS, Chu FF, et al. Glutathione peroxidase-2 and selenium decreased inflammation and tumors in a mouse model of inflammation-associated carcinogenesis whereas sulforaphane effects differed with selenium supply. Carcinogenesis 2012; 33:620-8; PMID:22180572; http://dx.doi.org/10.1093/carcin/bgr288
- Yang Y, Huang F, Ren Y, Xing L, Wu Y, Li Z, Pan H, Xu C. The anticancer effects of sodium selenite and selenomethionine on human colorectal carcinoma cell lines in nude mice. Oncol Res 2009; 18:1-8; PMID:19911698; http://dx.doi.org/10.3727/096504009789745647
- Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2009; 302:179-88; PMID:19584347; http://dx.doi.org/10.1001/jama.2009.976
- Guo R, Zhang Y, Turdi S, Ren J. Adiponectin knockout accentuates high fat diet-induced obesity and cardiac dysfunction: role of autophagy. Biochim Biophys Acta 2013; 1832:1136-48; PMID:23524376; http://dx.doi.org/10.1016/j.bbadis.2013.03.013
- Barrett CW, Singh K, Motley AK, Lintel MK, Matafonova E, Bradley AM, Ning W, Poindexter SV, Parang B, Reddy VK, et al. Dietary selenium deficiency exacerbates DSS-induced epithelial injury and AOM/DSS-induced tumorigenesis. PloS One 2013; 8:e67845; PMID:23861820; http://dx.doi.org/10.1371/journal.pone.0067845
- Ghadi FE, Malhotra A, Ghara AR, Dhawan DK. Selenium as a modulator of membrane stability parameters and surface changes during the initiation phase of 1,2-dimethylhydrazine induced colorectal carcinogenesis. Mol Cell Biochem 2012; 369:119-26; PMID:22752389; http://dx.doi.org/10.1007/s11010-012-1374-z
- Chen YC, Prabhu KS, Mastro AM. Is selenium a potential treatment for cancer metastasis? Nutrients 2013; 5:1149-68; PMID:23567478; http://dx.doi.org/10.3390/nu5041149
- Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer 2013; 12:86; PMID:23915189; http://dx.doi.org/10.1186/1476-4598-12-86
- Cooks T, Pateras IS, Tarcic O, Solomon H, Schetter AJ, Wilder S, et al. Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell 2013; 23:634-46; PMID:23680148; http://dx.doi.org/10.1016/j.ccr.2013.03.022
- Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol 2007; 8:49-62; PMID:17183360; http://dx.doi.org/10.1038/nrm2083
- Seven A, Civelek S, Inci E, Inci F, Korkut N, Burcak G. Evaluation of oxidative stress parameters in blood of patients with laryngeal carcinoma. Clin Biochem 1999; 32:369-73; PMID:10480452; http://dx.doi.org/10.1016/S0009-9120(99)00022-3
- Hrdina J, Banning A, Kipp A, Loh G, Blaut M, Brigelius-Flohé R. The gastrointestinal microbiota affects the selenium status and selenoprotein expression in mice. J Nutritional Biochem 2009; 20:638-48; PMID:18829286; http://dx.doi.org/10.1016/j.jnutbio.2008.06.009
