ABSTRACT
Background: Circulating tumor cells (CTCs) and microemboli (CTM) are attracting increasing attention in medical biology and clinical practice. However, the clinical relevance of CTCs in nasopharyngeal carcinoma (NPC) has not yet been ascertained, and no study has focused on the influence of Epstein-Barr virus (EBV) status on CTCs in NPC patients. These issues were therefore examined. Methods: Peripheral blood samples were prospectively obtained from 33 NPC patients before treatment. CTCs and CTM were captured using the Isolation by Size of Epithelial Tumor (ISET) method. Immunohistochemistry on CK5/6 (cytokeratin5/6) and P63, as well as in situ hybridization of EBERs (EBV-encoded RNAs) were used to validate the harvested tumor cells. Results: Out of 33 NPC patients, CTCs were detected in 22 cases (66.7%), and CTM were observed in 2 cases (6.1%). CTCs were presented in all stages of NPC patients but had no association with the TNM stages (all P > 0.05). The presence of CTCs appeared to correlate with EBV activation status. Among the total NPC patients, the EBV VCA-IgA levels in CTC-positive cases were higher than that in CTC-negative cases (negative vs. positive: 3.88 vs. 4.86, P = 0.016). A similar result was observed in the patients without distant metastasis (negative vs. positive: 3.76 vs. 4.95, P = 0.009). When the number of CTCs was considered, CTC counts were found to correlate with EBV VCA-IgA (R = 0.382, P = 0.041) and EBV DNA load (R = 0.396, P = 0.033) for all NPC patients as well as NPC patients without distant metastases. Conclusions: These findings strongly suggested detectable CTCs/CTM in all stages of NPC patients and support a positive correlation between CTCs and EBV activation in NPC patients.
Introduction
Nasopharyngeal carcinoma (NPC) is the most common type of head and neck cancer in the Southeast Asia.Citation1 Although radiotherapy is a very effective modality of treatment of non-disseminated NPC, some patients relapse after the treatment due to un-recognizable local or distant metastasis. For treatment options for metastatic NPC, circulating tumor cells (CTCs), a blood biomarker with a potential prognostic and predictive value, have attracted increasing attention in both medical biology and clinical practice.
CTCs are thought to be shed from the primary tumor and circulate through the bloodstream.Citation2,3 These rare cells can be enriched and detected via different technologies.Citation4,5,6 CTCs have been considered to be of prognostic value in various solid tumors, including metastatic breast, prostate, colorectal, and non-small-cell lung cancers.Citation7,8,9,10 Although there have been several available techniques used to detect CTCs, the study on CTCs in NPC patients is very limited. One recent published study has demonstrated detectable CTCs in the peripheral blood of NPC patients, in which the CTCs were screened based on the magnetic bead isolation technique and were defined as nucleated cells lacking CD45 expression.Citation11 Since CTCs in NPC patients is poorly understood, studies are needed to illustrate the status of CTCs in NPC for guiding treatment and prognosis.
Epstein-Barr virus (EBV) activation is a frequent event in the process of NPC carcinogenesis.Citation12 The pretreatment levels of circulating EBV DNA load and EBV-related antigens and antibodies are correlated with NPC tumor loads and are prognostic factors for NPC.Citation13,14,15 Currently, there is no report about the EBV status on CTCs in NPC patients. In the present study, we evaluate the association between EBV activation and CTC detection in NPC patients. We applied the method of Isolation by Size of Epithelial Tumor (ISET), which has been proven to be useful in detection of various cancer cells Citation16,17,18], to identify CTCs in NPC patients. Moreover, immunohistochemical staining using the CK5/6 (cytokeratin5/6) and P63 plus in situ hybridization using the EBV-specific biomarker of EBERs (EBV-encoded RNAs) was used to help differentiate NPC cells from normal blood cells.
Materials and methods
Patients and peripheral blood samples
This study included 33 patients who were diagnosed with NPC at the Sun Yat-sen University Cancer Center, Guangzhou, China, between December 2015 and April 2016. All patients were diagnosed with undifferentiated non-keratinizing nasopharyngeal carcinoma (the WHO type 3) confirmed by histopathology. For TNM-staging evaluation, all the patients underwent a PET/CT or radionuclide bone scan, chest X-ray or computed tomography of the chest, abdominal ultrasound, and skeletal scintigraphy. The TNM stage was evaluated according to the seventh edition of the American Joint Committee on Cancer (AJCC). Clinical data were collected from patients' medical records. We focused on patients' gender, age, EBV DNA load, EBV VCA-IgA, EBV EA-IgA, routine blood count, blood lipids, and blood coagulation parameters.
All enrolled patients were treated with standard therapy according to the NCCN guidelines. Before initiating treatment, 5 ml of peripheral blood from the antecubital vein was obtained for medical examination. Then 5 mL of peripheral blood was obtained in a BD Vacutainer tube (Lot. 367863; Becton, Dickinson & Co., Franklin, NJ) for CTC enumeration within 2 hours. Approval to review, analyze, and publish the data in this study was given by the Sun Yat-sen University Cancer Center Research Ethics Board. Written informed consent was obtained from all patients at their first visit.
CTCs/CTM detection and analysis
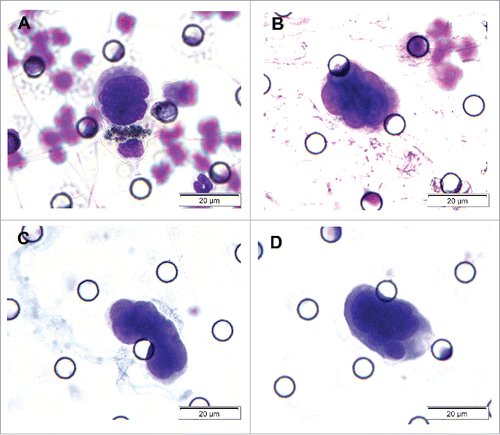
CTC analysis was performed using ISET assay as described in several previous studies.Citation16-18 Five milliliters of whole blood was diluted up to 8 ml with filtration buffer containing 0.2% paraformaldehyde. Then, each sample was harvested by a membrane with an 8-μm diameter and cylindrical pores. The membrane was stained with Romanowsky stain, washed with PBS, air-dried at room temperature, rinsed with TBS, and finally mounted with filter. Imaging of CTCs was captured with a confocal microscope (Olympus, DP130, Japan) with a 40× or 100× magnification objective. Cells isolated in this study were assigned as CTCs only if they met the following morphological criteria Citation4,17,18]: 1) the nuclear-cytoplasmic ratio was greater than 0.8; 2) the long diameter of nucleus was larger than 18 μm; 3) irregularity (thickened, sunken and wrinkled) of the nuclear membrane; 4) a large nucleoli or abnormal mitotic figures; or 5) the presence of tumor cell aggregations. CTC clusters formed by at least 3 tumor cells were called circulating tumor microemboli (CTM). All candidates' CTCs/CTM were blindly reviewed and identified independently by 2 senior cytopathologists.
Immunohistochemical staining of tumor cells
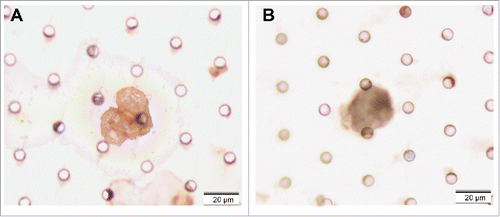
Slides prepared by ISET assay were immersed in 100% xylene for 3 hours at room temperature until cover glasses dropped off, and immersed in fresh xylene at room temperature for 5 minutes to remove the residual mounting medium on the slides. Immunohistochemical (IHC) staining against CK5/6 and P63 (Santa Cruz) was performed with commercial available antibodies according to the manufacturers' instructions. Cells were classified as CTCs when staining was positive for CK5/6 or P63 and the above-mentioned morphological criteria were fulfilled.
In situ hybridization (ISH) of EBERs
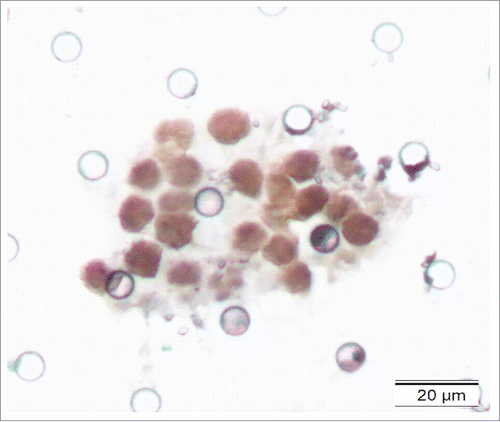
The cover glass and mounting medium on the filter membrane were removed as the aforementioned description. EBERs were detected using the EBV probe in situ hybridization kit (DIG-AP, A300K.9901, PanPath Company, Amsterdam, Netherlands) as described in previous reports.Citation19,20 Briefly, the process included the following steps: dehydration by a series of graded ethanol; pretreatment with 0.4% pepsin for 10 minutes; hybridization with digoxigenin-conjugated EBV (EBERs) probe at 37°C for 3 hours; signal detection using peroxidase-conjugated anti-digoxigenin antibody and 3,3′-diaminobenzidine (DAB); and counterstaining the sections with hematoxylin solution. The positive signals were brownish-yellow and localized within the nuclei.
Statistical analysis
The Fisher's Exact Test or Linear-by-Linear Association analysis was used for the comparison of CTCs positivity between 2 groups or among multiple groups. The Student t-test or ANOVA analysis was performed for quantitative variables between 2 groups or among multiple groups. Before analysis, the raw data of EBV DNA load was normalized by 103 and the titers of VCA-IgA and EA-IgA were log-transformed to be a normal distribution. The partial correlation coefficient between the numbers of CTCs and other quantitative variables was performed adjusted for sex and age. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS version 16.0), and P ≤ 0.05 was considered significant.
Results
Detection of CTCs/CTM in NPC patients
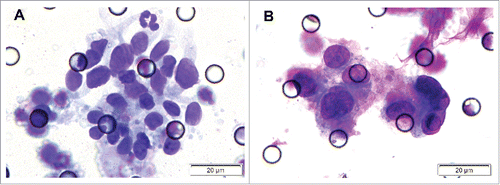
The study sample comprised 21 male and 12 female, with a mean age of 44.6 y (ranged from 16 to 69 years). Out of 33 NPC patients, 31 cases were being treated for the first time and 2 patients were re-admitted due to relapse after radiotherapy. The overall positive rate of CTCs was 66.7% (22/33) with a mean CTCs number of 2.06 cells (ranged from 0 to 12 cells) (). Two patients at T3N3M0 stage demonstrated CTM ().
To further characterize the CTCs/CTM detected by ISET assay, 2 CTC-positive samples and 2 CTM-positive samples were randomly selected for analysis by IHC and ISH staining. The samples were stained with CK5/6 and P63 (for epithelial cells), indicating the epithelia-origin of tumor cells. EBERS positive staining indicated EBV infection (–).
Association between CTCs and clinical stages of NPC patients
The occurrence of CTCs in the NPC patients' peripheral blood was evaluated in association with clinical stages. There was no statistically significant difference in CTCs positivity and CTCs number among different clinical stages, including T stage, N stage, M stage and a combined TNM stage (). CTCs were found in all clinical stages. This might explain why some patients at early stage relapsed after the local treatment. Among the 5 patients who had distant metastasis (confirmed by imaging and histopathology), 4 patients were CTCs positive, whereas one patient with multi-bone metastases did not show CTCs/CTM although it has been detected in triplicate. This patient was a 47-year-old Cantonese male, who was diagnosed of NPC at T1N3M1 IVc stage with multi-bone metastasis,. The patient had no special history and his vital signs were stable; however, his EBV DNA level was 1.02 × 107 copy/ml, indicating a large virus load.
Table 1. Association between CTCs and TNM stage in NPC patients.
Correlation between CTCs and EBV activation in NPC Patients
Circulating EBV DNA load, VCA-IgA and EA-IgA are the most common indicators of EBV activation. The relationship of CTCs with EBV status was analyzed. We observed that the presence of CTCs was associated with high EBV VCA-IgA levels in the all NPC patients (P = 0.016) and in the NPC patients without metastasis (P = 0.009) (). When the number of CTCs was considered, a positive correlation was observed between CTCs count and VCA-IgA titer (R = 0.382, P = 0.041), as well as between CTCs and EBV DNA (R = 0.396, P = 0.033) in all NPC patients (). Moreover, correlation coefficients were slightly elevated in the NPC patients without distant metastasis (). No analysis was performed for the NPC patients with metastasis because the sample size was not big enough.
Table 2. Association between CTCs and EBV activation in NPC Patients.
Table 3. Correlation between CTC numbers and EBV hematological features in NPC Patients.
Correlation between CTCs and hematological characteristics in NPC Patients
Because a correlation had been reported between CTCs/CTM and platelet counts in patients with esophageal squamous cell carcinoma Citation17], we explored whether CTCs were associated with hematological characteristics in the NPC patients (). In all 33 NPC patients, no significant associations were observed between the positive rate of CTCs and parameters of routine blood count, blood lipids and blood coagulation factors (all P > 0.05). In a small subgroup of 5 patients with distant metastasis, the positive rate of CTCs appeared to be significantly associated with eosinophilic counts (P = 0.007) and LDL levels (P = 0.006), which required further confirm with a large study sample.
Table 4. Association between CTCs and blood biochemical parameters.
Discussion
Despite the promising potential for CTCs to be a diagnostic, monitoring and prognostic biomarker for solid carcinoma, little is known about their role in NPC patients. Using an ISET assay, CTCs/CTM were detectable in 66.7% NPC patients in different clinical stages. We evaluated the relationship of CTCs with EBV activation in NPC. CTC counts were positively correlated with the levels of EBV DNA load and VCA-IgA titers in NPC. Such a link is of great importance for the monitoring of NPC development and progression.
In the past few years, the ISET method, which takes advantage of the physical and biologic properties of tumor cells, has facilitated the detection of CTCs.Citation21 This method has been widely used for various solid carcinomas, including liver, esophageal and breast cancers.Citation7,17,22 Harvested tumor cells by this technique are active and visible, allowing for identification of CTCs at morphological, immunocytologic, and genetic levels. By the staining of CK5/6, P63 and EBERs, tumor cells were further confirmed in this study. This method has high flexibility and could be recommended for CTC research in NPC.
It is unfortunate that we did not find any statistically significant relationship between CTCs and TNM stages of NPC. Feng L and his colleagues recently reported a positive rate of 52.6% for CTCs in NPC patients, in which they also showed no association for CTC number or positivity with TNM stages or other clinical parameters.Citation11 Although negative association was observed in the current study and previously published study, no confirmed conclusion could be drawn based on the currently available data. One of the reasons might be due to the small sample size. Therefore, further studies with large-scale study samples should be added to better address this issue.
EBV activation is a confirmed risk factor in NPC carcinogenesis. When EBV is activated, the levels of circulating EBV DNA load, VCA-IgA and EA-IgA were significantly elevated.Citation14,15,23 In this study, we also evaluated the influence of EBV status on the detection of CTCs. We observed positive associations between CTC counts and EBV DNA load and EBV VCA-IgA levels. These observations may indicate a biologic link between EBV and tumor cells shed from the nasopharynx. Previous studies indicated that patients with NPC having high levels of EBV VCA-IgA and EBV DNA loads are at greater risk of developing distant metastatic or recurrent NPC,Citation24,25 predicating a poorer prognosis.Citation26-28 In the incubation period of infection, EBV resides in lymphocytes of the nasopharyngeal mucosa. When EBV is activated, it may be released into the blood as circular DNA molecules due to the massive viral replication. Therefore, the EBV DNA load may be an effective and dynamic monitoring factor for NPC prognosis. The combined determination of CTCs and EBV DNA load or VCA-IgA may be used to better predict the relapse of NPC.
A major strength of the present study is that we found a positive correlation between the number of CTCs and EBV DNA load and VCA-IgA levels. Consistent results were found for all NPC patients and in patients without distant metastases, providing firm evidence of a link between CTCs and EBV activation in NPC. However, several limitations of this study should also be considered. The study sample is small especially for the subjects with distant metastasis. Therefore, no further analysis could be performed for NPC with distant metastasis. Another major limitation of this study is that the data were only obtained from a single center. These results warrants further validated in other data sets.
Conclusions
In summary, our study suggested detectable CTCs/CTM in NPC and supported a close link between CTCs and EBV activation. CTC counts were positively correlated with EBV DNA load and VCA-IgA levels. Although further clinical validation is needed, our results support the potential usefulness of CTCs as prognostic markers in NPC.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study is supported by grants from the Science and Technology Plan Project of International Cooperation of Guangdong Province [grant number 2016A050502011 and 2014A050503033]; National Natural Science Foundation of China [grant number 81572665 and 81602426]; Science and Technology Program of Guangdong Province [grant number 2013B021800141]; Medical Science and Technology Research Fund Project of Guangdong Province [grant number A2015003]; and Natural Science Foundation of Guangdong Province [grant number 2016A030310198].
References
- Wee JT, Ha TC, Loong SL, Qian CN. Is nasopharyngeal cancer really a “Cantonese cancer”? Chin J Cancer 2010; 29:517-26; PMID:20426903; https://doi.org/10.5732/cjc.009.10329
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 2009; 9:274-84; PMID:19308067; https://doi.org/10.1038/nrc2622
- Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massagué J. Tumor self-seeding by circulating cancer cells. Cell 2009; 139:1315-26; PMID:20064377; https://doi.org/10.1016/j.cell.2009.11.025
- Lecharpentier A, Vielh P, Perez-Moreno P, Planchard D, Soria JC, Farace F. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br J Cancer 2011; 105:1338-41; PMID:21970878; https://doi.org/10.1038/bjc.2011.405
- Chen W, Weng S, Zhang F, Allen S, Li X, Bao L, Lam RH, Macoska JA, Merajver SD, Fu J. Nanoroughened surfaces for efficient capture of circulating tumor cells without using capture antibodies. Acs Nano 2013; 7:566-75; PMID:23194329; https://doi.org/10.1021/nn304719q
- Stott SL, Hsu CH, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci U S A 2010; 107:18392-7; PMID:20930119; https://doi.org/10.1073/pnas.1012539107
- Farace F, Massard C, Vimond N, Drusch F, Jacques N, Billiot F, Laplanche A, Chauchereau A, Lacroix L, Planchard D, et al. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br J Cancer 2011; 105:847-53; PMID:21829190; https://doi.org/10.1038/bjc.2011.294
- Doyen J, Alix-Panabieres C, Hofman P, Parks SK, Chamorey E, Naman H, Hannoun-Lévi JM. Circulating tumor cells in prostate cancer: a potential surrogate marker of survival. Crit Rev Oncol Hematol 2012; 81:241-56; PMID:21680196; https://doi.org/10.1016/j.critrevonc.2011.05.004
- Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26:3213-21; PMID:18591556; https://doi.org/10.1200/JCO.2007.15.8923
- Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011; 29:1556-63; PMID:21422424; https://doi.org/10.1200/JCO.2010.28.7045
- Li F, Liu J, Song D, Zhang Q, Ding N, He X. Circulating tumor cells in the blood of poorly differentiated nasal squamous cell carcinoma patients: correlation with treatment response. Acta Otolaryngol 2016; 136(11):1164-7; PMID:27387761; https://doi.org/10.1080/00016489.2016.1258731
- Tsai CN, Tsai CL, Tse KP, Chang HY, Chang YS. The Epstein-Barr virus oncogene product, latent membrane protein 1, induces the downregulation of E-cadherin gene expression via activation of DNA methyltransferases. Proc Natl Acad Sci U S A 2002; 99:10084-9; PMID:12110730; https://doi.org/10.1073/pnas.152059399
- Lin JC, Wang WY, Chen KY, Wei YH, Liang WM, Jan JS, Jiang RS. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med 2004; 350:2461-70; PMID:15190138; https://doi.org/10.1056/NEJMoa032260
- Song C, Yang S. A meta-analysis on the EBV DNA and VCA-IgA in diagnosis of Nasopharyngeal Carcinoma. Pak J Med Sci 2013; 29:885-90; PMID:24353651.
- Li S, Deng Y, Li X, Chen QP, Liao XC, Qin X. Diagnostic value of Epstein-Barr virus capsid antigen-IgA in nasopharyngeal carcinoma: a meta-analysis. Chin Med J (Engl) 2010; 123:1201-5; PMID:20529563
- Hofman VJ, Ilie MI, Bonnetaud C, Selva E, Long E, Molina T, Vignaud JM, Fléjou JF, Lantuejoul S, Piaton E, et al. Cytopathologic detection of circulating tumor cells using the isolation by size of epithelial tumor cell method: promises and pitfalls. Am J Clin Pathol 2011; 135:146-56; PMID:21173137; https://doi.org/10.1309/AJCP9X8OZBEIQVVI
- Li H, Song P, Zou B, Liu M, Cui K, Zhou P, Li S, Zhang B. Circulating Tumor Cell Analyses in Patients With Esophageal Squamous Cell Carcinoma Using Epithelial Marker-Dependent and -Independent Approaches. Medicine (Baltimore) 2015; 94:e1565; PMID:26402816; https://doi.org/10.1097/MD.0000000000001565
- Hofman V, Long E, Ilie M, Bonnetaud C, Vignaud JM, Fléjou JF, Lantuejoul S, Piaton E, Mourad N, Butori C, et al. Morphological analysis of circulating tumour cells in patients undergoing surgery for non-small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathology 2012; 23:30-8; PMID:21210876; https://doi.org/10.1111/j.1365-2303.2010.00835.x
- Huang YH, Wu QL, Zong YS, Feng YF, Hou JH. Nasopharyngeal extranodal NK/T-cell lymphoma, nasal type: retrospective study of 18 consecutive cases in Guangzhou, China. Int J Surg Pathol 2011; 19:51-61; PMID:21134987; https://doi.org/10.1177/1066896910388806
- Wang L, Lin Y, Cai Q, Long H, Zhang Y, Rong T, Ma G, Liang Y. Detection of rearrangement of anaplastic lymphoma kinase (ALK) and mutation of epidermal growth factor receptor (EGFR) in primary pulmonary lymphoepithelioma-like carcinoma. J Thorac Dis 2015; 7:1556-62; PMID:26543602; https://doi.org/10.3978/j.issn.2072-1439.2015.05.11
- Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schütze K, Capron F, Franco D, Pazzagli M, Vekemans M, et al. Isolation by size of epithelial tumor cells : a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol 2000; 156:57-63; PMID:10623654; https://doi.org/10.1016/S0002-9440(10)64706-2
- Wu LJ, Pan YD, Pei XY, Chen H, Nguyen S, Kashyap A, Liu J, Wu J. Capturing circulating tumor cells of hepatocellular carcinoma. Cancer Lett 2012; 326:17-22; PMID:22842097; https://doi.org/10.1016/j.canlet.2012.07.024
- Tay JK, Chan SH, Lim CM, Siow CH, Goh HL, Loh KS. The role of epstein-barr virus DNA load and serology as screening tools for nasopharyngeal carcinoma. Otolaryngol Head Neck Surg 2016; 155(2):274-80; PMID:27143706; https://doi.org/10.1177/0194599816641038
- Tang LQ, Chen QY, Fan W, Liu H, Zhang L, Guo L, Luo DH, Huang PY, Zhang X, Lin XP, et al. Prospective study of tailoring whole-body dual-modality [18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol 2013; 31:2861-9; PMID:23857969; https://doi.org/10.1200/JCO.2012.46.0816
- Adham M, Greijer AE, Verkuijlen SA, Juwana H, Fleig S, Rachmadi L, Malik O, Kurniawan AN, Roezin A, Gondhowiardjo S, et al. Epstein-Barr virus DNA load in nasopharyngeal brushings and whole blood in nasopharyngeal carcinoma patients before and after treatment. Clin Cancer Res 2013; 19:2175-86; PMID:23493345; https://doi.org/10.1158/1078-0432.CCR-12-2897
- Lin JC, Chen KY, Wang WY, Jan JS, Liang WM, Tsai CS, Wei YH. Detection of Epstein-Barr virus DNA in the peripheral-blood cells of patients with nasopharyngeal carcinoma: relationship to distant metastasis and survival. J Clin Oncol 2001; 19:2607-15; PMID:11352952; https://doi.org/10.1200/jco.2001.19.10.2607
- Leung SF, Zee B, Ma BB, Hui EP, Mo F, Lai M, Chan KC, Chan LY, Kwan WH, Lo YM, et al. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol 2006; 24:5414-8; PMID:17135642; https://doi.org/10.1200/JCO.2006.07.7982
- Leung SF, Lo YM, Chan AT, To KF, To E, Chan LY, Zee B, Huang DP, Johnson PJ. Disparity of sensitivities in detection of radiation-naive and postirradiation recurrent nasopharyngeal carcinoma of the undifferentiated type by quantitative analysis of circulating Epstein-Barr virus DNA1,2. Clin Cancer Res 2003; 9:3431-4; PMID:12960133