ABSTRACT
Numb is a protein whose asymmetric segregation during cell division determines cell fate and has numerous functions relevant to multiple fields of study, including developmental neurobiology and cancer biology. Little is known about the role of Numb in esophageal squamous cell carcinoma (ESCC), the predominant histological esophageal carcinoma in Asian populations. In this study, we focused on the expression and biologic functions of Numb in the context of ESCC. From analysis of tissue microarrays with 212 patients, it was found that Numb was significantly upregulated in ESCC tissues compared with corresponding non-cancerous tissues. Kaplan-Meier survival analysis suggests that higher expression of Numb was significantly associated with a high tumor recurrence (p = 0.015) and poor overall post-surgical survival (p = 0.016). Using multiple Cox regression, the expression of Numb was determined to be an independent predictor of poor prognosis. When siRNA was used to knockdown Numb in ESCC cell lines, there was a consistent increase in caspase-3 dependent apoptosis and inhibition of cellular proliferation, as well as downregulation of expression of the cancer stem cell markers Oct-4, SOX-2 and Nanog. In addition, downregulated Numb expression was not significantly associated with the migration of ESCC cells. These results indicate that Numb acts as an oncoprotein and has potential as a novel prognostic biomarker and therapeutic target in ESCC patients.
Introduction
Among malignancies, human esophageal squamous cell carcinoma (ESCC) is one of the most aggressive and lethal cancers and accounts for more than 90% of esophageal cancer cases in China.Citation1-3 Despite improvements in diagnosis and multimodal treatments of ESCC in recent decades, patient prognosis is still poor and the overall 5-year survival of esophageal carcinoma patients ranges from only 15% to 25%.Citation3 There are few specific biomarkers currently known that can be used for early diagnosis, targeted therapies and assessing prognosis.
Historically, Numb was originally identified in Drosophila as a critical cell fate determinant.Citation4 In mammals, the Numb homolog, which is more structurally complex than Numb in Drosophila, has multiple functions, including in controlling asymmetric cell division and cell fate decisions, endocytosis, cell adhesion, cell migration and ubiquitination of substrates.Citation5 Recent studies have found that Numb is involved in several human malignancies, where it plays diverse roles and is expressed at different levels depending on the malignancy.Citation6-9 In our previous study, we found that Numb isoform 1 (NUMB-1) mRNA was downregulated in 66.7% of primary ESCC tissues and this low expression of NUMB-1 was significantly correlated with a high rate of tumor recurrence and poor overall post-surgical survival.Citation10 However, the biologic roles and clinical significance of the Numb protein in ESCC remain largely uncharacterized.
In this study, we found the Numb protein was significantly upregulated in ESCC tissues as compared with paired non-tumor samples. This rise in Numb protein expression was an independent indicator of a poor prognosis in ESCC patients. Furthermore, siRNA mediated abrogation of Numb expression inhibited ESCC cell proliferation as well as increased the number of apoptotic cells induced by cisplatin which was widely used in chemotherapy for ESCC patients.
Results
Upregulation of Numb protein in ESCC tissues and cell lines
212 patients diagnosed with ESCC joined our study. An initial survey of the 212 collected human ESCC tissues was performed by immunohistochemistry (IHC) on tissue microarrays to measure Numb protein expression in ESCC tissues and corresponding non-tumor tissues. The degree of IHC staining was evaluated using an H-score as described in the Materials and Methods. In our study, we observed a range of upregulation of Numb expression in ESCC tissues compared with corresponding non-tumor tissues (). We further investigated the expression of Numb in another 12 paired ESCC tissues using western blot (). Of these 12 samples, 10 had upregulated Numb expression compared with adjacent non-cancerous tissues. This confirmed the trend of Numb expression observed in our IHC experiments.
Figure 1. Numb expression in ESCC tissues and cell lines. (A) Immunohistochemistry (IHC) on tissue microarrays was used to measure Numb expression and upregulation in ESCC tissues. classes 1-3 correspond directly to Numb staining intensity. Images on left and right are magnified 100 × and 400 ×, respectively. (B) Upregulation of Numb protein in ESCC tissue compared with corresponding non-tumor tissue was confirmed by western blot. A representative image is shown. (C) Numb protein expression was evaluated by western blot in the different ESCC cell lines of KYSE30, KYSE140, EC9706, EC109, EC108 and TE-1, and normal esophageal tissue.
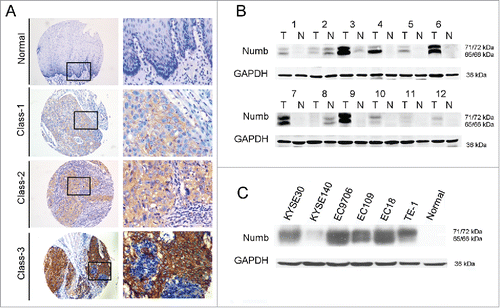
In addition, Numb expression was also detected by western blot in the ESCC cell lines KYSE30, KYSE140, EC9706, EC109, EC108 and TE-1 (). In comparison to normal esophageal tissue, ESCC cells had a range of upregulated Numb expression.
Relationship of Numb expression to clinicopathological features of ESCC patients
A goal of this study was to determine if there was a correlation between Numb expression and the clinical and pathological characteristics of ESCC patients. To this end, we took the median H-score of Numb expression as a cut-off value and, based on this, divided patients into either the low Numb group (H-score H < 4) or high Numb group (H-score ≥ 4). In our cohort, 193 out of 212 patients completed long-term follow-up. Relevant clinical and pathological data are shown in . No significant differences were found in age, sex, tumor differentiation grade, regional lymph node and clinical stage between 2 groups (p>0.05) ().
Table 1. Association between Numb protein expression and clinical characteristics in ESCC samples.
Numb upregulation correlates with poor prognosis in human ESCC
From the 193 patients in our study with complete follow-up information, we discovered that the high Numb group experienced a significantly shorter length of event-free survival (EFS) as compared with patients in the low Numb group (mean EFS: high Numb was 22.5 months and low Numb was 30.0 months, p = 0.015, log-rank test) and overall survival (OS) (mean OS: high Numb was 28.0 months and low Numb was 36.4 months, p = 0.016, log-rank test) (). In addition, we evaluated factors that may influence the clinical outcome of ESCC patients and found that lymph node metastasis (p = 0.002, HR: 1.825, 95% CI: 1.258-2.650), advanced clinical tumor stage (p = 0.001, HR: 1.921, 95% CI: 1.319-2.799) and high Numb expression (p = 0.018, HR: 1.590, 95% CI: 1.082-2.335) were all significant predictors of recurrence in ESCC patients. In terms of long-term survival, lymphatic metastasis (p = 0.001, HR: 2.035, 95% CI: 1.364-3.037), T stage (p = 0.039, HR: 1.596, 95% CI: 1.023-2.488), advanced tumor stage (p < 0.001, HR: 2.209, 95% CI: 1.477-3.304) and high Numb expression (p = 0.018, HR: 1.650, 95% CI: 1.088-2.503) were all predictors of a decrease in length of overall survival (). Further analysis using multiple Cox regression found that lymphatic metastasis (p = 0.006 for EFS; p = 0.004 for OS) as well as high Numb expression (p = 0.044 for EFS; p = 0.047 for OS) were independent factors suggestive of a poor prognosis ().
Figure 2. Increased Numb expression in ESCC tissue correlates with poor prognosis in patients. Numb protein levels in ESCC and adjacent normal tissues from 193 patients with complete long-term follow-up were measured by IHC. Kaplan-Meier analysis revealed that high Numb expression correlated with a significantly shorter (A) EFS (P = 0.015, log-rank test) and (B) OS time (P = 0.016, log-rank test) for all patients. p < 0.05 was considered significant.
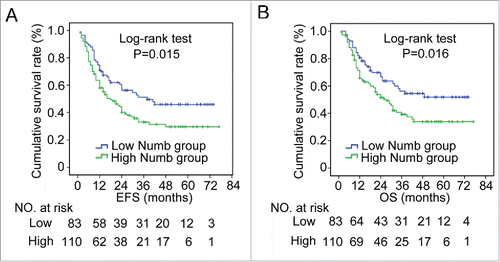
Table 2. Univariate analysis of influence of clinicopathological characteristics on ESCC patient prognosis.
Table 3. Cox multivariate analysis of influence of clinicopathological characteristics on ESCC patient prognosis.
Numb protein increased caspase-3 dependent apoptosis, inhibited proliferation, and downregulated the expression of cancer stem cell biomarkers of ESCC cells
Considering previous studies delineating the multiple functions Numb has in different human malignancies, we hypothesized that Numb plays an important role in tumor growth and metastasis of ESCC. To address this issue, we first effectively knocked Numb down in ESCC cell line, KYSE30, using siRNA interference (). MTT assays was performed to measure ESCC cell survival under different conditions, including treatment with the scr-siRNA negative control and Numb-siRNA. Cell proliferation was then assessed at days 1 to 5 post treatment. As shown in , ESCC cells treated with Numb-siRNA proliferated significantly less than the untreated cells or the cells treated with scr-siRNA. In apoptosis study, KYSE30 cells were incubated with Numb-siRNA or a siRNA negative control, scr-siRNA, for 4 hours, and then treated with cisplatin (which was a potent inducer of apoptosis for KYSE30 cells) at a range of concentrations for another 24 hours. Cells were stained with annexin V and propidium idodide (PI) then analyzed by flow cytrometry (FACS) to measure apoptosis, where apoptotic cells are classified as annexin V+ PtdIns- cells. As seen in and , there was a significant increase in the proportion of apoptotic ESCC cells following exposure to cisplatin as compared with the negative control cells. The mean proportion of apoptotic cells in ESCC vs. negative control cells following treatment with 10µg/ml, 20µg/ml or 40µg/ml cisplatin was 17.2% vs. 3.33%, 25.4% vs. 5.87%, and 49.0% vs. 18.3%, respectively (). The proportion of total cell death was also analyzed and shown in .
Figure 3. siRNA knockdown of endogenous Numb in KYSE30 cells inhibits proliferation, promotes apoptosis, and downregulates cancer stem cell biomarker expression. (A) Downregulation of Numb expression in KYSE30 cells using Numb-siRNA. (B) Using the MTT assay, it was determined that downregulation of Numb suppresses KYSE30 cell growth. (C-E) Following transfection with Numb-siRNA, KYSE30 cells were treated with a range of concentrations of cisplatin for 24 hours. Flow cytometry was performed to quantify the number of apoptotic cells and total dead cells following treatment. KYSE30 cells with lower expression of Numb had significantly more apoptotic cells and total dead cells than the negative control. Data are shown as mean ± SD and are from 3 independent experiments. (F-G) Western blots were performed to assess caspase-3 cleavage in KYSE30 cells following treatment with cisplatin. Decreased expression of Numb was associated with increased cleavage of caspase-3. (H) Decreased Numb expression resulted in lower levels of expression of Oct-4, Sox-2 and Nanog, which are biomarkers of cancer stem cells. All data are from at least 3 independent experiments. Differences were considered statistically significant at p < 0.05 versus the control.
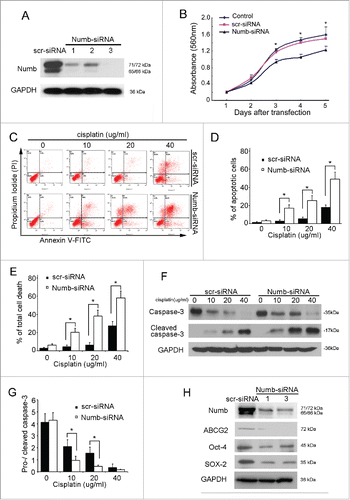
Caspase-3 is a cell death protease involved in both the extrinsic and intrinsic apoptotic pathways,Citation11 and is active only in its cleaved form. Therefore, we examined the protein levels of cleaved caspase-3 in ESCC cells and control cells following siRNA-mediated Numb knockdown and cisplatin treatment. The results showed that the level of cleaved caspase-3 appreciably increased following cisplatin treatment in a concentration dependent manner as compared with the control cells ( and ).
A number of factors contribute to the growth of tumors. In this study, we found a relationship between Numb expression and ESCC stem cell characteristics, where downregulation of Numb significantly inhibited the expression of the cancer stem cell biomarkers Oct4, SOX-2 and Nanog ().
The effect of Numb expression on the migration of ESCC cells
Scratch wound healing and Boyden chamber invasion assays were performed to measure the migration of KYSE30 cells expressing different levels of Numb. Overall, there were no significant differences between cells treated with Numb-siRNA and scr-siRNA, indicating there is no apparent association between Numb expression and the migration of ESCC cells (Fig. S1A-C). Consistent with this conclusion, immunoblot analysis revealed that downregulation of Numb did not affect the expression of src, E-cadherin or vemintin, which play important roles in the migration and metastasis of human tumors (Fig. S1D).
Discussion
Recently published studies have found an association between abnormal expression of Numb and human malignancies, and the role Numb plays depends on the type of tumor. Therefore, Numb has been suggested to be a tumor suppressor due to its downregulation in breastCitation6 and non-small cell lung cancers (NSCLC),Citation7 and early cerebellar granule cell progenitor (GCP) derived cancer cells.Citation12 However, it has also been suggested to be a potential tumor promotor due to its overexpression in astrocytomasCitation9 and cervical squamous carcinoma cells.Citation8 Overall, these findings suggest that the function of Numb is tumor specific. Based on IHC performed from a relatively large cohort of samples, we first found that Numb was overexpressed in human ESCC tissues and several ESCC cell lines. Further analysis revealed a significant association between this increased Numb expression and a poor ESCC patient prognosis. When we abrogated Numb expression in ESCC cells, rates of cisplatin-induced apoptosis significantly increased and cell survival decreased. Altogether, these results suggest that Numb may function as an oncoprotein in ESCC.
The cancer stem cell (CSC) theory is a conceptual link between human cancers and asymmetric cell division that hypothesizes that impairment of asymmetric division of stem cells results in an imbalance in self-renewal and differentiation, thus contributing to tumorigenesis.Citation13 Numb is the first intrinsic molecular determinant of cell fate, where it regulates asymmetric segregation during cell division, and has a range of functions relevant to multiple fields of study, including cancer biology.Citation5 Considering its role in many critical processes in stem cells, we hypothesized that the tumor promotor abilities of Numb protein in ESCC was linked to its affect in cancer stem cells (i.e. cancer-initiating cells). Based on the CSC theory of carcinogenesis, we found decreased expression of Numb in ESCC cells resulted in lower expression levels of Oct4, SOX-2 and Nanog, thus presenting a potential association between Numb expression and CSC characteristics in ESCC. However, the mechanisms by which Numb changes the properties of the CSCs and proliferation of ESCC cells remains unknown. Further studies into the roles of Numb protein in ESCC are currently ongoing.
This is the first report on Numb expression and its roles in ESCC. However, there are several weaknesses in our study that should be discussed. First, the predictive value of our findings concerning the prognosis of the ESCC patients was evaluated by retrospective study, and, therefore, the results need to be confirmed using a prospective study. Second, the functions of Numb in ESCC were examined only using siRNA interference of Numb expression. Numb overexpression experiments were technically challenging because the Numb protein in humans occurs in at least 6 alternatively spliced transcripts (Numb isoforms 1-6). Third, we elucidated the potential roles and functions of Numb protein in ESCC, but the exact mechanism by which Numb influences proliferation and apoptosis needs to be further studied. Furthermore, studies on the functions of different Numb isoforms in ESCC are also important avenues to pursue.
In conclusion, we found that Numb was significantly upregulated in ESCC tissues compared with normal tissues, and this increased expression was associated with a poor prognosis. Downregulation of Numb increased cisplatin-induced apoptosis of ESCC cells, inhibited cellular proliferation and the expression of cancer stem cell biomarkers. These results indicate Numb may be an oncogene in ESCC. Considering the varying functions of Numb in different human malignances, further investigation into the biologic functions and the corresponding mechanisms of Numb is needed. These studies would not only facilitate our understanding of ESCC development and progression, but may also provide potential therapeutic targets for ESCC treatment.
Materials and methods
Study population and follow-up
This study was performed according to a protocol approved by the Ethics Committee and Institutional Review Board of the First Affiliated Hospital of Sun Yat-sen University. 212 ESCC patients who underwent a radical operation at the First Affiliated Hospital of Sun Yat-sen University between January 2010 to December 2014 were enrolled. Of these patients, none had received neoadjuvant therapy before surgery. Patient pathology was reviewed to confirm histology and grade of ESCC. All ESCC cases were staged according to a modification of the American Joint Committee on Cancer (AJCC) 7th edition, 2010. Patient follow-up after surgery was performed at the follow-up center by trained interviewers using a standardized questionnaire during routine ambulatory treatment, clinic visits or telephone interviews. The interval between 2 consecutive follow-ups was typically less than 6 months. Radiographic imaging and histologic information from video-assisted gastroscopy were often used to confirm the follow-up data.
Tissue microarrays and IHC
Tissue microarray construction was performed as described previously.Citation14 Briefly, non-necrotic areas of interest in esophageal tumor tissue that had been embedded in paraffin were identified on corresponding haematoxylin and eosin (H&E) stained sections. The tumor tissues were cored and 0.8 mm diameter cores were transferred to new recipient paraffin blocks using a Tissue Microarrayer. Three cores from each tumor area and 2 cores from the corresponding normal mucosa of the same tissue block were arrayed for each case. Every 100 donor cores were formatted into one recipient block. H&E staining was used to verify all samples. IHC was performed as described previously.Citation15 Samples was embedded in the recipient block and cut by microtome into 4 μm thick tissue sections for immunostaining according to standard techniques. Samples were stained with a rabbit anti-Numb primary antibody (Cell signaling) at a 1:400 dilution. A negative control where the primary antibody was omitted during the procedure was included for each specimen. IHC staining was evaluated separately by 3 different members of our laboratory using the H-score.Citation16,Citation17 Tissue was scored based on the proportions and intensity of cell staining. The proportion of stained cells were scored as 0 (no stained cells), 1 (0-25% of cells were positively stained), 2 (26-50% were positively stained) and 3 (51-100% of cells were positively stained). The intensity of the staining was recorded as 0 (no staining), 1 (class 1 = light yellow), 2 (class 2 = yellow brown) and 3 (class 3 = brown). IHC scores were calculated by multiplying the scores for proportion of stained cells by the scores of the staining intensity. To determine the optimal cut-off point to distinguish between low and high Numb expression, we used the median IHC score (H = 4) for all ESCC tissues as a dichotomized indicator variable. Therefore, samples with an H < 4 were considered to have low Numb expression and samples with an H ≥ 4 had high Numb expression.
Western blots
Western blots were performed as described previously.Citation18 12 pairs of primary ESCC tumor tissues and matched adjacent normal tissues from the proximal resection margins were collected and stored in a liquid nitrogen tank immediately after surgical resection to be used for total protein extraction. Protein extraction from tissues and ESCC cells was performed according to standard techniques. Primary antibodies against Numb (Cell signaling), caspase-3 (Cell signaling), Sox-2(Cell signaling), Oct-4 (Prosci Inc.), Nanog (Santa Cruz Biotechnology) and GAPDH (Santa Cruz Biotechnology) were used for detection of protein. Western blotting data was quantified using the Image J software.
Cell culture and siRNA transfection
The ESCC cell lines of KYSE30, KYSE140, EC9706, EC109, EC108 and TE-1 were cultured in RPMI-1640 (Invitrogen, USA) medium or DMEM medium supplemented with 10% FBS at 37°C in a humidified 5% CO2 incubator.
Oligonucleotide siRNA duplexes were synthesized by Shanghai Gene Pharma (Shanghai, China). The sequences were 5’-GGACCTCATAGTTGACCAG-3’ for Numb-siRNA-1, 5’-GCCUUGCAAUUAGGCUAAATT-3’ for Numb-siRNA-2, and 5’-GGCCUGUAUUAGAGAUCAATT-3’ for Numb-siRNA-3. Transfection of siRNAs was performed using Lipofectamine 2000 reagent (Invitrogen, USA) according to the manufacturer's instructions.
Flow cytometry
Flow cytometry was performed according to previous reports.Citation19 Cells from different experimental conditions were harvested with EDTA-free trypsin, washed twice with PBS, and then centrifuged. After centrifugation, cells were costained with annexin V-FITC and propidium iodide (PtdIns) using an Apoptosis Detection Kit according to the manufacturer's instructions. Stained cells were quantified using a Cytomics FC 500 Flow Cytometer (Beckman Coulter, Fullerton, CA) and Cell QuestTM (BD Bio-sciences) software. Apoptotic cells were classified as annexin V+ PtdIns-.
Wound healing assay
Cells were seeded in 6-well plates and cultured under permissive conditions. After 90% confluence reached, and then starving the cells for 24 h in medium without FBS, the confluent cell monolayer was scratched with a pipette tip in a straight line. The debris was removed and the edge of the scratch was smoothed with PBS washing. The wound healing assays were performed in growth factor-free medium to exclude any proliferative differences. The gap was then inspected and photographed, at a 200× magnification by phase contrast microscopy, at the indicated times shown in the Figures. Three independent experiments were performed.
Boyden chamber assay
Cells were seeded in growth factor-free medium (no EGF or FBS) in the top chamber (Corning, USA), while medium containing EGF or FBS was added to the bottom chamber. The cells was cultured for 24h, and then cells on the undersurface of the chambers were fixed with 1% paraformaldehyde and stained with hematoxylin. The number of cells in 10 random fields of view, at 400× magnification, were counted for each filter. A minimum of 3 independent experiments was performed, and the data are presented as the mean ± SD.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 software (SPSS, Chicago, IL). Continuous variables are shown as mean ± standard deviation (SD) and categorical quantitative data are presented as counts and proportions. The statistical significance of clinical characteristics and follow-up data was assessed by Student's t test for parametric tests, and Mann-Whitney U test for non-parametric tests. Ratios of categorical variables were compared by Chi-square test. Univariate and multivariate Cox regression analyses were performed to determine the association of gathered clinical characteristics on ESCC patient outcomes. The length of overall survival (OS) and event-free survival (EFS) were evaluated by Kaplan-Meier analysis. All experiments were performed at least 3 times independently. Statistical significance was considered as p < 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary_Figure_1.docx
Download MS Word (225.1 KB)Acknowledgments
Zhenguo Liu, Canqiao Luo and Weixiong Yang contributed equally to this work. We would like to thank Fenghua Xu, Honghe Luo and Fotian Zhong for administrative support.
Additional information
Funding
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59(4):225-49; PMID:19474385; https://doi.org/10.3322/caac.20006
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003; 349(23):2241-52; PMID:14657432; https://doi.org/10.1056/NEJMra035010
- Pennathur A, et al. Oesophageal carcinoma. Lancet 2013; 381(9864):400-12; PMID:23374478; https://doi.org/10.1016/S0140-6736(12)60643-6
- Uemura T, Shepherd S, Ackerman L, Jan LY, Jan YN. numb, a gene required in determination of cell fate during sensory organ formation in Drosophila embryos. Cell 1989; 58(2):349-60; PMID:2752427; https://doi.org/10.1016/0092-8674(89)90849-0
- Gulino A, Di Marcotullio L, Screpanti I. The multiple functions of Numb. Exp Cell Res 2010; 316(6):900-6; PMID:19944684; https://doi.org/10.1016/j.yexcr.2009.11.017
- Colaluca IN, Tosoni D, Nuciforo P, Senic-Matuglia F, Galimberti V, Viale G, Pece S, Di Fiore PP. NUMB controls p53 tumour suppressor activity. Nature 2008; 451(7174):76-80; PMID:18172499; https://doi.org/10.1038/nature06412
- Westhoff B, Colaluca IN, D'Ario G, Donzelli M, Tosoni D, Volorio S, Pelosi G, Spaggiari L, Mazzarol G, Viale G, et al. Alterations of the Notch pathway in lung cancer. Proc Natl Acad Sci U S A 2009; 106(52):22293-8; PMID:20007775; https://doi.org/10.1073/pnas.0907781106
- Chen H, Chen X, Ye F, Lu W, Xie X. Symmetric division and expression of its regulatory gene Numb in human cervical squamous carcinoma cells. Pathobiology 2009; 76(3):149-54; PMID:19468255; https://doi.org/10.1159/000209393
- Yan B, Omar FM, Das K, Ng WH, Lim C, Shiuan K, Yap CT, Salto-Tellez M. Characterization of Numb expression in astrocytomas. Neuropathology 2008; 28(5):479-84; PMID:18384513; https://doi.org/10.1111/j.1440-1789.2008.00907.x
- Hong J, Liu Z, Zhu H, Zhang X, Liang Y, Yao S, Wang F, Xie X, Zhang B, Tan T, et al. The tumor suppressive role of NUMB isoform 1 in esophageal squamous cell carcinoma. Oncotarget 2014; 5(14):5602-14; PMID:24980814; https://doi.org/10.18632/oncotarget.2136
- Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ, et al. Apoptosis and cancer: mutations within caspase genes. J Med Genet 2009; 46(8):497-510; PMID:19505876; https://doi.org/10.1136/jmg.2009.066944
- Di Marcotullio L, Ferretti E, Greco A, De Smaele E, Po A, Sico MA, Alimandi M, Giannini G, Maroder M, Screpanti I, et al. Numb is a suppressor of Hedgehog signalling and targets Gli1 for Itch-dependent ubiquitination. Nat Cell Biol 2006; 8(12):1415-23; PMID:17115028; https://doi.org/10.1038/ncb1510
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 2006; 441(7097):1068-74; PMID:16810241; https://doi.org/10.1038/nature04956
- Aquino G, Pannone G, Santoro A, Liguori G, Franco R, Serpico R, Florio G, De Rosa A, Mattoni M, Cozza V, et al. pEGFR-Tyr 845 expression as prognostic factors in oral squamous cell carcinoma: a tissue-microarray study with clinic-pathological correlations. Cancer Biol Ther 2012; 13(11):967-77; PMID:22825335; https://doi.org/10.4161/cbt.20991
- Luo CQ, Yeung SC, Liu ZG, Meng J, Zhong FT, Cheng C. Pulmonary well-differentiated fetal adenocarcinoma with platelet-derived growth factor receptor (PDGFR)alpha expression. Cancer Biol Ther 2012; 13(14):1384-9; PMID:22986233; https://doi.org/10.4161/cbt.22253
- Finn RS, Press MF, Dering J, Arbushites M, Koehler M, Oliva C, Williams LS, Di Leo A. Estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2 (HER2), and epidermal growth factor receptor expression and benefit from lapatinib in a randomized trial of paclitaxel with lapatinib or placebo as first-line treatment in HER2-negative or unknown metastatic breast cancer. J Clin Oncol 2009; 27(24):3908-15; PMID:19620495; https://doi.org/10.1200/JCO.2008.18.1925
- Zhu H, Zhang H, Jin F, Fang M, Huang M, Yang CS, Chen T, Fu L, Pan Z. Elevated Orai1 expression mediates tumor-promoting intracellular Ca2+ oscillations in human esophageal squamous cell carcinoma. Oncotarget 2014; 5(11):3455-71; PMID:24797725; https://doi.org/10.18632/oncotarget.1903
- Cheng C, Liu ZG, Zhang H, Xie JD, Chen XG, Zhao XQ, Wang F, Liang YJ, Chen LK, Singh S, et al. Enhancing chemosensitivity in ABCB1- and ABCG2-overexpressing cells and cancer stem-like cells by an Aurora kinase inhibitor CCT129202. Mol Pharm 2012; 9(7):1971-82; PMID:22632055; https://doi.org/10.1021/mp2006714
- Ding X, Zhu F, Li T, Zhou Q, Hou FF, Nie J. Numb protects renal proximal tubular cells from puromycin aminonucleoside-induced apoptosis through inhibiting Notch signaling pathway. Int J Biol Sci 2011; 7(3):269-78; PMID:21448337; https://doi.org/10.7150/ijbs.7.269
