ABSTRACT
Chronic myelomonocytic leukemia (CMML) is a heterogeneous neoplastic hematologic disorder with worse overall survival. Half of CMML have mutations, but case with concomitant mutations of DNA methyltransferase 3A (DNMT3A) and Internal tandem duplications of the juxtamembrane domain of FLT3 (FLT3-ITD) in CMML was not reported before. We reported a 51-year-old man who had CMML with concomitant mutations in DNMT3A and FLT3-ITD.The patient received decitabine and sorafenib combined treatment. In this report, we reviewed DNMT3A mutation and FLT3 mutation, and we reviewed treatment of decitabine and sorafenib. This report is significant. First: This is the first report on CMML with double-mutations of DNMT3A and FLT3-ITD. Second: It shows the importance of targeted drug in combined treatment of CMML.
Introduction
Chronic myelomonocytic leukemia (CMML) is a heterogeneous neoplastic hematologic disorder characterized by peripheral blood monocytosis and is a tendency for acute myeloid leukemia (AML) transformation.Citation1 CMML is further subdivided into 2 subsets, CMML-1 and CMML-2, depending on the number of blasts plus promonocytes in the peripheral blood and bone marrow.Citation2 The majority of CMML patients are classified as World Health Organization (WHO) CMML-1.Citation3 Cytogenetic abnormalities are found in about 30% of patients with CMML, but none were specific.Citation2 The frequent affected genes in CMML are tet methylcytosine dioxygenase 2 (TET2) (50–60%), Serine And Arginine Rich Splicing Factor 2 (SFSR2) (36–46%), additional sex combs like 1 (ASXL1) (43–44%),Citation4 the frequency of affected DNMT3A is 5–10%, and the frequency of affected FLT3 is 1–3%.Citation4 There was no report about concomitant mutations of DNMT3A and FLT3-ITD in CMML. We reported a case of CMML-1 with concomitant mutations in DNMT3A and FLT3-ITD. The patient was treated with decitabine and sorafenib. We reviewed DNMT3A mutation and FLT3 mutation, and we reviewed treatment of decitabine and sorafenib in CMML. This report displayed the significant role of targeted drug in combined treatment of CMML.
Case report
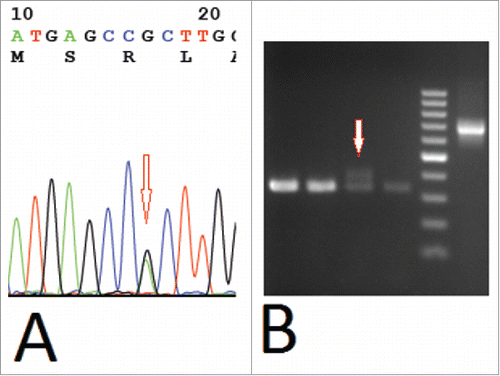
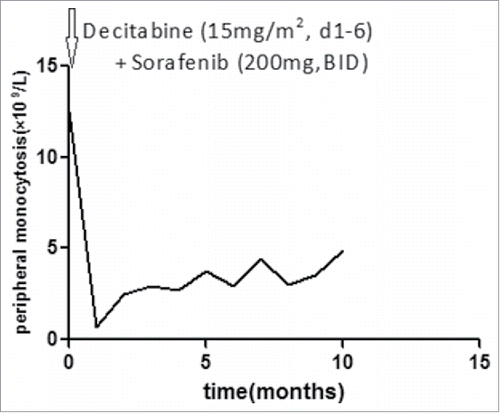
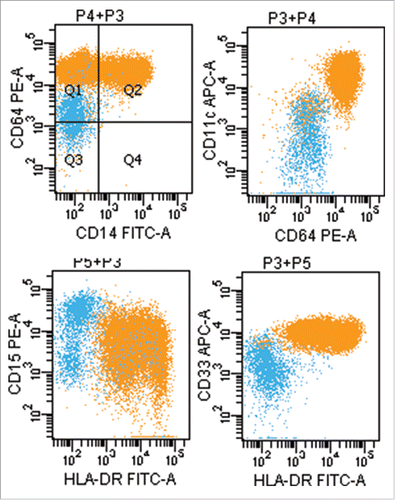
A previously healthy 51-year-old Chinese man presented to our hematology department in our hospital with dizzy for 20 d since October 10, 2014. The result of physical examination showed that there was no superficial lymph node enlargement except for mild splenomegaly. Ultrasound showed the thickness of spleen was 4.2cm (normal: 2–3.5cm).The laboratory data showed an increased level of white blood cell (WBC) [115 × 109/L (normal: 4–10 × 109/L)], increased percentages of monocytes (82 × 109/L) on peripheral blood analysis associated with decreased level of hemoglobin(HB) [112 g/L (normal: 120–160)] and normal level of platelet(PLT) [134 × 109/L (normal: 100–300 × 109/L)]. Level of lactate dehydrogenase (LDH) was increased [LDH 420U/L, (normal: 135–225)]. The characteristic of monocytes (folded nuclei and delicate nuclear chromatin) can be appreciated among the granulocytes in bone marrow smear, and bone marrow aspiration revealed obvious granulocytic component ().The blast percentage (including promocytes) is 8% in bone marrow and 4.2% in peripheral blood. The immunohistochemical staining in bone marrow biopsy specimen showed CD68+ CD15+CD34-MPO-(). Flow cytometric analysis of bone marrow revealed that 78% nucleated cells were monocytes, and there were 56% monocytes with phenotypic abnormalities (CD14-CD64+CD33+) ().Conventional cytogenetic analysis of bone marrow showed normal karyotype. The results from RT-PCR showed there were no fusion genes including BCR-ABL1, PML-RARα, AML1-ETO, CBFβ-MYH11, MLL-AF9, DEK-CAN, NPM1-MLF, FIP1L1-PDGFRA. Molecular genetics analysis revealed the R882H DNMT3A mutation and FLT3-ITD mutation () without mutation of C-kit exon8 and exon17, CEBPA, ASXL1 exon12, SF3B1 exon15, and SRSF2 exon1.The patient received decitabine(15mg/m2, d1–6, CHIA TAI TIANQING, China) and sorafenib (200mg,BID, NATCO, India) treatment after hydroxyureaoral administration decreased the leucocyte count. One month later, bone marrow aspiration revealed complete hematological remission, but mutational analysis was not performed. The patient rejected hospitalization again, so he received long-term treatment of oral sorafenib (200mg, BID). He took blood routine examination once every month and the results were no obvious abnormality. He had no discomfortableness, rash, abnormality of liver and renal function. The peripheral monocytosis did no increase significantly (). On August 14, 2015, the patient was again admitted to our department for fever and petechiae. The physical examination showed that there was an abscess on dorsum of left foot, and there were scattered petechiae on lower limbs without superficial lymph node enlargement and without hepatosplenomegaly. The laboratory data showed normal level of WBC [10 × 109/L (normal: 4–10 × 109/L)], normal level of HB [133 g/L (normal: 120–160)] and increased percentages of mature monocytes(49%) on peripheral blood analysis associated with decreased level of PLT [9 × 109/L (normal: 100–300 × 109/L)]. Bone marrow aspiration revealed obvious increased monocytes (mature monocyte 63%).The ratio of blasts (including promocytes) is 7% in bone marrow. Molecular genetics analysis revealed there was still the R882H DNMT3A mutation, but PCR did not detectFLT3-ITD mutation again. Although the patient was treated with meropenem and teicoplanin, he had persistent hyperpyrexia and died of disseminated intravascular coagulation at 4th day after the patient admitted to hospital. We guessed the uncontrolled infection should be fundamental cause.
Figure 1. Histopathology of bone marrow. (A) the Wright-Giemsa stain in bone marrow smear, the characteristic of monocytes (folded nuclei and delicate nuclear chromatin) is obvious. (B) a bone marrow biopsy specimen, the granulocytic component is most obvious in the biopsy specimen. (C,D) positive staining for CD15 and CD68 E, F: negativity for MPO and CD34.
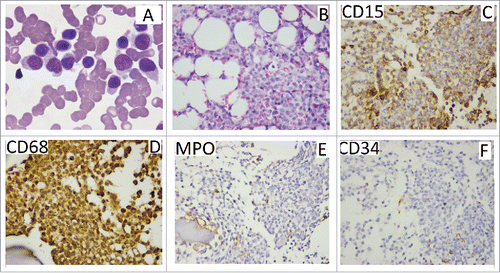
Figure 2. Phenotypic abnormalities of monocytes detected by a multicolor flow cytometric approach at initial diagnosis.
Discussion
CMML is described by WHO as a clonal disease of the haematopoietic stem cell represented by persistent monocytosis. In the light of the WHO 2008 classification, diagnosis of CMML needs sustained monocytosis for greater than 1.0 × 109/L for 3 months. In patients with obviously increased leukocyte counts, the monocytes should account for greater than 10% of the WBC.Citation5 Cases are divided into CMML-1 (<5% peripheral blasts and promonocytes, <10% bone marrow blasts and promonocytes) and CMML-2 (5–19% peripheral and 10–19% marrow blasts and promonocytes).Citation5 Our case met WHO criterion of CMML-1; and results of fusion genes such as BCR-ABL1 ruled out CML. The immunophenotyping analysis of this case showed immature abnormal monocytes without increased blasts. The immunophenotyping analysis did not play the critical role in diagnosis of CMML, but flow cytometric analysis is valuable as a tool for distinguishing CMML from reactive monocytosis.Citation6
With a median survival of only 20∼30 months and leukemic transformation rates(5 years) of 45%,the prognosis of CMML is poor overall.Citation7 Prognostic parameter recognized by WHO classification did not satisfy demand of treatment, especially for cases with normal karyotype. With whole-exome sequencing application, more and more mutated genes were found in CMML. Overall, about 50% of CMML has mutations in genes. The genes covered many cellular pathways, such as signaling, RNA splicing, transcription, cohesin complex DNA methylation, histone modifications.Citation4 In the future, these mutations may play important roles in the prognosis of CMML. ASXL1 mutations in CMML have predictable value; the median survival in cases of ASXL1 mutation (13.2 months) is significantly shorter than that of ASXL1 wild type (30.0 months).Citation8 SRSF2 mutations were linked with a statistically significant reduction in All survival (OS).Citation9 The genes such as DNMT3A and FLT3 were not studies further in CMML, but have been researched deeply in AML.
DNA methylation is an epigenetic modification that is important in cell differentiation (e.g. haematopoietic stem cell differentiation) and gene regulation. DNA methylation refers to the addition of a methyl group to the C5 position of the pyrimidine ring of cytosines to form 5–methylcytosine.Disrupted DNA methylation patterns in malignancies suggest a role in the development of cancer, but there was seldom evidence that genetic aberrations directly linked the DNA methylation machinery to malignancies. DNMT3A(encoding DNA methyltransferase 3A) is a critically important new tumor-suppressing gene. DNMT3A is a member of the DNA methyltransferase proteins that enzymatically add a methyl group to 5′cytosine in the CpG dinucleotides. The DNMT3A mutations resulted in loss-of-function with a dominant negative effect.Citation10 Present studies shows that DNMT3A mutation is related to tumorigenesis and prognosis of hematologic malignancies.Citation11 FLT3 is a receptor tyrosine kinase which is expressed on immature haematopoietic cells and takes part in the development of stem cells. The wild type FLT3 receptor is transmembrane protein, and this protein contains 5 extracellular immunoglobulin-like domains, a transmembrane domain, a JM domain and a split intracellular kinase domain. FLT3 is activated and is dimerized when FLT3 ligand binds to the extracellular domain of FLT3.Citation27 Subsequent phosphorylation of FLT3 activates downstream signaling pathways. Mutations of DNMT3A and FLT3 influenced the pathophysiology and clinical features of AML at a different angle.
DNMT3A mutation was intensively studied in AML (). Recent studies have found that frequency of DNMT3A mutations in AML is 17%∼30% and most of DNMT3A mutations are heterozygousCitation12,15,16,18 Besides Common DNMT3A R882 mutation, mutations of V897D, G543C and R478W are also found.Citation12,13 DNMT3A mutations in adult AML are associated with clinical features such as gender, age, white blood cell countCitation14 andtype of AML.Citation20 Although there were many studies, there was no definite conclusion about effect of DNMT3A mutations on AML prognosis ().Citation15-21,26
Table 1. DNMT3A and FLT3 in leukemia.
FLT3 is the most frequently mutated gene in AML (), with an approximately 28%∼34%of AML cases harboring FLT3 mutationsCitation28,29 FLT3 have 2 kinds of mutations: an internal tandem duplication (ITD) in the juxtamembrane domain and a point mutation of the tyrosine kinase domain (TKD). Estimated 20–25% of AML cases harbor FLT3-ITD mutations,Citation27 and FLT3-ITDmutations in adult AML are associated with clinical features such as having higher white blood cell count and higher peripheral blood blast cell count.Citation47 These mutations lead to constitutive kinase activation. Many studies showed that FLT3-ITD was associated with worse prognosis.Citation29
Although there were many studies about DNMT3A and FLT3 in AML, there were fewer reports on DNMT3A in CMML (). Abdel-Wahab did not detect DNMT3A mutations in 15 CMML.Citation23 Anna M. Jankowska analyzed DNMT3A mutations in CMML and CMML-derived AML. DNMT3A mutations were identified in 2 of 52 cases with CMML (4%), all with CMML-2 (2 of 16; 12%). 5 mutant cases were detected in cases with CMML-derived AML (6 of 20; 25%). The DNMT3A mutations were mostly heterozygous and they affected the R882 position.Citation22 The result from analysis of 75 CMML (8/75 DNMT3A mutations) showed OS was associated with mutations in DNMT3A(P = 0.015).Citation26 Another report from analysis of 312 CMML (5/227 DNMT3A mutations) showed ASXL1 mutations, not DNMT3A mutations, predicted inferior OS.Citation8 From the current research results, the frequency of DNMT3A mutation in CMML tends to increase in progression to AMLCitation22, 26 So, more clinical studies are needed to define the role of DNMT3A mutations in prognosis of CMML.
Reports of FLT3-ITD mutations were also less in CMML (). Wang analyzed 68 cases of CMML, and detected only one case with FLT3-ITD mutation.Citation30 Chen W analyzed 29 cases of CMML, and detected 3 cases with FLT3-ITD mutation.Citation31 At MD Anderson Cancer Center, analysis of FLT3 mutation was performed on 302 cases of CMML. 13 (4.3%) CMML patients had FLT3 mutations: 8 patients had FLT3-ITD mutations and 5 patients had FLT3-TKD mutations. There was no significant difference in disease characteristics between CMML with and without FLT3 mutations, and there was no significant difference in median OS between CMML with and without FLT3 mutation.Citation32 Shih LY evaluated FLT3-ITD mutation in 198 cases of MDS, and detected 3 cases with FLT3-ITD mutation from 51 CMML patients. There was no significant difference in clinicohematologic characteristics, cytogenetic characteristics, or international prognostic scoring system score among MDS patients with and without FLT3 mutation. But FLT3-ITD was identified as an independent predictor of reduced time to development of AML and reduced overall survival on multivariate analysis. The role of FLT3-ITD in CMML was not estimated because of fewer cases.Citation33 Although frequency of FLT3-ITD mutation was less in CMML, the result from murine model showed Flt3-ITD “knockin” mice developed myeloproliferative disease resembling CMML.Citation34
Concomitant mutations in DNMT3A and FLT3 were frequent in AML,Citation21 and ‘double-mutated' (FLT3/ITD+, DNMT3A+) patients were considered as having very poor prognosis.Citation35 Although there were no reports on CMML with concomitant mutations in DNMT3A and FLT3, we think that concomitant mutations in DNMT3A and FLT3-ITD also might bring poor prognosis in this patient.
Aberrant epigenome changes are now recognized to be vital in driving the development of leukemia. Acquisition of the mutation of DNMT3A in haematopoietic stem cells can lead to clonal amplification resulting in a pre-leukemic stem cell (pre-LSC) population. Pre-LSCs can become fully transformed into fully transformed leukemic stem cells with the acquisition of additional driver mutations (e.g., FLT3-ITD).Citation45 CMML with DNMT3A mutation may quickly transform into AML after the mutation of FLT3-ITD happened. One case reported before gave the direct evidence that a pre-leukemic clone can evolve to AML.Citation11 This allogenic bone marrow transplantation case revealed definitely the process of transplanted bone marrow cells with DNMT3A mutation (for one lymphoma patient) transforming into AML after the acquisition of additional driver mutation of FLT3-ITD. After the patient acquired complete remission by inductive treatment, only DNMT3A mutation could be detected without mutation of FLT3-ITD. After the patient relapsed, mutations of DNMT3A and FLT3-ITD could be detected again. Another research showed that DNMT3A deletion in oncogenic Ras models not only significantly promoted CMML progression but also led to transformation to AML.Citation25 We might give a reasonable speculation that DNMT3A might be trunk somatic mutation in CMMLCitation11,24; and DNMT3A mutant brought out CMML through disturbing gene expression/DNA methylation in mice.Citation24 FLT3-ITD mutation might be a key subsequent mutation which transforms CMML to acute leukemia. So the ‘double-mutated' (FLT3/ITD+, DNMT3A+) CMML transformed very quickly to acute leukemia and had a very poor prognosis. We hope there will be zooperal study and clinical study to approve the guess.
The case of ‘double-mutated' (FLT3/ITD+, DNMT3A+) CMML in our report received one cycle treatment of decitabine and sorafenib long-term oral, he got approximately 10 months' remission. This case gave us a good example of combined targeted drug treatment.
Decitabine, as hypomethylating agent, was approved for treatment of patients with MDS. Three studies have now been completed using decitabine specifically in patients with CMML. The results showed complete remission ranged from 10% to 58%Citation36, 37 Low-dose, dose-intensity schedule of decitabine optimized epigenetic modulation and clinical responses in MDS and CMML.Citation38 For CMML with normal karyotype, response to decitabine is highly variable. Specific molecular signatures were evaluated to predict decitabine response in CMML.Citation39 A case report showed combination regimen of low-dose (15mg/m2 intravenously daily for 5 days) decitabine, low-dose cytarabine, low-dose aclarubicin and granulocyte colony-stimulating factor successfully managed one case of AML transformed from CMML in the elderly.Citation40 Until now, the role of DNMT3A mutation was undefined in CMML treated with decitabine.
Sorafenib is a new oral tyrosine kinase inhibitor, and main targets of sorafenib are deregulated receptor tyrosine kinase pathways in cancer including the Ras/Raf/MEK pathway, vascular endothelial growth factor (VEGF) pathway, and FLT3. Sorafenib was used for advanced renal cell cancer and hepatocellular cancer and shows good clinical activity. Sorafenib had superior performance in AML with FLT3-ITD mutation; though there were FLT3 mutations resistant to sorafenib.Citation41 Tschan-Plessl A studied 17 patients with FLT3-ITD positive AML. These patients were treated with sorafenib (pre-transplant and post-transplant) combined with allogeneic HSCT. The result showed sorafenib with synergy of cGvHD can achieve high rates of sustained remission in high-risk patients.Citation42 There were 2 case reports about successful treatment of CMML using sorafenibCitation43,44 Mutations of DNMT3A and FLT3 influenced the pathophysiology of leukemia at a different angle, the combination of FLT3 and DNA methyltransferase inhibition is synergistically cytotoxic to FLT3/ITD AML cells.Citation48
The case in our report received decitabine and sorafenib combined treatment and got approximately 10 months' remission. When he admitted to hospital again, he did not transform to AML. We could still detect DNMT3A mutation, but could not detect FLT3 mutation. In the course of CMML transforming into AML, there was clone evolution. DNMT3A mutation should be trunk somatic mutation in CMMLCitation11, 45 FLT3-ITD mutation is pivotal subsequent cooperating mutations in development of AML.Citation45 The patient did not transform to AML after sorafenib treatment, we surmise loss of FLT3-ITD played an important role. DNMT3A mutations are persistent at long-term remission in adult patients with AML.Citation46 The results from decitabine treatment showed CMML complete remission ranged from 10% to 58%Citation36,37 and there were no reports on the effect of decitabine in CMML with DNMT3A mutation. This patient received only one course of decitabine treatment. After the patient got complete hematological remission induced by treatment of decitabine and sorafenib, the patient rejected treatment of decitabine; he only received treatment of oral sorafenib. Persistence of DNMT3A mutation implied existence of trunk tumor clone and led the relapse of CMML. Because the mutation of FLT3-ITD was controlled by sorafenib, the patient did not transform into AML for 10months. So sorafenib was very important for ‘double-mutated' (FLT3/ITD+, DNMT3A+) CMML. Treatment of sorafenib may effectively prolong ‘double-mutated' (FLT3/ITD+, DNMT3A+) CMML transforming into AML.
In conclusion, we reported one case of CMML with double-mutations of DNMT3A and FLT3-ITD, and this case showed the importance of targeted drug in combined treatment of CMML with FLT3 mutation.
Disclosure of potential conflicts of interest
The authors declare that they have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
Funding
This work was supported by a Grant from the National Natural Science Foundation of China (No 81370658).
References
- Padron E, Komrokji R, List AF. The clinical management of chronic myelomonocytic leukemia. Clin Adv Hematol Oncol 2014; 12:172-178. PMID:24927265.
- Sherdlow SH, Campo E, Harris NL. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. IARC; Lyon: 2008, 4th edition:76.
- Padron E, Garcia-Manero G, Patnaik MM, Itzykson R, Lasho T, Nazha A, Rampal RK, Sanchez ME, Jabbour E, Al Ali NH, et al. An international data set for CMML validates prognostic scoring systems and demonstrates a need for novel prognostication strategies. Blood Cancer J 2015; 5:e333; PMID:26230957; https://doi.org/10.1038/bcj.2015.53
- Zoi K, Cross NC. Molecular pathogenesis of atypical CML, CMML and MDS/MPN-unclassifiable. Int J Hematol 2015; 101:229-42; PMID:25212680; https://doi.org/10.1007/s12185-014-1670-3
- Padron E, Komrokji R, List AF. The clinical management of chronic myelomonocytic leukemia. Clin Adv Hematol Oncol 2014; 12:172-8. PMID:24927265
- Xu Y, McKenna RW, Karandikar NJ, Pildain AJ, Kroft SH. Flow cytometric analysis of monocytes as a tool for distinguishing chronic myelomonocytic leukemia from reactive monocytosis. Am J Clin Pathol 2005; 124:799-806; PMID:16203279; https://doi.org/10.1309/HRJ1XKTD77J1UTFM
- Such E, Germing U, Malcovati L, Cervera J, Kuendgen A, Della Porta MG, Nomdedeu B, Arenillas L, Luño E, Xicoy B, et al. Development and validation of a prognostic scoring system for patients with chronic myelomonocytic leukemia. Blood 2013; 121:3005-15; PMID:23372164; https://doi.org/10.1182/blood-2012-08-452938
- Itzykson R, Kosmider O, Renneville A, Gelsi-Boyer V, Meggendorfer M, Morabito M, Berthon C, Adès L, Fenaux P, Beyne-Rauzy O, et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J Clin Oncol 2013; 31:2428-36; PMID:23690417; https://doi.org/10.1200/JCO.2012.47.3314
- Meggendorfer M, Roller A, Haferlach T, Eder C, Dicker F, Grossmann V, Kohlmann A, Alpermann T, Yoshida K, Ogawa S, et al. SRSF2 mutations in 275 cases with chronic myelomonocytic leukemia (CMML).Blood 2012; 120:3080-8; PMID:22919025; https://doi.org/10.1182/blood-2012-01-404863
- Hou HA, Kuo YY, Liu CY, Chou WC, Lee MC, Chen CY, Lin LI, Tseng MH, Huang CF, Chiang YC, et al. DNMT3A mutations in acute myeloid leukemia: stability during disease evolution and clinical implications. Blood 2012; 119:559-568; PMID:22077061; https://doi.org/10.1182/blood-2011-07-369934
- Hahn CN, Ross DM, Feng J, Beligaswatte A, Hiwase DK, Parker WT, Ho M, Zawitkowski M, Ambler KL, Cheetham GD, et al. A tale of two siblings: two cases of AML arising from a single pre-leukemic DNMT3A mutant clone.Leukemia 2015; 9:1-4.; PMID:25748685; https://doi.org/10.1038/leu.2015.67
- Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, Kandoth C, Payton JE, Baty J, Welch J, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med 2010; 363:2424-33; PMID:21067377; https://doi.org/10.1056/NEJMoa1005143
- Yan XJ, Xu J, Gu ZH, Pan CM, Lu G, Shen Y, Shi JY, Zhu YM, Tang L, Zhang XW, et al. Exome sequencing identifi es somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nat Genet 2011; 43:309-15; PMID:21399634; https://doi.org/10.1038/ng.788
- Li Y, Zhu B. Acute myeloid leukemia with DNMT3A mutations. Leuk Lymphoma 2014; 55:2002-12; PMID:24283755; https://doi.org/10.3109/10428194.2013.869802
- Gale RE, Lamb K, Allen C, El-Sharkawi D, Stowe C, Jenkinson S, Tinsley S, Dickson G, Burnett AK, Hills RK, et al. Simpson's Paradox and the Impact of Different DNMT3A Mutations on Outcome in Younger Adults WithAcute Myeloid Leukemia.J Clin Oncol 2015; 33:2072-83; PMID:25964253; https://doi.org/10.1200/JCO.2014.59.2022
- Zare-Abdollahi D, Safari S, Movafagh A, Riazi-Isfahani S, Ghadyani M, Feyzollah HG, Nasrollahi MF, Omrani MD. A mutational and expressional analysis of DNMT3A in acute myeloid leukemia cytogenetic subgroups. Hematology 2015; 20:397-404; PMID:25592687; https://doi.org/10.1179/1607845415Y.0000000001
- Tie R, Zhang T, Fu H, Wang L, Wang Y, He Y, Wang B, Zhu N, Fu S, Lai X, et al. Association between DNMT3A mutations and prognosis of adults with de novo acute myeloid leukemia: a systematic review and meta-analysis. PLoS One 2014; 9:e93353; PMID:24936645; https://doi.org/10.1371/journal.pone.0093353
- Thol F, Damm F, Lüdeking A, Winschel C, Wagner K, Morgan M, Yun H, Göhring G, Schlegelberger B, Hoelzer D, et al. Incidence and prognostic influence of DNMT3A mutations in acute myeloid leukemia. J Clin Oncol 2011; 29:2889-96; PMID:21670448; https://doi.org/10.1200/JCO.2011.35.4894
- Ibrahem L, Mahfouz R, Elhelw L, Abdsalam EM, Soliman R. Prognostic significance of DNMT3A mutations in patients with acute myeloid leukemia. Blood Cells Mol Dis 2015; 54:84-9; PMID:25172541; https://doi.org/10.1016/j.bcmd.2014.07.015
- Shivarov V, Gueorguieva R, Stoimenov A, Tiu R. DNMT3A mutation is a poor prognosis biomarker in AML: results of a meta-analysis of 4500 AML patients. Leuk Res 2013; 37:1445-50; PMID:23962568; https://doi.org/10.1016/j.leukres.2013.07.032
- Gaidzik VI, Schlenk RF, Paschka P, Stölzle A, Späth D, Kuendgen A, von Lilienfeld-Toal M, Brugger W, Derigs HG, Kremers S, et al. Clinical impact of DNMT3A mutations in younger adult patients with acute myeloid leukemia: results of the AML Study Group (AMLSG). Blood 2013; 121:4769-77; PMID:23632886; https://doi.org/10.1182/blood-2012-10-461624
- Jankowska AM, Makishima H, Tiu RV, Szpurka H, Huang Y, Traina F, Visconte V, Sugimoto Y, Prince C, O'Keefe C, et al. Mutational spectrum analysis of chronic myelomonocytic leukemia includes genes associated with epigenetic regulation: UTX, EZH2, and DNMT3A. Blood 2011; 118:3932-41; PMID:21828135; https://doi.org/10.1182/blood-2010-10-311019
- Abdel-Wahab O, Pardanani A, Rampal R, Lasho TL, Levine RL, Tefferi A. DNMT3A mutational analysis in primary myelofibrosis, chronic myelomonocytic leukemia and advanced phases of myeloproliferative neoplasms. Leukemia 2011; 25:1219-20; PMID:21519343; https://doi.org/10.1038/leu.2011.82
- Xu J, Wang YY, Dai YJ, Zhang W, Zhang WN, Xiong SM, Gu ZH, Wang KK, Zeng R, Chen Z, et al. DNMT3A Arg882 mutation drives chronic myelomonocytic leukemia through disturbing gene expression/DNA methylation in hematopoietic cells. Proc Natl Acad Sci U S A 2014; 111:2620-5; PMID:24497509; https://doi.org/10.1073/pnas.1400150111
- Chang YI, You X, Kong G, Ranheim EA, Wang J, Du J, Liu Y, Zhou Y, Ryu MJ, Zhang J. Loss of Dnmt3a and endogenous Kras(G12D/+) cooperate to regulate hematopoietic stem and progenitor cell functions in leukemogenesis. Leukemia 2015; 29:1847-56; PMID:25801914; https://doi.org/10.1038/leu.2015.85
- Kar SA, Jankowska A, Makishima H, Visconte V, Jerez A, Sugimoto Y, Muramatsu H, Traina F, Afable M, Guinta K, et al. Spliceosomal gene mutations are frequent events in the diverse mutational spectrum of chronic myelomonocytic leukemia but largely absent in juvenilemyelomonocytic leukemia. Haematologica 2013; 98:107-13; PMID:22773603; https://doi.org/10.3324/haematol.2012.064048
- Stirewalt DL, Radich JP. The role of flt3 in haematopoietic malignancies. Nat Rev Cancer 2003; 3:650-65; PMID:12951584; https://doi.org/10.1038/nrc1169
- Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, Sonoda Y, Fujimoto T, Misawa S. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia 1996; 10:1911-8. PMID:8946930
- Port M, Böttcher M, Thol F, Ganser A, Schlenk R, Wasem J, Neumann A, Pouryamout L. Prognostic significance of FLT3 internal tandem duplication, nucleophosmin 1, and CEBPA gene mutations for acute myeloid leukemia patients with normal karyotype and younger than 60 years: a systematic review and meta-analysis. Ann Hematol 2014; 93:1279-86; PMID:24801015; https://doi.org/10.1007/s00277-014-2072-6
- Wang SA, Galili N, Cerny J, Sechman E, Chen SS, Loew J, Liu Q, Fadare O, Hasserjian R, Jones D, et al. Chronic myelomonocytic leukemia evolving from preexisting myelodysplasia shares many features with de novo disease. Am J Clin Pathol 2006; 126:789-97; PMID:17050076; https://doi.org/10.1309/FU04P779U310R3EE
- Chen W, Huang Q. Detection of FLT3/ITD, JAK2(V617F) and NPM1 gene mutations in chronic myelomonocytic leukemia. Leuk Res 2009; 33:e207-9; PMID:19540590; https://doi.org/10.1016/j.leukres.2009.05.015
- Daver N, Strati P, Jabbour E, Kadia T, Luthra R, Wang S, Patel K, Ravandi F, Cortes J, Qin Dong X, Kantarjian H, Garcia-Manero G. FLT3 mutations in myelodysplastic syndrome and chronic myelomonocytic leukemia. Am J Hematol 2013; 88:56-9; PMID:23115106; https://doi.org/10.1002/ajh.23345
- Shih LY, Lin TL, Wang PN, Wu JH, Dunn P, Kuo MC, Huang CF. Internal tandem duplication of fms-like tyrosine kinase 3 is associated with poor outcome in patients with myelodysplastic syndrome. Cancer 2004; 101:989-98; PMID:15329908; https://doi.org/10.1002/cncr.20440
- Lee BH, Tothova Z, Levine RL, Anderson K, Buza-Vidas N, Cullen DE, McDowell EP, Adelsperger J, Fröhling S, Huntly BJ, Beran M, Jacobsen SE, Gilliland DG. FLT3 mutations confer enhanced proliferation and survival properties to multipotent progenitors in a murine model of chronic myelomonocytic leukemia. Cancer Cell 2007; 12:367-80; PMID:17936561; https://doi.org/10.1016/j.ccr.2007.08.031
- Loghavi S, Zuo Z, Ravandi F, Kantarjian HM, Bueso-Ramos C, Zhang L, Singh RR, Patel KP, Medeiros LJ, Stingo F, et al. Clinical features of de novo acute myeloid leukemia with concurrent DNMT3A, FLT3and NPM1 mutations. J Hematol Oncol 2014 Oct 4; 7:74; PMID:25281355; https://doi.org/10.1186/s13045-014-0074-4
- Aribi A, Borthakur G, Ravandi F, Shan J, Davisson J, Cortes J, Kantarjian H. Activity of decitabine, a hypomethylating agent, in chronic myelomonocytic leukemia. Cancer 2007; 109:713-7; PMID:17219444; https://doi.org/10.1002/cncr.22457
- Wijermans PW, Rüter B, Baer MR, Slack JL, Saba HI, Lübbert M. Efficacy of decitabine in the treatment of patients with chronic myelomonocytic leukemia (CMML). Leuk Res 2008; 32:587-91; PMID:17881052; https://doi.org/10.1016/j.leukres.2007.08.004
- Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O'Brien S, Cortes J, Faderl S, Bueso-Ramos C, Ravandi F, Estrov Z, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood 2007; 109:52-7; PMID:16882708; https://doi.org/10.1182/blood-2006-05-021162
- Meldi K, Qin T, Buchi F, Droin N, Sotzen J, Micol JB, Selimoglu-Buet D, Masala E, Allione B, Gioia D, et al. Specific molecular signatures predict decitabine response in chronic myelomonocytic leukemia. J Clin Invest 2015; 125:1857-72; PMID:25822018; https://doi.org/10.1172/JCI78752
- Deng Q, Li JY, Liu PJ, Zhao MF. Successful management of acute myeloid leukemia transformed from chronic myelomonocytic leukemia in the elderly by a combination regimen of decitabine and cytarabine, aclarubicin and granulocyte colony-stimulating factor: A case report. Oncol Lett 2015; 9:1217-20. PMID:25663885; https://doi.org/10.3892/ol.2015.2870
- Smith CC, Lin K, Stecula A, Sali A, Shah NP. FLT3 D835 mutations confer differential resistance to type II FLT3 inhibitors. Leukemia 2015; 29:2390-2; PMID:26108694; https://doi.org/10.1038/leu.2015.165
- Tschan-Plessl A, Halter JP, Heim D, Medinger M, Passweg JR, Gerull S. Synergistic effect of sorafenib and cGvHD in patients with high-risk FLT3-ITD+AML allows long-term disease control after allogeneic transplantation. Ann Hematol 2015; 94:1899-905; PMID:26233683; https://doi.org/10.1007/s00277-015-2461-5
- Kosmider O, Chapuis N, Kaltenbach S, Coriat R, Boudou Rouquette P, Willems L, Chesnais V, Radford-Weiss I, Bardet V, Mayeux P, et al. Sustained leukemia-free state and molecular response to sorafenib in a patient with chronic myelomonocytic leukemia in transformation driven by homozygous FLT3-ITD malignant hematopoiesis. Clin Lymphoma Myeloma Leuk 2013; 13:347-50; PMID:23246161; https://doi.org/10.1016/j.clml.2012.11.007
- von Bubnoff N, Rummelt C, Menzel H, Sigl M, Peschel C, Duyster J. Identification of a secondary FLT3/A848P mutation in a patient with FLT3-ITD-positive blast phase CMML and response to sunitinib and sorafenib. Leukemia 2010; 24:1523-5; PMID:20520641; https://doi.org/10.1038/leu.2010.122
- Chan SM, Majeti R. Role of DNMT3A, TET2, and IDH1/2 mutations in pre-leukemic stem cells in acute myeloid leukemia. Int J Hematol 2013; 98:648-57; PMID:23949914; https://doi.org/10.1007/s12185-013-1407-8
- Pløen GG, Nederby L, Guldberg P, Hansen M, Ebbesen LH, Jensen UB, Hokland P, Aggerholm A. Persistence of DNMT3A mutations at long-term remission in adult patients with AML. Br J Haematol 2014; 167:478-86; PMID:25371149; https://doi.org/10.1111/bjh.13062
- Mori Y, Yoshimoto G, Kumano T, Miyamoto T, Iino T, Takenaka K, Iwasaki H, Harada N, Kinukawa N, Nagafuji K, et al. Distinctive expression of myelomonocytic markers and down-regulation of CD34 in acute myelogenous leukaemia with FLT3 tandem duplication and nucleophosmin mutation. Eur J Haematol 2007; 79:17-24; PMID:17598835; https://doi.org/10.1111/j.1600-0609.2007.00866.x
- Chang E, Ganguly S, Rajkhowa T, Gocke CD, Levis M, Konig H. The combination of FLT3 and DNA methyltransferase inhibition is synergistically cytotoxic to FLT3/ITD acute myeloid leukemia cells. Leukemia 2016; 30:1025-32; PMID:26686245; https://doi.org/10.1038/leu.2015.346